Abstract
BACKGROUND AND PURPOSE: Imaging of patients with a clinical diagnosis of mandibular osteoradionecrosis (ORN) is often performed to support that clinical suspicion, evaluate the extent of the disease, or exclude coexistent tumor recurrence. The purpose of our study was to describe the clinical, MR imaging, and CT features of five patients with mandibular ORN associated with prominent soft-tissue abnormality in the adjacent masticator muscles.
METHODS: The MR and CT examinations of five patients with mandibular ORN associated with soft-tissue abnormalities in the adjacent masticator muscles were reviewed. All patients had received external beam radiotherapy for primary head and neck malignancies, with a total radiation dose range of 60 Gy to 69 Gy in 30 to 38 fractions.
RESULTS: CT revealed the typical osseous findings of cortical disruption, trabecular disorganization, and fragmentation in all five patients. Abnormal diffuse enhancement of the adjacent masseter and pterygoid muscles was noted in all patients. Four patients had prominent mass-like thickening of these muscles adjacent to the osseous abnormality. Of the three patients who underwent MR imaging, all showed homogeneous abnormal T1 hypointensity, T2 hyperintensity, and intense enhancement of the bone marrow in the involved mandible. The masticator muscles adjacent to the osseous abnormality also showed abnormal T2 hyperintensity and intense diffuse enhancement on MR images.
CONCLUSION: Mandibular ORN can be associated with prominent soft-tissue thickening and enhancement in the adjacent musculature. These changes can appear mass-like and are not related to tumor recurrence or metastatic disease.
Mandibular osteoradionecrosis (ORN) is a serious complication of radiation therapy for neoplasms of the parotid gland, oral cavity, oropharynx, and nasopharynx. It has a widely varying reported incidence of 5% to 22% (1−4). Chronic and progressive mandibular ORN can lead to significant problems of deglutition, mastication, and speech, with detrimental effects on quality of life. The diagnosis of mandibular ORN is primarily based on clinical symptoms and signs (5). Patients are often referred for imaging, however, to confirm the diagnosis, to evaluate the extent of the process for management decisions, or to exclude malignant entities such as tumor recurrence and metastatic disease. The purpose of our study was to describe the clinical, MR imaging, and CT findings of five patients with proved mandibular ORN associated with significant soft-tissue abnormality in the adjacent masticator muscles.
Methods
A retrospective review of the clinical data and imaging studies of five patients with mandibular ORN and associated soft-tissue abnormality was made. The clinical data are summarized in the Table herein. Four patients were male and one was female, with an age range of 17 to 74 years (mean age, 54 years). All patients had received external beam radiotherapy for primary head and neck malignancies, with a total radiation dose range of 60 Gy to 69 Gy in 30 to 38 fractions. The interval between the initiation of radiation therapy and the diagnosis of ORN ranged from 1 to 8 years (mean, 4.4 years). The diagnosis of mandibular ORN was confirmed pathologically by fine-needle aspiration biopsy in two patients and surgery in one and was based on stable and typical clinical and radiologic findings at follow-up in the remaining two patients. The duration of follow-up, with no evidence of tumor recurrence, ranged from 1.1 to 4.5 years (mean, 2.9 years).
Summary of clinical data of patients with mandibular osteoradionecrosis

A total of four MR and seven CT examinations was evaluated. CT was performed in all five patients. Three of these patients also underwent MR imaging. CT scanning was performed on GE scanners (Milwaukee, WI) after the IV administration of contrast medium. Axial 3 × 3-mm-thick sections were obtained in all studies. Additional 3 × 3-mm-thick coronal images were obtained for two patients. Soft-tissue and bone windows were generated for all studies. All MR examinations were performed using GE 1.5-T units. Precontrast T1-weighted (400−660/9−15 [TR/TE]) and T2-weighted (3700−6000/105 [TR/TEeff]) images, as well as contrast-enhanced (0.1 mmol/kg) T1-weighted images, were reviewed.
