Abstract
BACKGROUND AND PURPOSE: Multi-section CT has great potential for use in vascular studies. Our purpose was to determine the accuracy of multi-section CT angiography in detecting cerebral aneurysms compared with digital subtraction angiography or surgery.
METHODS: One hundred consecutive patients who underwent multi-section CT angiography and either digital subtraction angiography or surgery were included in the study. Multi-section CT angiography and digital subtraction angiography results were evaluated independently by different neuroradiologists who performed aneurysm detection, quantitation, and characterization by using 2D multiplanar reconstructions, 3D maximum intensity projection, and volume-rendered techniques.
RESULTS: When using intra-arterial digital subtraction angiography or surgery, 113 aneurysms (true positives and false negatives) were detected in 83 of the 100 patients. A total of 106 aneurysms (true positives) were confirmed by using digital subtraction angiography or surgery, or both. Seven aneurysms were missed when using multi-section CT angiography. Eight aneurysms were not confirmed by digital subtraction angiography and were considered to be false positive evaluations. The sensitivity for detecting aneurysms <4 mm, 4 to 10 mm, and >10 mm on a per-aneurysm basis was 0.84 (95% confidence interval: 0.72, 0.92), 0.97 (95% confidence interval: 0.91, 0.99), and 1.00 (95% confidence interval: 0.88, 1.00), respectively. The sensitivity, specificity, and accuracy of multi-section CT angiography for detecting aneurysms on a per-patient basis were 0.99 (95% confidence interval: 0.96, 1.00), 0.88 (95% confidence interval: 0.69, 0.94), and 0.98 (95% confidence interval: 0.95, 1.00), respectively.
CONCLUSION: Multi-section CT angiography has a high sensitivity in detecting aneurysms (especially aneurysms >3 mm). However, CT angiography is currently not sensitive enough to replace digital subtraction angiography.
One of the most frequent causes of subarachnoid hemorrhage is ruptured aneurysm (1–4). Ruptured aneurysms not only cause subarachnoid hemorrhage but can also cause subdural or intracerebral hematomas (5–8). Intracranial hematomas may exacerbate deterioration of the patient’s condition and may require emergency surgical decompression. Cerebral digital subtraction angiography has been used as the criterion standard for aneurysm detection. The main advantages of digital subtraction angiography are the high resolution achieved by most systems, generally providing 0.3 mm resolution by using a 1024 × 1024 matrix, and the lack of venous contamination. Digital subtraction angiography has several disadvantages other than time delay, including invasiveness because of the required arterial puncture and intra-arterial catheter manipulation, high skill level to perform the procedure, and relatively high cost. The risk of permanent neurologic complication associated with digital subtraction angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation has been reported to be 0.07% (9).
Helical CT angiography is a noninvasive volumetric imaging technique. Images can be relatively safely obtained without the need for arterial puncture or catheter manipulation. CT angiography is not associated with significant patient risks other than those associated with the administration of iodinated contrast media. Once the images are acquired, CT angiography data can be evaluated in almost unlimited projections in 2D and 3D formats. This is an important advantage of CT angiography over conventional digital subtraction angiography, although during the past few years, it has become more commonplace to perform 3D rotational angiography. For these reasons, helical cerebral CT angiography has been widely used in the detection of intracranial aneurysms, with a reported sensitivity of 70–96% (10–15).
Multi-section CT scanners are fundamentally improved over single section CT scanners in three ways: faster speed, longer distance, and better section thickness (16). The high gantry speed of the multi-section CT scanner permits helical scanning to be performed with a smaller section thickness than is possible with conventional helical CT. As a result, nearly isovolumetric data (i.e., improved resolution in the z axis) can be obtained (17). The purpose of this study was to assess the diagnostic accuracy of four-section CT angiography for the detection of cerebral aneurysms.
Methods
From August 1999 through August 2003, 218 consecutive patients who underwent unenhanced CT of the head and multi-section CT angiography were retrospectively evaluated. Among the 218 patients, 100 (44 men and 56 women; median age, 52 years; age range, 24–76 years) who underwent multi-section CT angiography and digital subtraction angiography or surgery were included in the study. Fifty-four (54%) of these 100 patients had subarachnoid hemorrhage, and multi-section CT angiography was performed usually within an hour after the determination of subarachnoid hemorrhage to evaluate for the presence of aneurysms. If the CT angiography was performed during off-hours, the study was evaluated by a neuroradiology fellow or neuroradiology staff, usually within an hour. In 18 patients, multi-section CT angiography was performed to evaluate aneurysms suspected based on CT findings (n = 8) or MR angiography findings (n = 10), which were obtained for nonspecific symptoms such as headache, dizziness, or visual disturbances. In 17 patients, the indication for multi-section CT angiography was headache. The remaining 11 patients had a variety of indications, including visual disturbances, ischemia, vertigo, tumor, trauma, ptosis, mental status changes, hemiparesis, and dizziness. All patients underwent multi-section CT angiography according to the same protocol (described below).
