Abstract
Preoperative chemoradiotherapy (CRT) for rectal cancer contributes to tumor down-staging and decreases locoregional recurrence. However, each patient shows a significantly different response to CRT. Therefore, the identification of predictive factors to CRT response would be beneficial to avoid unnecessary treatment. Cancer immunity in patients has been suggested to play an important role in the eradication of the tumor by CRT. In the present study, the utility of CD8+ and forkhead box P3 (FoxP3)+ tumor-infiltrating lymphocytes (TILs) and the expression of a novel immuno-regulatory factor, lactadherin (MFG-E8), in predicting CRT effectiveness in patients with rectal cancer was examined. A total of 61 patients with rectal cancer, who underwent curative resection following CRT were included in the study. The numbers of CD8+ and FoxP3+ TILs in a biopsy taken before CRT and MFG-E8 expression level in the specimens obtained at the time of the surgery after CRT were examined using immunohistochemical staining, and their association with clinicopathological characteristics, including patient survival, was determined. The tumors with more CD8+ TILs in the biopsy samples before CRT showed a significantly more favorable CRT response. The patients with tumors and a higher number of CD8+ TILs before CRT also exhibited significantly longer disease-free and overall survival times. Higher MFG-E8 expression level in post-CRT specimens was significantly associated with favorable CRT response; however, no significant association was found with any other clinicopathological characteristics, including survival time. The number of CD8+ TILs before CRT was a valuable predictor for CRT response and was associated with favorable prognosis in patients with lower rectal cancer and who were treated with CRT. High MFG-E8 expression level after CRT was also associated with a favorable CRT response.
Keywords: CD8+ TIL, FoxP3+ TIL, MFG-E8, rectal cancer, chemoradiotherapy, immunohistochemistry
Introduction
Preoperative chemoradiotherapy (CRT) has been widely accepted as a standard therapy for advanced lower rectal cancer and previous studies have shown that it contributed to tumor down-staging and decreased postoperative locoregional recurrence (1-3). Notably, each patient showed significantly different responses to CRT, with some cases showing little or no response (4,5). Therefore, identification of predictive factors for CRT response would be beneficial for selecting measures of treatment to avoid adverse events, such as radiation dermatitis, hematologic toxicity, and enteritis, which may be associated with CRT (1-3).
Previous studies have suggested that the host immune system might play an important role in the eradication of the tumor using preoperative CRT and that some immunological markers might predict the tumor response to CRT (6,7). Shinto et al (8) reported positive and negative associations between CRT response and the density of intraepithelial infiltration of CD8+ T cells and forkhead box P3 (FoxP3)+ regulatory T cells, respectively. Some studies have also reported the association between FoxP3+, CD4+, and CD8+ tumor-infiltrating lymphocytes (TILs) and CRT response in rectal cancer (9-12).
Lactadherin (MFG-E8) is a secreted glycoprotein with pleiotropic functions, including enhancement of phagocytosis of apoptotic cells by macrophages, anti-inflammatory effects, and VEGF-dependent angiogenesis (13-15). MFG-E8-knockout mice were reported to develop autoimmune and inflammatory diseases, due to the accumulation of dead cells (16). Furthermore, it plays an important role in the regulation of cancer immunity of the host and enhances tumorigenicity (16). Jinushi et al (17) reported that MFG-E8 promoted phagocytosis of apoptotic cells by macrophages and induced immune tolerance by the secretion of regulatory T cells, inducing cytokines. This phenomenon has been observed in some human tumors, such as cancer of the ovary, bladder, and prostate; malignant melanoma; and colorectal cancer (18-22). Notably, MFG-E8 expression has been reported to be a poor prognostic factor in malignant melanoma (21). Kusunoki et al (23) reported that MFG-E8 promoted tumor growth in a mouse model of colon cancer, and that MFG-E8 expression in advanced colorectal cancer was higher compared with that in adenoma or early colorectal cancer. Furthermore, Zhao et al (22) revealed that MFG-E8 expression in patients with colorectal cancer who were not treated with preoperative therapy was associated with lymph node metastasis, distant metastasis, and poor cancer-specific survival. However, the significance of MFG-E8 expression in patients with rectal cancer treated with preoperative CRT has not been clarified so far. Therefore, CD8+, FoxP3+ TILs, and MFG-E8 expression level in patients with rectal cancer and treated with preoperative CRT was investigated, and the association between their expression levels and clinicopathological features, response to CRT, and patient prognosis was also analyzed.
Materials and methods
Patients and specimens
A total of 61 patients, who underwent curative resection following CRT for T3-T4 lower rectal cancer at the Department of Surgical Oncology, University of Tokyo (Tokyo, Japan) between March 2001 and October 2009 were retrospectively analyzed. Written informed consent was obtained from all the patients. Tumor depth, nodal status, and presence of distant metastases were determined using colonoscopy, computed tomography, and magnetic resonance imaging.
The patients received a total radiation dose of 50.4 Gy (1.8 Gy in 28 fractions) and concomitant chemotherapy (oral administration of tegafur-uracil, 300 mg/m2/day and leucovorin, 75 mg/day for 28 days from the beginning to the end of irradiation). Total mesorectal excision, with lymph node dissection, was performed following an interval of 6-8 weeks post-CRT. All patients underwent regular follow-up examinations following surgery. Tumor markers were examined every 3 months, and abdominal and chest computed tomography was performed every 6 months. Total colonoscopy was performed annually.
