Abstract
BACKGROUND AND PURPOSE: MR venography is often used to examine the intracranial venous system, particularly in the evaluation of dural sinus thrombosis. The purpose of this study was to evaluate the use of MR venography in the depiction of the normal intracranial venous anatomy and its variants, to assess its potential pitfalls in the diagnosis of dural venous sinus thrombosis, and to compare the findings with those of conventional catheter angiography.
METHODS: Cerebral MR venograms obtained in 100 persons with normal MR imaging studies were reviewed to determine the presence or absence of the dural sinuses and major intracranial veins.
RESULTS: Systematic review of the 100 cases revealed transverse sinus flow gaps in 31% of the cases, with 90% of these occurring in the nondominant transverse sinus and 10% in the codominant transverse sinuses. No flow gaps occurred in the dominant transverse sinuses. The superior sagittal and straight sinuses were seen in every venogram; the occipital sinus was seen in only 10%. The vein of Galen and internal cerebral veins were also seen in every case; the basal veins of Rosenthal were present in 91%.
CONCLUSIONS: Transverse sinus flow gaps can be observed in as many as 31% of patients with normal MR imaging findings; these gaps should not be mistaken for dural sinus thrombosis.
The use of cerebral MR venography is increasing in frequency as a noninvasive means of evaluating the intracranial venous system. This technique is particularly useful in the diagnosis of venous sinus thrombosis, which at times can be difficult. The purpose of this study was to evaluate the normal intracranial venous anatomy and its variants as depicted by MR venography in order to facilitate the interpretation of these examinations and thereby to help avoid potential pitfalls in the diagnosis of dural venous sinus thrombosis.
Methods
Using cerebral MR venography, we prospectively examined 100 patients (ages, 9 days to 83 years) who had had normal findings on MR examinations. Patients with any intracranial abnormality, congenital anomaly, venous thrombosis, tumor, or previous craniotomy were excluded from the study. All MR venograms were performed on 1.5-T scanners using a contiguous 2D time-of-flight (TOF) MR angiographic technique and an inferior saturation band to eliminate signal from arterial structures. Sections with a thickness of 1.5 mm were acquired in the coronal plane using the following parameters: 50/8.4 (TR/TE), 60° flip angle, 210-mm field of view, and 256 × 128 matrix. All MR venographic source images thus obtained were postprocessed using the maximum (pixel) intensity projection (MIP) method, generating 12 MIP projections at 15° increments. Using a schematic diagram as a template, we systematically reviewed the resulting venograms in each case for the presence or absence of the transverse sinuses and the major intracranial veins, including the internal cerebral veins, the basal veins of Rosenthal, and the vein of Galen. Ten additional patients who had undergone both MR venography and conventional catheter angiography were also included in the study. The MR venograms in these 10 cases were compared with their corresponding conventional catheter angiograms, with emphasis on the transverse sinuses.
Results
Of the 100 MR venograms obtained, the transverse sinus was found to be right, left, and codominant in 59%, 25%, and 16% of the cases, respectively (Fig 1). Flow gaps within the transverse sinuses were observed in 31% of the studies. Of these, 90% (28/31) occurred in the nondominant transverse sinus. In the remaining 10% (3/31), flow gaps were seen in transverse sinuses that were considered codominant. Notably, no flow gaps were observed on the dominant side. In 48% (15/31) of the cases showing transverse sinus flow gaps, the length of the gap was less than or equal to one third the length of the ipsilateral sinus. Of the remaining 16 cases showing transverse sinus flow gaps, eight (26%) had flow gaps that were greater than one third but less than two thirds the length of the ipsilateral transverse sinus, and the remaining eight (26%) had transverse sinus flow gaps that were greater than two thirds the length of the ipsilateral transverse sinus (Fig 2). While the superior sagittal and straight sinuses were each observed in every study, the inferior sagittal sinus was seen in only 52% of the studies. An occipital sinus was observed in 10% of the cases. The vein of Galen was observed in all 100 cases, as were the paired internal cerebral veins; the paired basal veins of Rosenthal were seen in 91% of the cases. Also of note, the right vein of Trolard was observed in 37% of the cases, and the left vein of Trolard was seen in 34%; the right anastomotic vein of Labb´e was seen in 91% of the studies, and the left anastomotic vein of Labb´e was present in 96% (Fig 3). Examination of the 10 additional cases in which both an MR venogram and a conventional catheter angiogram were available revealed three cases in which the right dominant transverse sinuses were seen on both studies but small, hypoplastic (yet patent) nondominant transverse sinuses were seen only on the conventional catheter angiograms. In these three cases, the corresponding MR venograms failed to show a complete sinus on the nondominant side, but instead showed flow gaps (Fig 4). In four of these 10 cases, right transverse dominance was found on both studies, without a flow gap in the nondominant transverse sinus. In two cases, codominance was shown on both studies, again without a flow gap; and in one case, a left dominant transverse sinus was depicted on both studies, also without a flow gap.
fig 1.