Multi-section CT angiograms were obtained with a four-channel multi-row detector CT scanner (Somatom Plus Four Volume Zoom; Siemens Medical Systems, Forchheim, Germany). Typically, CT angiography was initiated 15 to 20 seconds after the start of an IV infusion of nonionic iodinated contrast material. Contrast material (ioversol, [Optiray 320]; Mallinckrodt Inc., St. Louis, MO) was injected at a rate of 5 mL/s, for a total scanning time of 15 to 20 seconds, with the use of a power injector (Medrad, Pittsburgh, PA) and an 18- or 20-gauge needle inserted in the antecubital vein. The volume of iodinated contrast material in each study was typically 90 to 100 mL. The scanning parameters included 120 kV, 225 mA, section thickness of 1.25 mm, reconstruction interval of 1 mm, and table speed of 2 to 3 mm/s. The scan revolution time was 0.5 seconds. Data for CT angiography were obtained in a caudocranial direction. 3D reconstructions were performed on Vitrea 2 workstations (Vital Images Inc., Plymouth, MN) by using preset volume-rendered and maximum intensity projection display algorithms. It took approximately 2 to 3 seconds to generate the 3D reconstructions on the workstation. Afterward, the neuroradiologists (M.T., A.M.) could manipulate the images in a near-infinite number of projections with varying amounts of time needed for review (usually 5–10 minutes). The source images and multiplanar reformations also were reviewed on the workstations by an experienced neuroradiologist.
Digital subtraction angiography was performed with femoral catheterization by the Seldinger technique with a biplane digital subtraction angiography unit (Toshiba CAS-30B; Toshiba Co., Tochigi-Ken, Japan). Three- or four-vessel angiograms were obtained in anteroposterior, lateral, and bilateral oblique projections (+45 degrees and −45 degrees) for each catheterization. Six to 9 mL of nonionic contrast material (Optiray 320) was used for each injection. Digital subtraction angiography was performed with a 33-cm field of view and a 1024 × 1024 matrix. The spatial resolution was 0.32 × 0.32 mm. The size of each aneurysm was calculated after correction of magnification factor on digital subtraction angiography. The largest diameter of each aneurysm was measured and graded as large (>10 mm), medium (4–10 mm), or small (<4 mm). The presence or absence of aneurysms and the aneurysm location and size, when present, were established by an experienced neuroradiologist who did not participate in interpretation of the multi-section CT angiography findings for the same patient. Digital subtraction angiography findings, when available, or operative and surgical results were accepted as the criterion standard.
For statistical analysis, 2 × 2 tables of the true positive, false positive, true negative, and false negative cases of multi-section CT angiography, as compared with digital subtraction angiography or surgery results, were constructed. Sensitivity, specificity, positive and negative predictive values, and accuracy were calculated on per-aneurysm and per-patient bases. The detection of at least one aneurysm was assumed positive for the 100 patients (i.e., the diagnosis of any one aneurysm would lead to conventional angiography, coil neuroembolization, or surgery) when calculating the statistical data on a per-patient basis. Exact 95% confidence intervals based on binomial probabilities were calculated.
Results
When using intra-arterial digital subtraction angiography or surgery, 113 aneurysms were detected in 83 of the 100 patients (Figs 1–3). Eight aneurysms that were identified on multi-section CT angiograms were not found on digital subtraction angiograms and were considered as false positive results. Classification of the sites and sizes of the aneurysms, characteristics of the aneurysms, and the patient data are summarized in Table 1. In 15 (six men and nine women; median age, 42 years; age range, 25–72 years) of the 100 patients, no aneurysm was identified by using multi-section CT angiography and digital subtraction angiography. The main indication for multi-section CT angiography for these patients was subarachnoid hemorrhage (10 [67%] of 15 patients). The second most common indication was headache (three [20%] of 15 patients).