All specimens were fixed in 10% formalin at room temperature for 24 h and embedded in paraffin. The histopathological findings were confirmed by several pathologists affiliated to the Department of Pathology, University of Tokyo (Tokyo, Japan), who were blinded to the samples. Data was collected from the patients' medical records, which were prospectively generated. The TNM classification was determined according to the Union for International Cancer Control, 9th edition (24). The post-CRT histological tumor regression grade was evaluated according to the Japanese Classification of Colorectal Carcinoma, 9th edition (Grade 0, no necrosis or regressive change; 1, >33.3% vital residual tumor cells; 2, <33.3% vital residual tumor cells; and 3, no vital residual tumor cells) (25). In the analysis of CD8 and FoxP3, specimens were evaluated before CRT. For MFG-E8 expression level, specimens were evaluated after CRT. Furthermore, in the analysis of MFG-E8, 5 cases with no residual cancer cells after preoperative CRT (Grade 3) were excluded.
The present study was approved by the Ethics Committee of the University of Tokyo on July 29, 2014 [approval no. 10476-(1)] and written informed consent was obtained from all the patients.
CD8, FoxP3, and MFG-E8 immunohistochemical staining
The tumor specimens were cut into 4-µm thick sections and immunohistochemically stained, as previously described (9). Primary anti-CD8 (cat. no. 4B11; dilution 1:50; Leica Biosystems) and anti-FoxP3 (cat. no. 236/E7; dilution 1:100; Abcam) mouse monoclonal antibodies, and the anti-MFG-E8 antibody [cat. no. 14A-11B; dilution 1:120; supplied by The Institute of Medical Science, University of Tokyo, (Tokyo, Japan)] were utilized (26). Normal tonsil tissue was used as the positive control for CD8, FoxP3, and normal breast tissue was used as the positive control for MFG-E8, and obtained from autopsy specimens, which were donated to the Division of Surgical Oncology, University of Tokyo for research purposes, and written informed consent was provided.
Evaluation of CD8, FoxP3, and MFG-E8 immunostaining
The number of immunoreactive lymphocytes was counted under a light microscope in three randomly selected fields, at x400 magnification, as previously described (9). Immunoreactive TIL densities and the median values of all samples were set as thresholds. Each sample was classified as ‘high’ if it was above the median and ‘low’ if it was below.
MFG-E8 expression was assigned scores according to the highest staining intensity, as described previously (26). The criteria for the scoring were as follows: 0, Negative staining or trace; 1, moderate; and 2, strong. Based on the scores, all sections were divided into two groups: High expression (a score of 2) or low expression (a score of 0 and 1). Analysis was performed by 2 observers independently, in a blinded manner, and interobserver agreement was confirmed using κ-statistics. Any discrepancies were resolved by discussion. The association of their expression level with CRT response, clinicopathological features and patient prognosis was subsequently analyzed.
Statistical analysis
The association between the density of TILs and CRT response was evaluated using a Wilcoxon signed rank test, while the association between MFG-E8 expression level and clinicopathological features was evaluated using a χ2, Fisher's exact, or unpaired t-tests, as appropriate. Overall survival (OS; defined as the time from surgery to death from any cause) and disease-free survival (DFS; defined as the time from surgery to cancer recurrence, secondary cancer, or death from any cause), were analyzed using the Kaplan-Meier method and log-rank test. Multivariate analysis was performed using Cox proportional hazards regression analysis. Interobserver agreement was confirmed using κ-statistics. P<0.05 was considered to indicate a statistically significant difference. All analyses were performed using JMP v11.0 software (SAS Institute Inc.).
Results
Clinicopathological analysis
The clinicopathological features of the patients are shown in Table I. The median age was 61.4 years (range, 33-78 years) and 39 patients (63.9%) were male. After preoperative CRT, 35 (57.4%) patients had T3-4 tumors. A total of 44 patients (72.1%) showed papillary carcinoma or well-differentiated adenocarcinoma histology, while 9 (14.8%) and 7 (11.5%) patients had lymph node and distant metastases (3 to the liver, 2 to the lung, 1 to the brain, and 1 paraaortic lymph node metastases), respectively. Based on the response to CRT classification, 35 (57.4%), 21 (34.4%), and 5 (8.2%) patients were categorized as Grades 1, 2 and 3. The median follow-up period was 5.8 years (range, 0.8-10.9 years).
Table I.