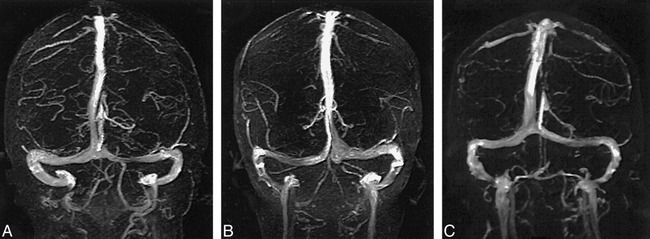
A–C, Transverse sinuses were found to be right (A), left (B), and codominant (C) in 59%, 25%, and 16% of the cases examined, respectively.
fig 2.
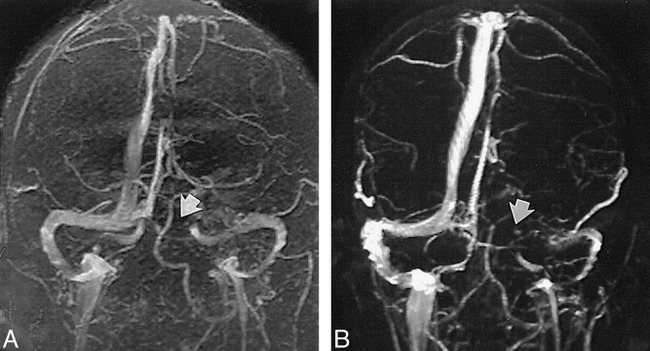
A and B, Length of flow gap is approximately one third (A, arrow) or two thirds (B, arrow) the size of the ipsilateral sinus
fig 3.
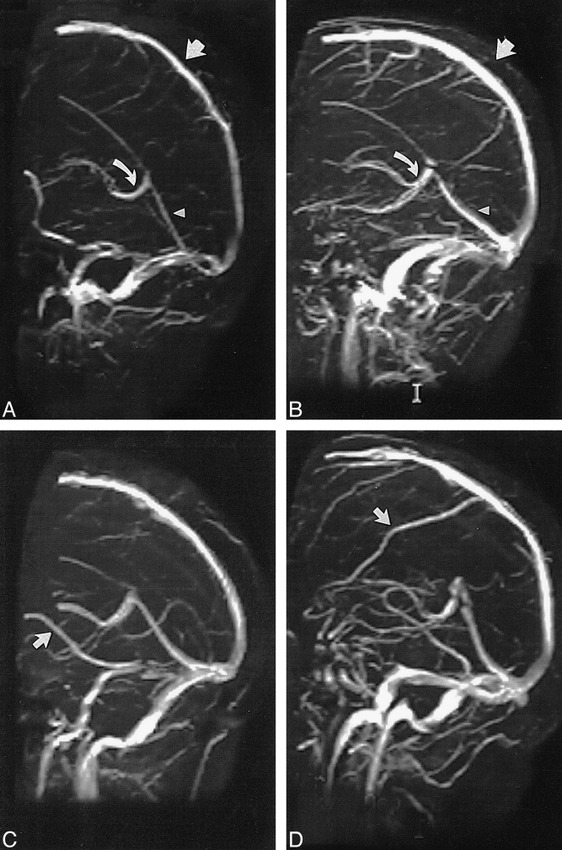
A and B, The superior sagittal sinus (straight arrow), straight sinus (arrowhead), and vein of Galen (curved arrow) are clearly depicted, and were seen in all 100 cases studied, without flow gaps.
C and D, The right vein of Labb´e (C, arrow) was seen in 91% of the cases. The right vein of Trolard (D, arrow), depicted as a large tributary to the superior sagittal sinus, was seen in only 37% of the cases.
fig 4.