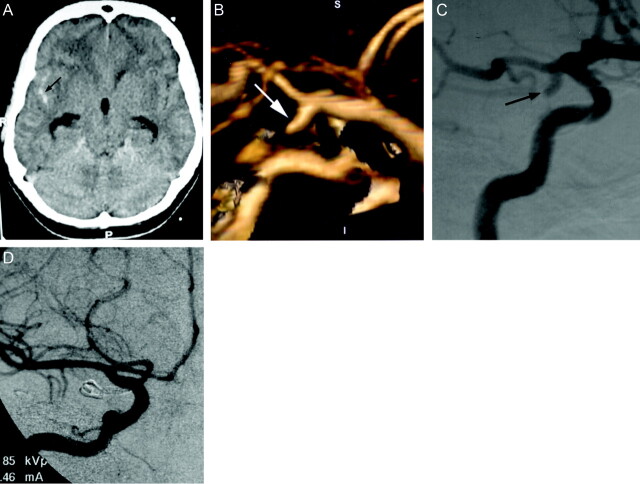
Fig 1.
Images obtained in a 45-year-old woman with severe headache.
A, Unenhanced CT scan of the head shows subarachnoid hemorrhage in the right sylvian fissure (arrow), with mild hydrocephalus. R, right; P, posterior.
B, 3D volume-rendered image (lateral) shows inferiorly and posteriorly directed saccular aneurysm at the origin of the right posterior communicating artery (arrow). S, superior; I, inferior; P, posterior; A, anterior.
C, Preoperative right internal carotid digital subtraction angiogram (right anterior oblique projection) shows inferiorly and laterally directed saccular aneurysm at the origin of the posterior communicating artery (arrow).
D, Intraoperative right internal carotid digital subtraction angiogram (anteroposterior projection) shows successful clip placement in the posterior communicating artery aneurysm.
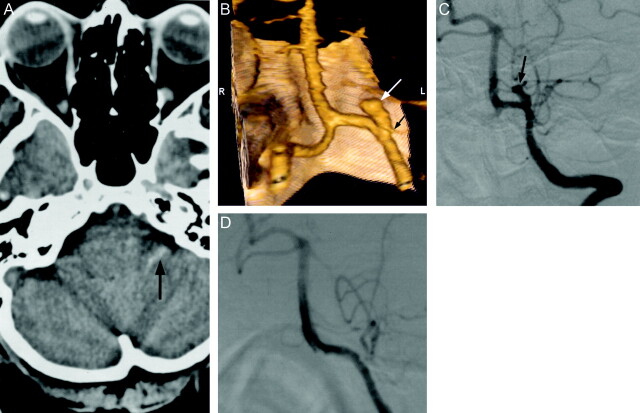
Fig 2.
Images obtained in a 49-year-old woman with severe headache.
A, Unenhanced CT scan of the head shows subarachnoid hemorrhage in the left cerebellopontine angle cistern (arrow).
B, 3D volume-rendered image (posteroanterior) shows superiorly, medially, and anteriorly directed saccular aneurysm (white arrow), which is incorporated into the origin of posterior inferior cerebellar artery (black arrow). S, superior; I, inferior; R, right; L, left.
C, Preoperative left vertebral artery digital subtraction angiogram (anteroposterior projection) shows saccular aneurysm projecting superiorly and medially at the origin of the posterior inferior cerebellar artery. Note the hypoplastic P1 segment of the posterior cerebral artery.
D, Intraoperative left vertebral artery digital subtraction angiogram (anteroposterior projection) shows successful clip placement in the aneurysm without occlusion of the posterior inferior cerebellar artery.
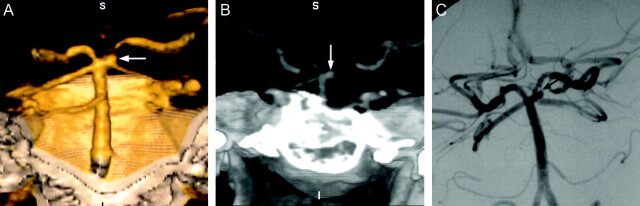
Fig 3.
Images obtained in a 41-year-old woman with headache.
A, 3D volume-rendered image (posteroanterior) shows aneurysm at the origin of the left superior cerebellar artery (arrow). S, superior; I, inferior; R, right; L, left.
B, Maximum intensity projection image (anteroposterior) shows aneurysm at the origin of the left superior cerebellar artery (arrow). S, superior; I, inferior; R, right; L, left.
C, Left vertebral artery digital subtraction angiogram (anteroposterior projection) shows aneurysm at the origin of the left superior cerebellar artery. This patient underwent attempted treatment by GDC embolization. However, the aneurysm was thrombosed during the procedure before coil embolization. The patient was followed up and did not have any significant complaint as of the time of this writing.