Characteristics of the patients with rectal cancer.
| Characteristics | Value |
|---|---|
| Sex, n (%) | |
| Male | 39 (63.9) |
| Female | 22 (36.1) |
| Mean age ± SD, years | 61.4±9.6 |
| pT stagea, n (%) | |
| T0-2 | 26 (42.6) |
| T3-4 | 35 (57.4) |
| Histological type, n (%) | |
| Well | 44 (72.1) |
| Mod | 16 (26.2) |
| Muc | 1 (1.6) |
| Lymphatic invasion, n (%) | |
| Present | 5 (8.2) |
| Abscent | 56 (91.8) |
| Vascular invasion | |
| Present | 31 (50.8) |
| Abscent | 30 (49.1) |
| Lymph node metastasis, n (%) | |
| Present | 9 (14.8) |
| Abscnet | 52 (85.2) |
| Distant metastasis, n (%) | |
| Present | 7 (11.5) |
| Abscent | 54 (88.5) |
| TNM stagea, n (%) | |
| 0-II | 49 (80.3) |
| III-IV | 12 (19.7) |
| Tumor regression gradeb, (%) | |
| 1 | 35 (57.4) |
| 2 | 21 (34.4) |
| 3 | 5 (8.2) |
aAccording to TMN classification of malignant tumors, 8th edition according to Union for the Iinternational Cancer Control.
bAccording to Japanese Classification of Colorectal Carcinoma, 9th edition. Well, well-differentiated adenocarcinoma; Mod, moderately-differentiated adenocarcinoma; Muc, mucinous adenocarcinoma.
CD8, FoxP3 and MFG-E8 expression level
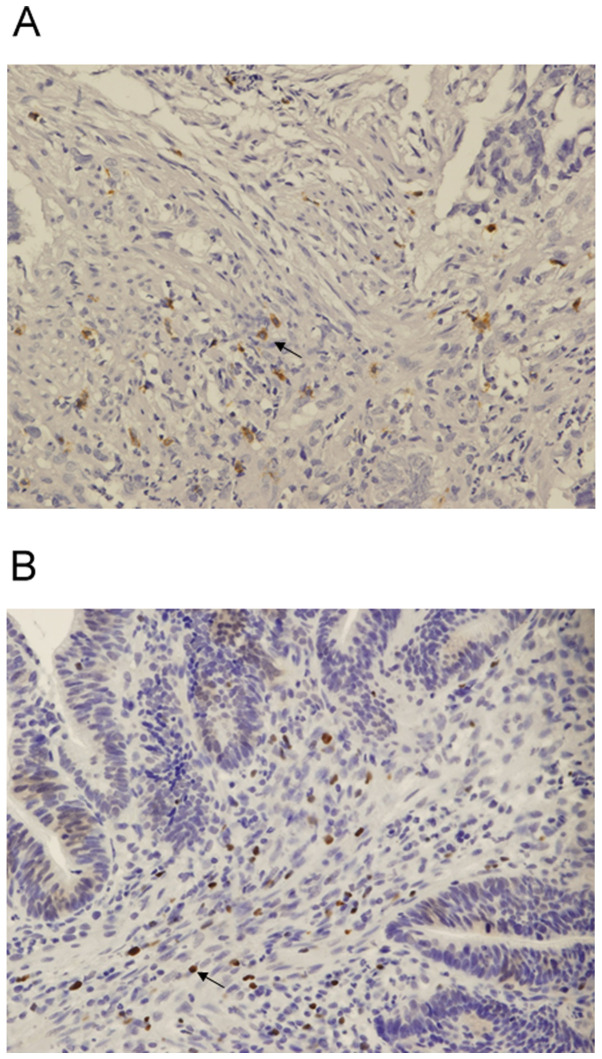
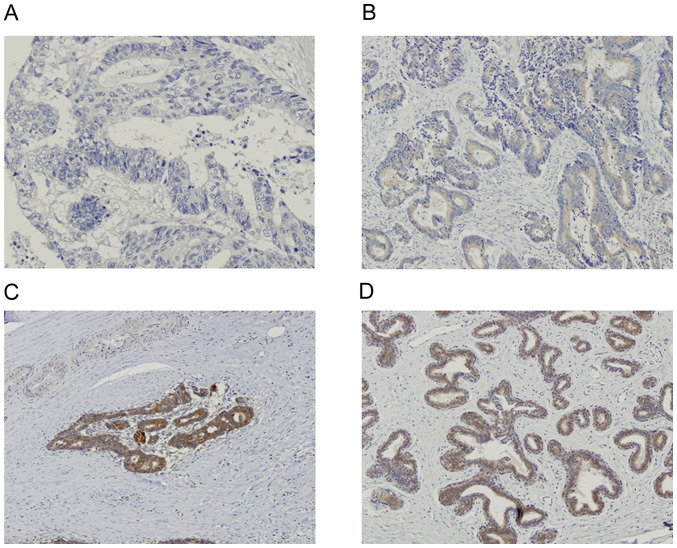
CD8 expression was detected in the cell membrane, while FoxP3 was detected in the nucleus (Fig. 1A and B, respectively). MFG-E8 expression level was detected in the cytoplasm of normal breast tissue, which was used as the positive control (Fig. 2A). MFG-E8 expression was also detected in the cytoplasm of rectal cancer tissue (Fig. 2B-D). Based on the immunohistochemistry scoring, 23 (41.1%) patients were categorized as MFG-E8 high.
Figure 1.
Immunohistochemical staining of CD8 and FoxP3 in biopsy samples from patients with lower rectal cancer. (A) Immunohistochemical staining of CD8. CD8 expression level was detected in the cell membrane. (B) Immunohistochemical staining of FoxP3. FoxP3 expression level was detected in the nucleus. Magnification, x100. FoxP3, forkhead box P3.
Figure 2.
Immunohistochemical staining of lactadherin. (A) Negative staining with a score of 0. (B) Moderate staining with a score of 1. (C) Strong staining with a score of 2. (D) Positive control (normal breast tissue). Magnification, x100.