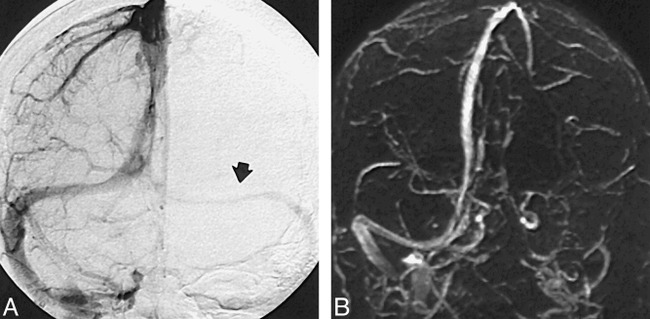
A and B, Venous phase of a conventional intra-arterial catheter angiogram clearly shows flow within the nondominant transverse sinus (A, arrow), whereas corresponding MR venogram (B) shows a flow gap
Discussion
Dural venous sinus thrombosis, seen in a number of conditions, including dehydration, hypercoagulable states, infection, tumor invasion, and in conjunction with oral contraception, may be a cause of neurologic deterioration. This diagnosis has traditionally been made during the venous phase of conventional catheter angiography, which has been considered the standard of reference (1, 2). This is, however, an invasive procedure with well-known associated risks (3). Recent reports have suggested that perhaps MR imaging and MR angiography may be able to replace conventional angiography in the diagnosis of dural venous sinus thrombosis (4–6). Indeed, in our series, we found that the distribution of transverse sinus dominance was in excellent agreement with the distributions reported in the literature, which were based on conventional intraarterial methods (7–10). Furthermore, we found that in every MR venographic examination performed, there was full visualization of the superior sagittal sinus, the straight sinus, and the vein of Galen, as well as the dominant transverse sinus, without evidence of a flow gap. High rates of visualization were also noted for the basal veins of Rosenthal as well as for an anastomotic vein of Labb´e (Fig 3). Our data also show that while there was excellent depiction of the aforementioned vessels, transverse sinus flow gaps were observed in 31% of nondominant transverse sinuses in patients whose MR studies were normal. We believe that these transverse sinus flow gaps, which were found only in nondominant transverse sinuses, are artifactual in nature. This supposition is supported by conventional catheter angiograms, which show the presence of hypoplastic, but patent, nondominant transverse sinuses in cases in which the corresponding MR venograms failed to show sinus continuity. The observation of such flow gaps could potentially lead to diagnostic difficulties when the issue of venous sinus thrombosis is in question. To interpret MR angiograms accurately and avoid such potential pitfalls in diagnosis, one must be aware of the technical limitations of 2D-TOF imaging itself. These limitations, which may give rise to flow gaps, are mainly related to artifacts resulting from slow intravascular blood flow, in-plane flow, and complex blood flow patterns, with the added problem of the MR angiographic postprocessing method used.
Although 2D-TOF imaging is exquisitely sensitive to slow flow, it nevertheless has a minimum threshold below which sufficient signal from flowing blood cannot be obtained. If flowing spins within the selected 2D slice slab are not replenished by TR, which is usually on the order of 45 to 50 milliseconds, these spins become subject to repeated pulses and become saturated, as do the surrounding stationary tissues, resulting in signal loss from the selected slice. This signal loss, if severe, can result in the production of artifactual flow gaps within a vessel. To overcome this problem, it is desirable to set the slice thickness as small as possible, typically on the order of 1.0 to 1.5 mm. This minimum through-plane velocity, which can be determined by the simple relation V = d/TR, where V is the minimum velocity of flowing blood (cm/s), TR is the pulse repetition time (in ms), and d is the slice thickness (in mm), is approximately 3 cm/s, and in actual practice, rarely becomes an issue with MR venography.
To overcome artifactual signal loss resulting from in-plane vascular flow, it is desirable to orient the acquisition plane perpendicular to the long axis of the vessel being imaged. Because the bulk of the intracranial venous flow is in an anteroposterior direction, toward the torcular Herophili, slice acquisitions in the coronal plane satisfy the requirement of perpendicular orientation during acquisition for a majority of the intracranial dural sinuses. Although this improves signal from vessels that are oriented predominantly in the sagittal plane (superior sagittal sinus, straight sinus, and so on), signal loss can become a potential problem in the region of the torcular Herophili and posterior aspect of the superior sagittal and transverse sinuses, as these segments gradually become coplanar with the imaging plane. Conversely, 2D-TOF imaging in the sagittal plane alone, which would be expected to improve the signal and enhance visualization of these posterior regions, would be suboptimal for showing the superior sagittal sinus, straight sinus, inferior sagittal sinus, portions of the sigmoid sinuses, and so forth. In an attempt to overcome the technical limitations associated with both acquisition planes, one may be tempted to prescribe a doubly oblique plane (ie, 45°) to both the coronal and sagittal planes. At first glance this may seem to be a solution for obtaining full coverage of the sinuses and thereby for eliminating in-plane orientation, but it becomes apparent that no one rectilinear acquisition plane can be made simultaneously orthogonal to all segments of each sinus or vessel. Furthermore, even if a doubly oblique acquisition plane were to be used in obtaining an MR venographic data set, there currently are software limitations that make it impracticable and difficult to reorient the data set back into standard anatomic position which is (currently) necessary before MIP projections can be generated. As described above, the current approach of using a coronal acquisition plane is favored because of its maximum coverage of the predominantly anteroposterior direction of dural sinus flow.