TABLE 1:
Characteristics of Patients and Aneurysms
| Aneurysm Size (mm) | Aneurysm Number | Mean Age ± SD (yr) | % Female | Most Common Indication for MSCTA (%) | Most Common Location (%) |
|---|---|---|---|---|---|
| <4 | 36 | 50 ±12 | 60 | Subarachnoid hemorrhage (46) | MCA Bi/Tri (19) |
| PcoA (14) | |||||
| AcoA (14) | |||||
| 4–10 | 63 | 55 ±11 | 60 | Subarachnoid hemorrhage (58) | PcoA (22) |
| AcoA (13) | |||||
| MCA Bi/Tri (8) | |||||
| >10 | 22 | 53 ±14 | 45 | Subarachnoid hemorrhage (41) | MCA Bi/Tri (18) |
| ICA SC (18) | |||||
| PcoA (14) |
Note.—MSCTA indicates multi-section CT angiography; MCA Bi/Trif, bifurcation/trifurcation of middle cerebral artery; PcoA, posterior communicating artery; AcoA, anterior communicating artery; ICA SC, supraclinoid segment of internal carotid artery.
The most common location for aneurysms <4 mm was the middle cerebral artery (bi-/trifurcation) (19%). The primary location for aneurysms between 4 and 10 mm was the posterior communicating artery (22%). The most common locations for aneurysms >10 mm were the bi-/trifurcation of the middle cerebral artery (18%) and the supraclinoid segment of the internal carotid artery (18%). Thirty-one (27%) of 113 aneurysms were read as <4 mm; 60 (53%) were read as being between 4 and 10 mm; and 22 (20%) were read as >10 mm. Five of the eight false positive aneurysms were <4 mm, and three were between 4 and 10 mm. A total of 106 (94%) of 113 aneurysms that were found by using digital subtraction angiography or surgery were detected by multi-section CT angiography (true positives). True positive and false negative statistical results on a per-aneurysm basis are presented in Table 2 . The most frequent location of false positive interpretation was the supraclinoid segment of the internal carotid artery (Table 3). Seven aneurysms (false negatives) were missed when using multi-section CT angiography. The primary reason for missing aneurysms on multi-section CT angiograms (seven total) seems to be the close proximity to bone in addition to the small size of the aneurysms (four aneurysms). Additionally, another single basilar tip aneurysm was missed because of its very small size. One aneurysm was not identified because of the large amount of hemorrhage obscuring the aneurysm. Another aneurysm was not identified on multi-section CT angiograms because of an atypical configuration (Table 4). In retrospective evaluation, only the aneurysm with atypical configuration (a 4-mm posterior communicating artery aneurysm) was clearly identified. Other aneurysms that were missed on multi-section CT angiography could not be clearly identified in retrospective evaluation.
TABLE 2:
Aneurysm Detection by Multi-Section CT Angiography
| True Positive | False Negative | |
|---|---|---|
| <4 mm | 26 | 5 |
| 4–10 mm | 58 | 2 |
| >10 mm | 22 | 0 |
| Total | 106 | 7 |
TABLE 3:
Characteristics of False Positive Evaluations (n=8)
| Location | Size (mm) |
|---|---|
| Supraclinoid segment of ICA | 4 |
| Supraclinoid segment of ICA | 3 |
| Supraclinoid segment of ICA | 2 |
| Bi-Trifurcation of MCA | 4 |
| P2 segment of PCA | 6 |
| ICA (para-ophthalmic) | 2 |
| Anterior communicating artery | 2 |
| Posterior communicating artery | 2 |
Note.—ICA indicates internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery.
TABLE 4:
Data for Missed Aneurysms (n = 7)
| Location | Size (mm) | Main Reason for Missed Aneurysm |
|---|---|---|
| Posterior communicating artery | 4 | Atypical configuration |
| Posterior inferior cerebellar artery | 4 | Obscured by hemorrhage |
| ICA (para-ophthalmic) | 3 | Small, adjacent to bone |
| Posterior communicating artery | 2 | Very small, adjacent to bone |
| ICA (cavernous segment) | 2 | Very small, adjacent to bone |
| Anterior choroidal artery | 2 | Very small, adjacent to bone |
| Basilar tip | 1 | Very small |
Note.—ICA indicates internal carotid artery.