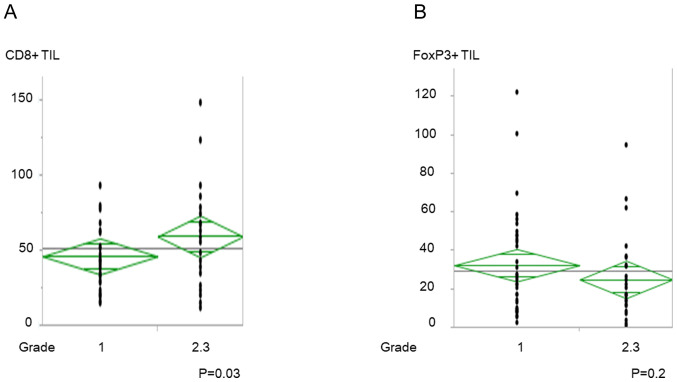
Association between CD8+ and FoxP3+ TILs, and CRT response
The increased number of CD8+ TILs before CRT was significantly associated with good CRT response (P=0.03; Fig. 3A); however, there was no significant association with FoxP3+ TILs (Fig. 3B). CD8/FoxP3+ TIL ratio was also associated with good CRT response (P=0.04; data not shown).
Figure 3.
Numbers of CD8+ and FoxP3+ TILs and histological response to CRT. (A) CD8+ TILs and (B) FoxP3+ TILs and histological response to CRT. TIL, tumor-infiltrating lymphocytes; CRT, preoperative chemoradiotherapy.
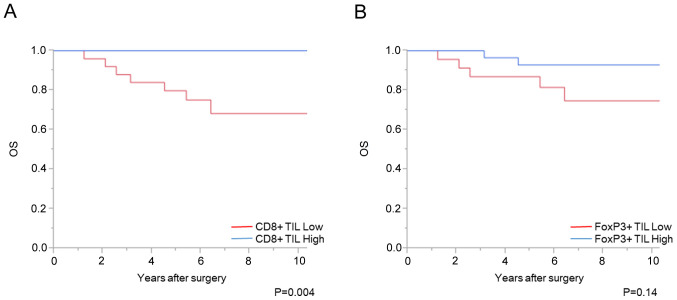
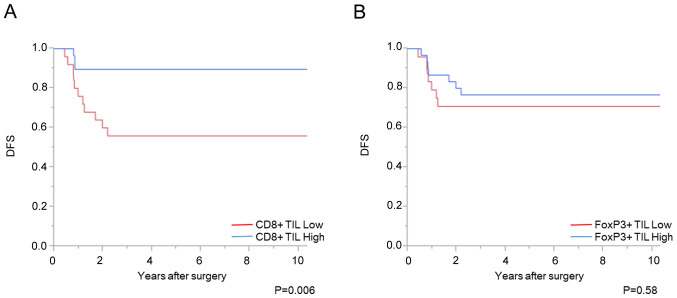
Association between CD8+ and FoxP3+ TILs, and patient prognosis
A total of 7 cases with distant metastasis were excluded from the prognostic analysis. The increased number of CD8+ TILs before CRT was significantly associated with favorable OS and DFS times (P<0.01 and P<0.01, respectively); however, there was no significant association with the number of FoxP3+ TILs (Figs. 4 and 5). Univariate analysis showed that the density of CD8+ TIL before CRT, vascular invasion and pathological T stage was associated with DFS time (P<0.01, P=0.04, and P<0.01, respectively). Using a multivariate analysis, the density of CD8+ TIL before CRT, in addition to pathological T stage, was independently associated with DFS time (Table II). With respect to OS time, univariate analysis showed that the density of CD8+ TIL before CRT and pathological T stage was associated with OS time (P<0.01 and P<0.01, respectively). In addition, multivariate analysis showed that the density of CD8+ TIL before CRT was independently associated with OS time (P=0.01), although 7 patients died.
Figure 4.
Numbers of CD8+ and FoxP3+ TILs and OS time. Association between (A) CD8+ TILs and (B) FoxP3+ TILs and OS time. TILs, tumor-infiltrating lymphocytes; OS, overall survival; FoxP3, forkhead box P3.
Figure 5.
Numbers of CD8+ and FoxP3+ TILs and DFS time. Association between (A) CD8+ TILs and (B) FoxP3+ TILs with DFS time. TILs, tumor-infiltrating lymphocytes; DFS, disease-free survival; FoxP3, forkhead box P3.
Table II.
Univariate and multivariate analysis between disease free survival and clinicopathological characteristics.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Charactertistic | HR | 95% CI | P-value | HR | 95% CI | P-value |
| pT stage (T3-4 vs. T0-2) | 6.5 | 1.8-41.8 | <0.01 | 4.2 | 1.02-28.68 | 0.046 |
| Lymphatic invasion (present vs. absent) | 4.3 | 0.23-22.1 | 0.25 | |||
| Vascular invasion (present vs. absent) | 3.2 | 1.08-11.84 | 0.04 | 1.6 | 0.50-6.27 | 0.45 |
| Lymph node metastasis (present vs. absent) | 2 | 0.45-6.31 | 0.33 | |||
| CD8+ TIL (high vs. low) | 0.2 | 0.04-0.64 | <0.01 | 0.3 | 0.06-0.94 | 0.038 |
| FoxP3+ TIL (high vs. low) | 0.74 | 0.25-2.17 | 0.58 | |||
TIL, tumor-infiltrating lymphocytes. HR, hazard ratio.