Results
All of the patients were symptomatic on the same side from which imaging abnormalities were revealed at the time of scanning. They all presented with swelling and pain, except for one who had dysesthesia, numbness, and tingling along the distribution of the inferior alveolar nerve. Three patients had trismus, and two developed complications of fistula formation and pathologic mandibular fracture. The mandibular ORN was ipsilateral to the original tumor site in four patients and contralateral in one. In all patients, the site of development of ORN was within the radiation field, but not immediately adjacent to the primary tumor.
The CT scans of all five patients showed cortical disruption, with disorganization and loss of trabeculation of the spongiosa of the mandible (Figs 1, 2). The osseous abnormalities involved the body (premolar and molar regions) of the mandible in one patient and the ramus in one, whereas the remaining three patients had involvement of both the body and ramus. One patient had a pathologic fracture of the body of the mandible. Air was seen in the masticator space of a patient who developed a cutaneous fistula. Diffuse abnormal enhancement of the masseter and pterygoid muscles adjacent to the osseous changes in the mandible was noted in all five patients when compared with the contralateral nonenhancing normal masticator muscles (Fig 2B). The enhancement was intense in three patients and mild in two. There was prominent mass-like thickening of these muscles in the masticator space in all patients except one.
fig 1.
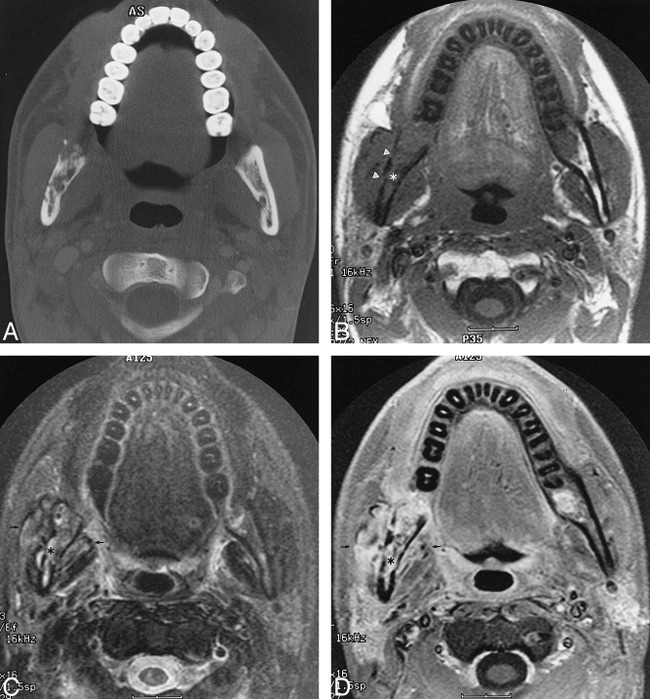
Images of a 17-year-old male patient who underwent radiation for left-sided poorly differentiated squamous cell carcinoma of the nasopharynx.
A, Axial CT scan (bone window) shows ORN involving the mandibular ramus contralateral to the primary tumor site with cortical disruption and trabecular disorganization.
B, Unenhanced T1-weighted (400/9 [TR/TE]) MR image shows abnormal homogeneous signal hypointensity of the bone marrow of the right mandible (asterisk) and cortical disruption (arrowheads). Note that the right masticator muscles appear slightly larger than the contralateral side.
C, Axial T2-weighted (4000/105 [TR/TEeff]) MR image shows abnormal hyperintense signal of the marrow (asterisk) and adjacent masseter and pterygoid muscles (arrows).
D, Contrast-enhanced fat-saturated T1-weighted (400/9 [TR/TE]) MR image shows diffuse intense enhancement of the marrow (asterisk) and adjacent musculature (arrows).
fig 2.
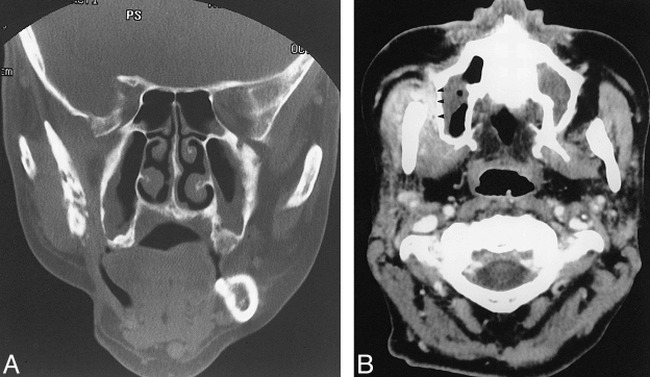
Images of a 49-year-old man treated for right-sided soft palate squamous cell carcinoma.