The sensitivities of multi-section CT angiography on a per-aneurysm basis for aneurysms <4 mm, between 4 and 10 mm, and >10 mm were 0.84 (26 of 31; 95% confidence interval: 0.72, 0.92), 0.97 (58 of 60; 95% confidence interval: 0.91, 0.99), and 1.00 (22 of 22; 95% confidence interval: 0.88, 1.00), respectively. Details of the accuracy of multi-section CT angiography on a per-aneurysm basis are explained in Table 5. The sensitivity, specificity, and accuracy of detection of aneurysms on a per-patient basis were 0.99 (95% confidence interval: 0.96, 1.00), 0.88 (95% confidence interval: 0.69, 0.94), and 0.98 (95% confidence interval: 0.95, 1.00), respectively. The positive predictive value and negative predictive value of detecting aneurysms on multi-section CT angiography on a per-patient basis were 0.98 (95% confidence interval: 0.96, 0.99) and 0.94 (95% confidence interval: 0.74, 0.99), respectively.
TABLE 5:
Accuracy of Multi-Section CT Angiography on a per-Aneurysm Basis
| Size (mm) | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| <4 | 0.84(26/31) | 0.75(15/20) | 0.84 | 0.75 | 0.80 |
| 0.72, 0.92 | 0.56, 0.88 | 0.72, 0.92 | 0.56, 0.88 | 0.69, 0.91 | |
| 4–10 | 0.97(58/60) | 0.83(15/18) | 0.95 | 0.88 | 0.94 |
| (0.91, 0.99) | 0.64, 0.92 | 0.90, 0.98 | 0.68, 0.97 | 0.88, 0.99 | |
| >10 | 1.00(22/22) | 1.00(15/15) | 1.00 | 1.00 | 1.00 |
| (0.88, 1.00) | 0.83, 1.00 | 0.88, 1.00 | 0.83, 1.00 | 0.84, 1.00 |
Note.—PPV indicates positive predictive value; NPV, negative predictive value. Numbers in parenthesis indicate numbers of aneurysms, and numbers on second line indicate 95% confidence intervals.
For 10 (19%) of 54 patients with subarachnoid hemorrhage, no aneurysms were detected by using multi-section CT angiography and digital subtraction angiography. The hemorrhages in each of these patients were adjacent to the brain stem and were considered to be nonaneurysmal perimesencephalic hemorrhages. In 42 (78%) of 54 patients with subarachnoid hemorrhage, aneurysms were confirmed by digital subtraction angiography or surgery. In two (4%) patients with subarachnoid hemorrhage, although multi-section CT angiography detected aneurysms (anterior communicating artery, 2 mm; internal carotid artery supraclinoid segment, 3 mm), these aneurysms could not be confirmed by using digital subtraction angiography and were considered as a false positive evaluation for the purpose of this study. Of 42 patients with subarachnoid hemorrhage and aneurysm shown by digital subtraction angiography or surgery, 26 (62%) were female and 16 (38%) were male patients. A total of 53 aneurysms were detected via digital subtraction angiography or surgery in the 42 patients who had subarachnoid hemorrhage. Of the 53 aneurysms in patients with subarachnoid hemorrhage, nine (17%) were <4 mm in size, 35 (66%) were between 4 and 10 mm, and nine (17%) were >10 mm.
Discussion
The performance of helical CT has improved during the past 8 years, with faster gantry rotation, more powerful X-ray tubes, and superior interpolation algorithms. However, probably the most significant advance in CT angiography has been the recent introduction of multi-section CT scanners. Currently capable of acquiring multiple channels of helical data simultaneously, multi-section CT scanners have achieved the greatest incremental gain in scan speed since the development of helical CT. These machines have profound implications for clinical CT scanning.
Fundamental theoretical advantages of multi-section CT over single section CT include substantially shorter acquisition times, retrospective creation of thinner or thicker sections from the same raw data, improved 3D rendering with diminished artifacts, and decreased contrast dose. Although these features will likely be important to many applications of CT scanning (16, 18–21), the greatest impact has been on CT angiography. The ability of acquiring thinner overlapping images with a multi-section CT scanner, without increasing the data acquisition time, theoretically allows improved quality and spatial resolution of the 2D and 3D reconstructions.