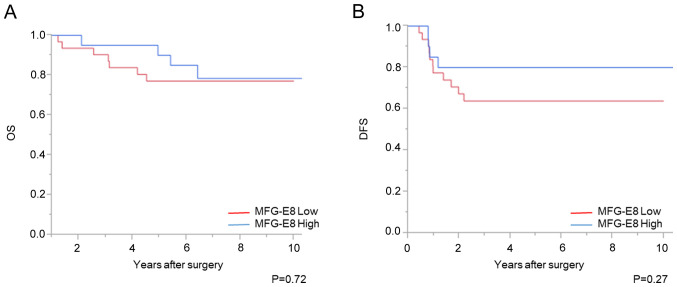
Association between MFG-E8 expression level and clinicopathological features
High MFG-E8 expression after CRT was significantly associated with high tumor regression grade (P<0.01; Table III). However, no significant difference with OS or DFS time was found between the patient groups with high and low MFG-E8 expression level (Fig. 6).
Table III.
Association between MFG-E8 expression level and clinicopathological characteristics.
| MFG-E8+ (n=23) | MFG-E8- (n=33) | ||||
|---|---|---|---|---|---|
| Characteristic | Number | Percentage | Number | Percentage | P-value |
| Sex | |||||
| Male | 13 | 56.5 | 25 | 75.8 | 0.21 |
| Female | 10 | 43.5 | 8 | 24.2 | |
| Mean age ± SD, years | 59.6±10.4 | 63.4±8.7 | 0.16 | ||
| pT stagea | |||||
| T0-2 | 8 | 34.8 | 13 | 39.4 | 0.73 |
| T3-4 | 15 | 65.2 | 20 | 60.6 | |
| Histological type | |||||
| Well | 18 | 78.3 | 21 | 63.6 | 0.24 |
| Mod | 5 | 21.7 | 11 | 33.3 | |
| Muc | 0 | 0 | 1 | 3.1 | |
| Lymphatic invasion | |||||
| Present | 1 | 4.3 | 4 | 12.1 | 0.30 |
| Abscent | 22 | 95.7 | 29 | 87.9 | |
| Vascular invasion | |||||
| Present | 11 | 47.8 | 20 | 60.6 | 0.34 |
| Abscent | 12 | 52.2 | 13 | 39.4 | |
| Lymph node metastasis | |||||
| Present | 3 | 13.0 | 6 | 18.2 | 0.60 |
| Abscent | 20 | 87.0 | 27 | 81.8 | |
| Distant metastasis | |||||
| Present | 3 | 13.0 | 3 | 9.1 | 0.37 |
| Abscent | 20 | 87.0 | 30 | 90.9 | |
| TNM stagea | |||||
| I-II | 18 | 78.3 | 26 | 78.8 | 0.96 |
| III-IV | 5 | 21.7 | 7 | 21.2 | |
| Tumor regression gradeb | |||||
| 1 | 9 | 39.1 | 26 | 78.8 | <0.01 |
| 2 | 14 | 60.9 | 7 | 21.2 | |
aAccording to TMN classification of malignant tumors, 8th edition according to Union for the Iinternational Cancer Control.
bAccording to Japanese Classification of Colorectal Carcinoma, 9th edition. Well, well-differentiated adenocarcinoma; Mod, moderately differentiated adenocarcinoma; Muc, mucinous adenocarcinoma; MFG-E8, lactadherin.
Figure 6.
Expression level of MFG-E8 and patient prognosis. Association of MFG-E8 expression with (A) OS and (B) DFS times. MFG-E8, lactadherin; OS overall survival; DFS, disease-free survival.
Discussion
Preoperative CRT for advanced lower rectal cancer decreased post-operative locoregional recurrence and contributed to tumor down-staging, which lead to an increased sphincter-preservation rate (27). Furthermore, previous reports described the usefulness of the ‘watch and wait’ strategy or local excision after CRT (28,29). Therefore, the identification of predictive factors for CRT response is important to determine whether preoperative CRT should be performed.
Firstly, using biopsy samples before CRT, the association between TIL infiltration density, MFG-E8 expression level, and CRT response was investigated. Teng et al (11) and Anitei et al (12) demonstrated that patients with high CD8+ TIL density showed favorable CRT responses. FoxP3+ TILs suppressed the host immune system, contrary to cytotoxic CD8+ TILs. Therefore, we hypothesized that both CD8+ and FoxP3+ TIL densities could be valuable indicators. Shinto et al (8) reported the effectiveness of intraepithelial CD8/FoxP3 TILs ratio in patients with short-term preoperative CRT regimen (20 Gy administered in 5 daily doses of 4 Gy, with the administration of tegafur/uracil at 400 mg/day and surgery was performed ~30 days after CRT). However, their results may not be applicable to long-term preoperative CRT, with a higher radiation dose and a longer period before surgery [50.4 Gy (1.8 Gy in 28 fractions) with concomitant chemotherapy and surgery was performed 6-8 weeks after CRT]. Therefore, the association between TIL density and CRT response using long-term preoperative CRT samples was determined in the present study. CD8+ TIL density, but not FoxP3+ TIL density, was associated with CRT response. The association between the CD8/FoxP3 TIL ratio and CRT response was determined; however, it was not a more effective indicator than CD8+ TIL density alone. Thus, we hypothesized that FoxP3+ TILs may not play an important role in long-term preoperative CRT. Furthermore, McCoy et al (30) recently reported that there was no association between TIL density before CRT, including CD8+ and FoxP3+ TIL densities, and CRT response. A further study, with a larger sample size, would, therefore, be required to validate the results from the present study. It was difficult to determine the grade of MFG-E8 expression level using small biopsy samples obtained before CRT and surgery, which showed heterogenous expression patterns. Therefore, further study using other methodology, such as PCR will also be required.