A, Coronal CT scan (bone window) shows cortical disruption and fragmentation of the right ramus.
B, Contrast-enhanced CT scan shows prominent thickening and enhancement of the adjacent masticator muscles. The buccal space is also involved (arrowheads).
MR imaging was performed in three of the five patients. The bone marrow of the involved portions of the mandible had abnormal signal in all three patients. These areas showed abnormal homogeneous low signal intensity on the T1-weighted sequence and high signal intensity on the T2-weighted sequence (Fig 1B-C). There was diffuse intense enhancement of the marrow after the IV administration of contrast medium in all studies (Fig 1D). Cortical disruption of the mandible was also evident. All three patients showed abnormal T2 hyperintensity of the masseter and pterygoid muscles adjacent to the abnormal bone, with diffuse intense enhancement of these muscles on the contrast-enhanced sequence when compared with the contralateral normal muscles of mastication (Fig 1B-D). Two patients also had prominent thickening of the masseter and pterygoid muscles adjacent to the osseous changes of ORN.
Discussion
The clinical diagnosis of mandibular ORN is primarily based on clinical symptoms and signs of ulceration or necrosis of the overlying mucous membrane with exposure of necrotic bone (5). Radiographic imaging of mandibular ORN is usually performed to confirm the diagnosis in clinically suspect patients with new onset of trismus or pain to evaluate the extent of mandibular involvement for surgical planning or follow-up, or when there is clinical confusion with possible tumor recurrence or metastatic disease. It has been said in the literature that the “absence of a soft-tissue mass strongly favors radiation necrosis” and that the “presence of a soft-tissue mass favors the diagnosis of sarcoma or tumor recurrence” (6). Our data, however, suggest that this is not necessarily true. Mandibular ORN can be associated with significant soft-tissue thickening and enhancement in the adjacent masticator muscles. This may appear mass-like. Therefore, the presence of a soft-tissue “mass” or other soft-tissue imaging abnormality adjacent to abnormal bone does not exclude the diagnosis of mandibular ORN. Because such soft-tissue abnormality may be misinterpreted as tumor recurrence, correlation should be made with the typical osseous findings of mandibular ORN on CT scans, which include cortical disruption, disorganization of trabeculation, and osseous fragmentation (7).
The development of osseous and associated soft-tissue abnormality at a site distant or contralateral to the primary tumor makes ORN more likely. Patient 5 in our study developed findings contralateral to the original site of the primary tumor, supporting the diagnosis of mandibular ORN. Of course, because synchronous or metachronous lesions can also occur in cancer patients, it is wise to ascertain that a lesion is within the radiation field before entertaining a diagnosis of ORN.
The interval after the initiation of radiation therapy and the onset of mandibular symptoms does not help to exclude ORN, particularly during the first 2 years. The risk of developing ORN is greatest during the first 6 to 12 months after radiation therapy (5, 8−10). Most tumor recurrences also tend to occur during the first 2 years after treatment (11). The risk of ORN, however, persists for years because the ability of the mandible to repair itself remains compromised (12). Thus, the development of symptoms more than 2 years after treatment may suggest ORN. Three of our patients developed mandibular ORN more than 2 years after treatment, with one developing mandibular problems as long as 8 years after therapy.
In the three patients from whom biopsies or surgical specimens were obtained, the typical histologic features of ORN were present, consisting of hypovascular or hypocellular osteonecrosis and the absence of active inflammation (12). In the appropriate clinical setting, these features do not generally present diagnostic difficulty to the pathologist.