Single section CT angiography has had limited sensitivity in detecting aneurysms <3 mm (10, 22). Wintermark et al (23) recently reported sensitivity, specificity, and accuracy of 94.8%, 95.2%, and 94.9%, respectively, in detecting aneurysms by using multi-section CT angiography on a per-aneurysm basis. Excellent correlation was shown between aneurysm size assessed by multi-section CT angiography and digital subtraction angiography in that study; however, the exact values of sensitivity, specificity, and accuracy, as related to aneurysm size, were not available. In the current study, the sensitivity, specificity, and accuracy of detecting aneurysms <4 mm were 0.84, 0.75, and 0.80, respectively. Also, five aneurysms <4 mm were missed by multi-section CT angiography in the current study. In addition to small size, the main reason aneurysms <4 mm were missed was their close proximity to bone (Table 4). For aneurysms >3 mm, detection accuracy was much higher and was consistent with the literature (10, 11). The sensitivity, specificity, and accuracy of detecting aneurysms between 4 and 10 mm were 0.97, 0.83, and 0.94, respectively. Two aneurysms >3 mm were missed by multi-section CT angiography. One of them was a 4-mm posterior communicating artery aneurysm, which had atypical configuration. The other was a 4-mm aneurysm located in the posterior caudal loop of the posterior inferior cerebellar artery. The apparent reason this aneurysm was missed was large hemorrhage in the posterior fossa obscuring the visualization of the distal posterior inferior cerebellar artery branches. For aneurysms >10 mm, the accuracy was 1.00. The 22 aneurysms were detected with 100% sensitivity. The largest was 40 mm in diameter, located in the proximal segment of the basilar artery.
In this study, the multi-section technique enables sub-millimeter resolution of vascular structures providing high accuracy of detection of intracranial aneurysms that have a diameter ≥4 mm (97% sensitivity). Our study showed that even for aneurysms <4 mm, the sensitivity and accuracy can be as high as 84% and 80%, respectively. The greatest improvement in detection of cerebral aneurysms with CT angiography seems to be in small size aneurysms in our study compared with the previous single section CT angiography studies. As the size of the aneurysm increases, the sensitivity of detecting aneurysms does not significantly differ between our results and the results of the previous single section CT angiography studies. This may be related to an equivalent amount of volumetric data being obtained by single section CT angiography, and although single section CT angiography takes longer, equivalent data may be obtained in an increased amount of time. However, it is likely that more isotropic data and sub-millimetric resolution with higher spatial resolution of multi-section CT angiography compared with single section CT angiography is more important in the detection of small size aneurysms.
Important preoperative information, such as aneurysm dimensions and relation to the parent artery, is well visualized by using CT angiography. Gonzales-Darder et al (24) reported the microsurgical management of cerebral aneurysms based on CT angiography without preoperative digital subtraction angiography in 46 cases. Their results showed the valuable anatomic information provided by CT angiography. The sensitivity for diagnosis of ruptured aneurysms in their series was 100%, and the overall sensitivity was 90.4% (24). In the present study, eight patients with aneurysms in multi-section CT angiography underwent surgery without digital subtraction angiography, and surgery confirmed the size and location of the aneurysms. The smallest sized aneurysm in this surgical group measured 2 mm and was located in the M1 segment of the middle cerebral artery.
Recently, 3D rotational digital subtraction angiography has entered clinical use because of its shorter reconstruction time and more robust equipment (25). 3D digital subtraction angiography and digital subtraction angiography together can depict more intracranial aneurysms than digital subtraction angiography alone (26, 27). Traditionally, 2D digital subtraction angiography has been considered the criterion standard in detection of cerebral aneurysms. However, finding more aneurysms together with 3D rotational digital subtraction angiography supported that 2D digital subtraction angiography alone is not necessarily an ideal criterion standard for imaging cerebral aneurysms. In our study, eight presumed aneurysms (noted on CT angiograms), including those in two patients with subarachnoid hemorrhage, were not shown by digital subtraction angiography. Unfortunately, these patients did not undergo surgery and probable false negative results of digital subtraction angiography could not be confirmed. These aneurysms were considered as false positive interpretations of multi-section CT angiography for the purpose of this study. Five of the eight aneurysms were <4 mm. The most common location of false positive aneurysms was the supraclinoid segment of the internal carotid arteries. We speculate that the main reason for false positive interpretation of the aneurysms in the supraclinoid segment was low location (adjacent to the clinoid process) of the aneurysms, and the clinoid process may have caused bony artifacts with false aneurysm appearance. In addition, digital subtraction angiography procedures were performed by using the conventional method without 3D rotational angiography in all these patients. The sensitivity of detecting aneurysms on multi-section CT angiograms may have been even higher (especially for those <4 mm) if 3D digital subtraction angiography had been performed in these patients. Therefore, the theoretical criterion standard for detecting cerebral aneurysms should be preferably surgery or at least the combination of 3D rotational and 2D digital subtraction angiography.