Secondly, using surgically resected specimens after CRT, immunology-related responses on the tumor microenvironment were analyzed. With respect to TlLs, a considerable number of TILs were detected in normal tissue in post-CRT specimens, and the distribution of TILs varied as a result of prominent fibrosis caused by CRT. Therefore, accurate TIL density after CRT was difficult to evaluate. Shinto et al (8) evaluated TILs in specimens after short-term CRT; however, we hypothesized that the different total radiation dose and time between CRT and surgery resulted in different results in the present study. High MFG-E8 expression level in post-CRT specimens was significantly associated with a favorable CRT response; however, no significant difference in patient prognosis was observed. In previous trials (1-3), preoperative CRT decreased postoperative locoregional recurrence, although CRT did not improve the survival time in the patients. In the present study, MFG-E8 expression level was associated with high tumor regression grade, although no significant difference with OS was found, which was consistent with previous reports (1-3). With respect to DFS, we hypothesized that MFG-E8 expression might be associated with high tumor regression grade and DFS, however no significance differences were observed, which may be due to small sample size. Jinushi et al (31) reported MFG-E8 induced resistance to chemotherapy and irradiation in melanoma cells from the secretion of regulatory T cells, inducing cytokines, and MFG-E8 induced resistance to chemotherapy in melanoma cells through Akt. Therefore, we hypothesized that MFG-E8 could be associated with resistance to CRT in rectal cancer. Surprisingly, high MFG-E8 expression level in post-CRT specimens was significantly associated with favorable CRT response. This type of association was not found in the recent study on esophageal cancer (26). In the present study, it could not be determined whether MFG-E8 expression level after CRT reflected the MFG-E8 expression in samples before CRT or it was induced by CRT. The association between MFG-E8 expression level and TIL density was also not evaluated; however, high MFG-E8 expression level in esophageal squamous cell carcinoma was associated with a low CD8+/FoxP3+ TILs ratio (26). We hypothesized that this may be due to the local microenvironment of the type of cancer. In esophageal cancer, lipopolysaccharide, a component of gram-negative bacterial cell walls, participates in the oncogenic process. Lipopolysaccharide has been reported to activate the nuclear factor-κ B signaling pathway, and to promote the release of inflammation-associated mediators, including interleukin (IL) 1β, IL6, IL8 and tumor necrosis factor-α (32,33). However, the gut microbiome in the colon is a complex community in the human body, and microbiota metabolites have either tumorigenic or anti-tumorigenic effects. Lipopolysaccharide has also been reported to be associated with tumorigenesis in colorectal cancer (34), and Ladoire et al (35) previously described that numerous bacterial species produce proinflammatory cytokines, which led to carcinogenesis. They also demonstrated that by suppressing inflammation, FoxP3+ TILs could be anti-tumorigenic in colorectal cancer. Furthermore, microbes in colorectal cancer have been reported to induce epithelial-to-mesenchymal transition though various signaling pathways, such as TGFβ, Wnt and Notch (34). On the other hand, butyric acid and urolithins, which are also generated from colonic bacteria have been reported to be anti-tumorigenic by inhibiting the Wnt signaling pathway (36,37). The association between MFG-E8 expression level and TILs density could not be investigated; however, Jinushi et al (17,38) reported that MFG-E8 enhanced regulatory T cell activity. Thus, it may be possible that MFG-E8 expression level in patients with rectal cancer affects regulatory T cell activity and CRT response.
With respect to the prognosis of patients, several meta-analyses investigating patients with colorectal cancer and who did not receive preoperative therapy revealed that the density of CD8+ and FoxP3+ TILs was associated with favorable patient prognosis (39). Whereas, in patients with rectal cancer who received CRT, the impact of TILs on patient prognosis has not been fully elucidated. Teng et al (11) reported that pre-CRT CD8+ TIL density was associated with favorable patient prognosis, which is similar to the result in the present study. Shinto et al (8) reported that high post-CRT CD8+ TIL density, and not pre-CRT CD8+ TIL density, was associated with favorable patient prognosis. However, in the study by Shinto et al (8), the patients received a short course of CRT, and immunological response on tumor microenvironment could be different between their study and the present study. McCoy et al (10,30) evaluated FoxP3+ TIL density in pre- and post-CRT samples and reported that post-CRT FoxP3+ TIL density, but not pre-CRT FoxP3+ TIL density, was associated with worsening prognosis. However, TILs in the post-CRT samples were not investigated in the present study as aforementioned. Therefore, further studies investigating TIL density after CRT are warranted. With respect to MFG-E8, Zhao et al (22) recently reported that MFG-E8 expression level in colorectal cancer was associated with advanced tumor depth, lymph node metastasis, and poor patient survival. However, there was no significant association between MFG-E8 expression level, clinicopathological features, and patient prognosis. To the best of our knowledge, this is the first report evaluating MFG-E8 expression level in patients with lower rectal cancer treated with preoperative CRT, and MFG-E8 expression level could be affected by CRT. Therefore, the results from the study by Zhao et al (22) may not be applicable to rectal cancer cases with CRT, and further study is required.