The MR imaging appearance of mandibular ORN has received little attention in the literature (13, 14). Two patients in the series from Fujita et al (13) had homogeneous low signal intensity on their T1-weighted images and high signal intensity on their T2-weighted images of the mandible. Bachmann et al (14) reported 11 patients with mandibular ORN who had abnormal T1 hypointensity, T2 hyperintensity, and marked enhancement of the bone marrow after the administration of contrast medium. These studies, however, did not address the MR imaging features or the significance of associated soft-tissue changes in mandibular ORN. Our study shows similar bone marrow signal changes and enhancement of the involved portions of the mandible. Furthermore, MR imaging may also show abnormal T2 signal intensity, enhancement, and thickening of the masseter and pterygoid muscles associated with mandibular ORN.
Conclusion
Our study shows that mandibular ORN can be associated with significant soft-tissue thickening and enhancement in the muscles of mastication, which are not related to tumor recurrence or metastatic disease. These soft-tissue changes may appear mass-like and represent a potential pitfall to the unwary radiologist. Correlation with clinical and other radiographic findings, such as the well-described osseous changes of mandibular ORN on CT scans, will help to avoid misdiagnosis.
Footnotes
Presented at the 1998 ASHNR 32nd Annual Meeting, Phoenix, AZ.
Address reprint requests to Lawrence E. Ginsberg, MD, MD Anderson Cancer Center, Department of Radiology, Box 57, 1515 Holcombe Boulevard, Houston, TX 77030.
References
- 1.MacDougall JA, Evans AM, Lindsay RK. Osteoradionecrosis of the mandible and its treatment. Am J Surg 1963;106:816-818 [DOI] [PubMed] [Google Scholar]
- 2.Bedwinek JM, Shukovsky LJ, Fletcher GH, Daley TE. Osteonecrosis in patients treated with definitive radiotherapy for squamous cell carcinomas of the oral cavity and naso- and oropharynx. Radiology 1976;119:665-667 [DOI] [PubMed] [Google Scholar]
- 3.Morrish RB, Chan E, Silverman S, Meyer J, Fu KK, Greenspan D. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer 1981;47:1980-1983 [DOI] [PubMed] [Google Scholar]
- 4.Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10-year study: part I: factors influencing the onset of necrosis. Int J Radiat Oncol Biol Phys 1980;6:543-548 [DOI] [PubMed] [Google Scholar]
- 5.Epstein JB, Wong FLW, Stevenson-Moore P. Osteoradionecrosis: clinical experience and a proposal for classification. J Oral Maxillofac Surg 1987;45:104-110 [DOI] [PubMed] [Google Scholar]
- 6.Dalinka MK, Mazzeo VP. Complications of radiation therapy. Rev Diagn Radiol 1985;23:235-267 [PubMed] [Google Scholar]
- 7.Hermans R, Fossion E, Ioannides C, Van den Bogaert W, Ghekiere J, Baert AL. CT findings in osteoradionecrosis of the mandible. Skel Radiol 1996;25:31-36 [DOI] [PubMed] [Google Scholar]
- 8.Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10-year study: part II: dental factors: onset, duration and management of necrosis. Int J Radiat Oncol Biol Phys 1980;6:549-553 [DOI] [PubMed] [Google Scholar]
- 9.Beumer J, Harrison R, Sanders B, et al. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck Surg 1984;6:819-827 [DOI] [PubMed] [Google Scholar]
- 10.Starcke EN, Shannon IL. How critical is the interval between extractions and irradiation in patients with head and neck malignancy. Oral Surg 1977;43:333-337 [DOI] [PubMed] [Google Scholar]
- 11.Mancuso AA, Hanafee WN. Oral cavity and oropharynx including tongue base, floor of mouth and mandible. In: Mancuso AA, Hanafee WN, eds. Computed Tomography and Magnetic Resonance Imaging of the Head and Neck 2nd ed. Baltimore, Md: Williams & Wilkins; 1985;371-375
- 12.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983;41:283-288 [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Harada K, Masaki N, et al. MR imaging of osteoradionecrosis of the mandibule following radiotherapy for head and neck cancers. Nippon Acta Radiologica 1991;51:892-900 [PubMed] [Google Scholar]
- 14.Bachmann G, Robler R, Klett R, Rau WS, Bauer R. The role of magnetic resonance imaging and scintigraphy in the diagnosis of pathologic changes of the mandible after radiation therapy. Int J Oral Maxillofac Surg 1996;25:189-195 [DOI] [PubMed] [Google Scholar]