Although the sensitivity of detecting aneurysms (especially small size aneurysms) increases with the use of multi-section CT scanners, there are several reasons why CT angiography has not replaced digital subtraction angiography in aneurysm detection. Probably, the most important reason is that ruptured aneurysms cause substantial death and disability, and the consequences of failing to properly diagnose a ruptured aneurysm are potentially dire. The relatively small risk of permanent neurologic complication is justified by the potentially dire consequences of failing to diagnose a ruptured cerebral aneurysm. Another reason why CT angiography has not replaced digital subtraction angiography in aneurysm detection is that cerebral aneurysms increasingly undergo endovascular treatment. This treatment necessitates digital subtraction angiography, so in patients who are expected to be candidates for endovascular therapy, CT angiography would be an unnecessary extra step. Also, CT angiography does not have as much spatial resolution as digital subtraction angiography has. Small, yet vital, arteries (such as perforating arteries) adjacent to aneurysms are important for treatment planning and may not be revealed by CT angiography.
We recognize that our study has some limitations. With a high suspicion of the presence of aneurysms, a high rate of accuracy could result because of observer expectation bias. Because of the inclusion criteria, the population we studied had a high prevalence of aneurysms (83%), which might cause such bias. Previous publications have theorized that increasing disease prevalence can lead to an apparent improvement in the sensitivity and specificity of a diagnostic examination (28). Such an increased prevalence could have led to an apparent improvement in the sensitivity and specificity of multi-section CT angiography in our study, because none of the patients was asymptomatic. Our study population included patients with a variety of symptoms (which may have been related to aneurysm), although most (54%) had subarachnoid hemorrhage. Therefore, the relatively high prevalence of subarachnoid hemorrhage in our population may have influenced the sensitivity and specificity of detection of cerebral aneurysms by multi-section CT angiography.
Another limitation of our study was that not all patients who had multi-section CT angiography underwent digital subtraction angiography. If there was a low suspicion of cerebral aneurysm depending on the patient’s symptoms and if the multi-section CT angiography results were negative, the clinicians were occasionally reluctant to proceed to digital subtraction angiography. For example, only 100 of 218 patients who had multi-section CT angiograms underwent digital subtraction angiography or surgery in our study population. This may also have been a cause of expectation bias and overestimation of the sensitivity.
Another limitation of our study was that each study (multi-section CT angiography or digital subtraction angiography) was evaluated by only one neuroradiologist who did not participate in the evaluation of corresponding multi-section CT angiography or digital subtraction angiography in the same patient, and hence interobserver variability could not be calculated. This may be important in the reproducibility of the results of our study.
Conclusion
Multi-section CT angiography is a promising noninvasive screening method for the detection of cerebral aneurysms. With the increase in the number of detectors, more isotropic voxel data will be available, likely reducing partial volume effect. This will further increase the quality of 2D and 3D CT angiography images. Faster gantry speed will lessen the amount of necessary IV contrast material and will result in better discrimination of arteries from venous structures. However, CT angiography is currently not sensitive enough to replace digital subtraction angiography for the detection of cerebral aneurysms. Digital subtraction angiography plus rotational digital subtraction angiography remain the criterion standard method in detecting cerebral aneurysms.
References
- 1.King JT Jr. Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am 1997;7:659–668 [PubMed] [Google Scholar]
- 2.Horiuchi T, Tanaka Y, Hongo K, Kobayashi S. Aneurysmal subarachnoid hemorrhage in young adults: a comparison between patients in the third and fourth decades of life. J Neurosurg 2003;99:276–279 [DOI] [PubMed] [Google Scholar]
- 3.Russell SM, Lin K, Hahn SA, Jafar JJ. Smaller cerebral aneurysms producing more extensive subarachnoid hemorrhage following rupture: a radiological investigation and discussion of theoretical determinants. J Neurosurg 2003;99:248–253 [DOI] [PubMed] [Google Scholar]