The present study has several limitations. Firstly, TIL density was evaluated using pre-CRT samples, whereas MFG-E8 expression level was evaluated using post-CRT samples. Therefore, the association between MFG-E8 expression level and T cell immunity was not clarified. Secondly, this was a retrospective study with a relatively small number of patients. Therefore, prospective studies, with a larger number of patients are required.
In conclusion, the results from the present study suggest that the number of CD8+ TILs before CRT could be a valuable predictor for CRT response, and high CD8+ TIL density before CRT was associated with favorable prognosis in patients with lower rectal cancer who were treated with CRT. High MFG-E8 expression level after CRT was found to be associated with a favorable CRT response.
Acknowledgements
Not applicable.
Funding Statement
Funding: This research was supported by Grants-in-Aid for Scientific Research (A: Grant no. 16H02672, C: Grant nos. 16K07143, 16K07161, 17K10620, 10621 and 17K10623) from the Japan Society for the Promotion of Science and by the Project for Cancer Research and Therapeutic Evolution (grant no. 16cm0106502h0001) from the Japan Agency for Medical Research and Development.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
YH performed the experiment and wrote the manuscript. SK evaluated IHC scoring and offered advice for IHC analyses. TM evaluated IHC scoring. HS, HI, SE, KM, MK, KS, YS, TN, TT, KK, KH and HN involved in acquiring clinicopathological features of patients. TU offered advice for the evaluation of IHC scoring. HT and SI were responsible for the study design and the revised manuscript. All authors revised the manuscript and approved the final version. YH and SI confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the University of Tokyo on July 29, 2014 [approval no. 10476-(1)] and written informed consent was provided by all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish rectal cancer trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 2.Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 3.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. EORTC Radiotherapy Group Trial 22921. [DOI] [PubMed] [Google Scholar]
- 4.Topova L, Hellmich G, Puffer E, Schubert C, Christen N, Boldt T, Wiedemann B, Witzigmann H, Stelzner S. Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum. 2011;54:401–411. doi: 10.1007/DCR.0b013e3182070efb. [DOI] [PubMed] [Google Scholar]
- 5.Tomono A, Yamashita K, Kanemitsu K, Sumi Y, Yamamoto M, Kanaji S, Imanishi T, Nakamura T, Suzuki S, Tanaka K, Kakeji Y. Prognostic significance of pathological response to preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Int J Clin Oncol. 2016;21:344–349. doi: 10.1007/s10147-015-0900-x. [DOI] [PubMed] [Google Scholar]
- 6.Tada N, Kawai K, Tsuno NH, Ishihara S, Yamaguchi H, Sunami E, Kitayama J, Oba K, Watanabe T. Prediction of the preoperative chemoradiotherapy response for rectal cancer by peripheral blood lymphocyte subsets. World J Surg Oncol. 2015;13(30) doi: 10.1186/s12957-014-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara S, Iinuma H, Fukushima Y, Akahane T, Horiuchi A, Shimada R, Shibuya H, Hayama T, Yamada H, Nozawa K, et al. Radiation-induced apoptosis of peripheral blood lymphocytes is correlated with histological regression of rectal cancer in response to preoperative chemoradiotherapy. Ann Surg Oncol. 2012;19:1192–1198. doi: 10.1245/s10434-011-2057-9. [DOI] [PubMed] [Google Scholar]
- 8.Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M, Yamamoto J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21 (Suppl 3):S414–S421. doi: 10.1245/s10434-014-3584-y. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6(49) doi: 10.1186/1748-717X-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCoy MJ, Hemmings C, Miller TJ, Austin SJ, Bulsara MK, Zeps N, Nowak AK, Lake RA, Platell CF. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer. 2015;113:1677–1686. doi: 10.1038/bjc.2015.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, Zhang J, Yu J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721–732.e1. doi: 10.1016/j.trsl.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 14.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 15.Miyasaka K, Hanayama R, Tanaka M, Nagata S. Expression of milk fat globule epidermal growth factor 8 in immature dendritic cells for engulfment of apoptotic cells. Eur J Immunol. 2004;34:1414–1422. doi: 10.1002/eji.200424930. [DOI] [PubMed] [Google Scholar]
- 16.Li BZ, Zhang HY, Pan HF, Ye DQ. Identification of MFG-E8 as a novel therapeutic target for diseases. Expert Opin Ther Targets. 2013;17:1275–1285. doi: 10.1517/14728222.2013.829455. [DOI] [PubMed] [Google Scholar]