Complex flow patterns, such as flow separation with vortex flow, can generate hemodynamic conditions that are difficult to image and that may contribute to intravascular signal loss. If, however, the T1 of blood were to be lowered through the use of MR contrast agents, detection of intravascular signal by TOF methods could be improved.
Whereas TOF techniques rely mainly on flow-related enhancement for producing vascular images, phase-contrast MR angiography uses velocity-induced phase shifts imparted upon the moving spins to distinguish flowing blood from the surrounding stationary tissue. Although excellent background suppression is a major advantage, and quantitative determination of blood velocities may be possible, phase-contrast MR angiography may require long imaging times and an a priori estimate of blood flow velocity; it may also be more sensitive to signal loss due to turbulence or intravoxel dephasing.
Finally, after acquisition of the stack of contiguous 2D-TOF slices, the image volume is subjected to the MIP ray tracing algorithm to present the MR venograms in a format similar to that generated by conventional angiography. Although robust and computationally fast, MIP is a suboptimal algorithm and suffers from its own limitations, often generating vascular images that exhibit an intrinsic underestimation of vessel caliber, which corresponds to overestimation of vessel stenoses and flow gaps. In an attempt to address and overcome these shortcomings of MIP, investigations into alternative postprocessing methods and ray-tracing algorithms are currently in progress (11).
Conclusion
Our study has shown that when using 2D-TOF MR venography, flow gaps in nondominant transverse sinuses can be observed in up to 31% of patients with normal MR imaging findings, and that such flow gaps should therefore be judged with caution when the diagnosis of dural sinus thrombosis is in question. Furthermore, since no flow gaps, artifactual or otherwise, were observed in the dominant transverse sinus, the superior sagittal sinus, the straight sinus, or the vein of Galen, such a finding should raise suspicion of an underlying pathologic etiology.
Footnotes
Address reprint requests to C. Roger Bird, MD, Barrow Neurological Institute, St. Joseph's Hospital & Medical Center, 350 W. Thomas Rd., Phoenix, AZ 85013.
References
- 1.Yasargil MG, Damur M. Thrombosis of the cerebral veins dural sinuses. In: Newton TH, Potts DJ, eds Radiology of the Skull and Brain: Angiography St. Louis: Mosby-Year Book; 1974;2:2375-2400 [Google Scholar]
- 2.Casey SO, Alberico RA, Patel M, et al. Cerebral CT venography. Radiology 1996;198:163-170 [DOI] [PubMed] [Google Scholar]
- 3.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401-1407 [PMC free article] [PubMed] [Google Scholar]
- 4.Padayachee TS, Bingham JB, Graves MJ, Colchester AC, Cox TC. Dural sinus thrombosis: diagnosis and follow-up by magnetic resonance angiography and imaging. Neuroradiology 1991;33:165-167 [DOI] [PubMed] [Google Scholar]
- 5.Mattle HP, Wentz KU, Edelman RR, et al. Cerebral venography with MR. Radiology 1991;178:453-458 [DOI] [PubMed] [Google Scholar]
- 6.Chakeres DW, Schmalbrock P, Brogan M, Yuan C, Cohen L. Normal venous anatomy of the brain: demonstration with gadopentate dimeglumine in enhanced 3-D MR angiography. AJNR Am J Neuroradiol 1990;11:1107-1118 [DOI] [PubMed] [Google Scholar]
- 7.Browning H. The confluence of dural venous sinuses. Am J Anat 1953;93:307-329 [DOI] [PubMed] [Google Scholar]
- 8.Dora F, Zileli T. Common variations of the lateral and occipital sinuses at the confluens sinuum. Neuroradiology 1980;20:23-27 [DOI] [PubMed] [Google Scholar]
- 9.Bisaria KK. Anatomic variations of venous sinuses in the region of the torcular Herophili. J Neurosurg 1985;62:90-95 [DOI] [PubMed] [Google Scholar]
- 10.Das AC, Hasan M. The occipital sinus. J Neurosurg 1970;33:307-311 [DOI] [PubMed] [Google Scholar]
- 11.Ayanzen RH, Keller PJ, Heiserman JE. A novel postprocessing method for 2D TOF MRA. In: Proceedings of the International Society for Magnetic Resonance in Medicine. 1997;1849