- 4.Rinkel GJ. Treatment of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2003;2:12. [DOI] [PubMed] [Google Scholar]
- 5.Ohkuma H, Shimamura N, Fujita S, Suzuki S. Acute subdural hematoma caused by aneurysmal rupture: incidence and clinical features. Cerebrovasc Dis 2003;16:171–173 [DOI] [PubMed] [Google Scholar]
- 6.Kobata H, Tanaka H, Tada Y, Nishihara K, Fujiwara A, Kuroiwa T. Intracerebral hematoma due to ruptured nontraumatic middle meningeal artery aneurysm: case report. Neurol Med Chir (Tokyo) 2001;41:611–614 [DOI] [PubMed] [Google Scholar]
- 7.Nowak G, Schwachenwald D, Schwachenwald R, Kehler U, Muller H, Arnold H. Intracerebral hematomas caused by aneurysm rupture: experience with 67 cases. Neurosurg Rev 1998;21:5–9 [DOI] [PubMed] [Google Scholar]
- 8.Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg 1997;87:170–175 [DOI] [PubMed] [Google Scholar]
- 9.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317–320 [DOI] [PubMed] [Google Scholar]
- 10.Korogi Y, Takahashi M, Katada K, et al. Intracranial aneurysms: detection with three-dimensional CT angiography with volume rendering: comparison with conventional angiographic and surgical findings. Radiology 1999;211:497–506 [DOI] [PubMed] [Google Scholar]
- 11.White PM, Teasdale EM, Wardlaw JM, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohort. Radiology 2001;219:739–749 [DOI] [PubMed] [Google Scholar]
- 12.Anderson GB, Steinke DE, Petruk KC, Ashforth R, Findlay JM. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery 1999;45:1315–1322 [DOI] [PubMed] [Google Scholar]
- 13.Ogawa T, Okudera T, Noguchi K, et al. Cerebral aneurysms: evaluation with three-dimensional CT angiography. AJNR Am J Neuroradiol 1996;17:447–454 [PMC free article] [PubMed] [Google Scholar]
- 14.Alberico RA, Patel M, Casey S, Jacobs B, Maguire W, Decker R. Evaluation of the circle of Willis with three-dimensional CT angiography in patients with suspected intracranial aneurysms. AJNR Am J Neuroradiol 1995;16:1571–1580 [PMC free article] [PubMed] [Google Scholar]
- 15.White PM, Wardlaw JM, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology 2000;217:361–370 [DOI] [PubMed] [Google Scholar]
- 16.Rubin GD. MDCT imaging of the aorta and peripheral vessels [Suppl]. Eur J Radiol 2003;45:S42−S49 [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Nair S, Sano H, et al. Multi-slice 3D-CTA: an improvement over single slice helical CTA for cerebral aneurysms. Acta Neurochir (Wien) 2002;144:715–722 [DOI] [PubMed] [Google Scholar]
- 18.Ropers D, Baum U, Pohle K, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation 2003;107:664–666 [DOI] [PubMed] [Google Scholar]
- 19.Banerjee S. Multi-slice/helical computed tomography for lung cancer screening. Issues Emerg Health Technol 2003;48:1–4 [PubMed] [Google Scholar]
- 20.Werner A, Diehl SJ, Farag-Soliman M, Duber C. Multi-slice spiral CT in routine diagnosis of suspected acute left sided colonic diverticulitis: a prospective study of 120 patients. Eur Radiol 2003;13:2596–2603 [DOI] [PubMed] [Google Scholar]
- 21.Coche E, Pawlak S, Dechambre S, Maldague B. Peripheral pulmonary arteries: identification at multi-slice spiral CT with 3D reconstruction. Eur Radiol 2003;13:815–822 [DOI] [PubMed] [Google Scholar]
- 22.McFadzean RM, Teasdale EM. Computerized tomography angiography in isolated third nerve palsies. J Neurosurg 1998;88:679–684 [DOI] [PubMed] [Google Scholar]
- 23.Wintermark M, Uske A, Chalaron M, et al. Multislice computerized tomography angiography in the evaluation of intracranial aneurysms: a comparison with intraarterial digital subtraction angiography. J Neurosurg 2003;98:828–836 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Darder JM, Pesudo-Martinez JV, Feliu-Tatay RA. Microsurgical management of cerebral aneurysms based in CT angiography with three-dimensional reconstruction (3D-CTA) and without preoperative cerebral angiography. Acta Neurochir (Wien) 2001;143:673–679 [DOI] [PubMed] [Google Scholar]
- 25.Bidaut LM, Laurent C, Piotin M, et al. Second-generation three-dimensional reconstruction for rotational three-dimensional angiography. Acad Radiol 1998;5:836–849 [DOI] [PubMed] [Google Scholar]
- 26.Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002;23:1199–1205 [PMC free article] [PubMed] [Google Scholar]
- 27.Tanoue S, Kiyosue H, Kenai H, Nakamura T, Yamashita M, Mori H. Three-dimensional reconstructed images after rotational angiography in the evaluation of intracranial aneurysms: surgical correlation. Neurosurgery 2000;47:866–871 [DOI] [PubMed] [Google Scholar]
- 28.Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med 1997;16:981–991 [DOI] [PubMed] [Google Scholar]