- 17.Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibaldi L, Leyman S, Nicolas A, Notebaert S, Dewulf M, Ngo TH, Zuany-Amorim C, Amzallag N, Bernard-Pierrot I, Sastre-Garau X, Théry C. New blocking antibodies impede adhesion, migration and survival of ovarian cancer cells, highlighting MFGE8 as a potential therapeutic target of human ovarian carcinoma. PLoS One. 2013;8(e72708) doi: 10.1371/journal.pone.0072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugano G, Bernard-Pierrot I, Laé M, Battail C, Allory Y, Stransky N, Krumeich S, Lepage ML, Maille P, Donnadieu MH, et al. Milk fat globule-epidermal growth factor-factor VIII (MFGE8)/lactadherin promotes bladder tumor development. Oncogene. 2011;30:642–653. doi: 10.1038/onc.2010.446. [DOI] [PubMed] [Google Scholar]
- 20.Soki FN, Koh AJ, Jones JD, Kim YW, Dai J, Keller ET, Pienta KJ, Atabai K, Roca H, McCauley LK. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J Biol Chem. 2014;289:24560–24572. doi: 10.1074/jbc.M114.571620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oba J, Moroi Y, Nakahara T, Abe T, Hagihara A, Furue M. Expression of milk fat globule epidermal growth factor-VIII may be an indicator of poor prognosis in malignant melanoma. Br J Dermatol. 2011;165:506–512. doi: 10.1111/j.1365-2133.2011.10409.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Q, Xu L, Sun X, Zhang K, Shen H, Tian Y, Sun F, Li Y. MFG-E8 overexpression promotes colorectal cancer progression via AKT/MMPs signalling. Tumour Biol. 2017;39(1010428317707881) doi: 10.1177/1010428317707881. [DOI] [PubMed] [Google Scholar]
- 23.Kusunoki R, Ishihara S, Tada Y, Oka A, Sonoyama H, Fukuba N, Oshima N, Moriyama I, Yuki T, Kawashima K, et al. Role of milk fat globule-epidermal growth factor 8 in colonic inflammation and carcinogenesis. J Gastroenterol. 2015;50:862–875. doi: 10.1007/s00535-014-1036-x. [DOI] [PubMed] [Google Scholar]
- 24.Brierley JD, Gospodarowicz MK, Wittekind C (eds) The TNM classification of malignant tumours, 8th edition. Wiley Blackwell, Oxford, 2017. [Google Scholar]
- 25. Japanese classification of colorectal carcinoma. Kanehara & Co., Ltd., Tokyo, 2018. [Google Scholar]
- 26.Kanemura T, Miyata H, Makino T, Tanaka K, Sugimura K, Hamada-Uematsu M, Mizote Y, Uchida H, Miyazaki Y, Takahashi T, et al. Immunoregulatory influence of abundant MFG-E8 expression by esophageal cancer treated with chemotherapy. Cancer Sci. 2018;109:3393–3402. doi: 10.1111/cas.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane CH, Skibber JM, Feig BW, Vauthey JN, Thames HD, Curley SA, Rodriguez-Bigas MA, Wolff RA, Ellis LM, Delclos ME, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97:517–524. doi: 10.1002/cncr.11075. [DOI] [PubMed] [Google Scholar]
- 28.Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, Gama-Rodrigues J. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319–1329. doi: 10.1016/j.gassur.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Yu CS, Yun HR, Shin EJ, Lee KY, Kim NK, Lim SB, Oh ST, Kang SB, Choi WJ, Lee WY, et al. Local excision after neoadjuvant chemoradiation therapy in advanced rectal cancer: A national multicenter analysis. Am J Surg. 2013;206:482–487. doi: 10.1016/j.amjsurg.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 30.McCoy MJ, Hemmings C, Anyaegbu CC, Austin SJ, Lee-Pullen TF, Miller TJ, Bulsara MK, Zeps N, Nowak AK, Lake RA, Platell CF. Tumour-infiltrating regulatory T cell density before neoadjuvant chemoradiotherapy for rectal cancer does not predict treatment response. Oncotarget. 2017;8:19803–19813. doi: 10.18632/oncotarget.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, Dranoff G, Tahara H. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Francois F, Pei Z. Molecular pathways: Pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012;18:2138–2144. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng C, Bai C, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics. 2018;16:33–49. doi: 10.1016/j.gpb.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: The paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González-Sarrías A, Giménez-Bastida JA, Núñez-Sánchez MÁ, Larrosa M, García-Conesa MT, Tomás-Barberán FA, Espín JC. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur J Nutr. 2014;53:853–864. doi: 10.1007/s00394-013-0589-4. [DOI] [PubMed] [Google Scholar]
- 38.Jinushi M, Nakazaki Y, Carrasco DR, Draganov D, Souders N, Johnson M, Mihm MC, Dranoff G. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889–8898. doi: 10.1158/0008-5472.CAN-08-2147. [DOI] [PubMed] [Google Scholar]
- 39.Kong JC, Guerra GR, Pham T, Mitchell C, Lynch AC, Warrier SK, Ramsay RG, Heriot AG. Prognostic impact of tumor-infiltrating lymphocytes in primary and metastatic colorectal cancer: A Systematic review and meta-analysis. Dis Colon Rectum. 2019;62:498–508. doi: 10.1097/DCR.0000000000001332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.