Abstract
BACKGROUND AND PURPOSE: The incidence of middle turbinate pneumatization, or concha bullosa, has been well described in the literature. However, to our knowledge, no study has evaluated concha bullosa in relation to nasal septal deviation. We sought to analyze the incidence of concha bullosa and any correlation with nasal septal deviation and paranasal sinus disease.
METHODS: Three neuroradiologists retrospectively reviewed findings of 1095 consecutive paranasal sinus CT studies conducted between 2001 and 2002. All examinations were performed for evaluation of a symptom referable to the sinonasal region. Paranasal sinus inflammatory disease was identified and graded as mild, moderate, or severe. Sphenoid, ethmoid, maxillary, and frontal sinuses were each graded separately on both sides. If a concha bullosa was present, it was graded in size as small, moderate, or large. If bilateral concha were present, sizes were compared and when one was larger, it was identified as dominant. When nasal septal deviation was present, it was graded as mild, moderate, or severe. The direction of nasal septal deviation was identified as the face of the convex surface.
RESULTS: There was a clear association between the presence of a unilateral concha, or a dominant concha (in the case of bilateral concha), and the presence of nasal septal deviation (P < .0001). Moreover, there was a significant relationship between the presence of concha bullosa and deviation of the nasal septal to the contralateral side (P < .0001). This inverse association was present regardless of the size of the concha bullosa or degree of septal deviation. In every case, there was some preservation of air channels between the dominant concha and the nasal septum. Seventy-three percent of patients with concha bullosa had paranasal sinus inflammatory disease; 78% of patients without concha bullosa also had some form of inflammatory disease.
CONCLUSION: Concha bullosa is a common anatomic variant. There is a strong association between the presence of a concha bullosa and contralateral deviation of the nasal septum. Nasal septal deviation away from the dominant concha, with preserved adjacent air channels, suggests that the deviation is not a direct result of mass effect from the concha. No increased incidence of paranasal sinus disease exists in patients with concha bullosa.
Several authors have assessed the relationship between sinonasal anatomic variants and the incidence of sinusitis. The incidence of Haller cells has been reported to vary from 2–45%, and although some reports have found a statistically significant relationship between maxillary sinusitis and medium or large Haller cells (1), it is generally believed that the presence of a Haller cell is not related to sinusitis (2, 3). The incidence of agger nasi cells has been reported to vary from 3% to almost 100%, and its presence has been firmly associated with frontal sinusitis (2). The incidence of Onodi cells varies from 3.4–51%. Although not associated with sinusitis, its presence does pose an increased incidence of surgical complications (2, 4). There are two anatomic variants, the middle turbinate concha bullosa and nasal septal deviation; however, adequate documentation of their anatomy and etiology has not appeared in the literature either to support or refute their roles in sinusitis. The incidence of CT findings positive for concha bullosa has varied from 14–53%, and the relationship of concha bullosa to paranasal sinus disease continues to be debated (5, 6). Similarly, the role of nasal septal deviation in the etiology of sinusitis remains unclear. Our empirical observations regarding CT signs of paranasal sinus have suggested that when a concha bullosa was present, the nasal septum was deviated with a convexity to the opposite side and the airway between the concha and septum maintained. Herein we evaluate these observations to assess the relationship among concha bullosa, nasal septal deviation, and sinusitis in a large series that would yield statistically relevant data.
Methods
We retrospectively searched our radiology database for all paranasal sinus CT findings obtained between January 1, 2000, and December 31, 2002. The study was performed with institutional review board and HIPAA approval number GCO 03–0407. CT was performed on three CT scanners (GE Medical Systems HiSpeed CT/I or GE Medical Systems LightSpeed Ultra, GE Medical Systems, Milwaukee, WI; and Siemens Sensation 4, Siemens, Erlangen, Germany) to obtain 2.5–3.0-mm axial and coronal studies of the paranasal sinuses. Nasal vasoconstrictors were not administered. All patients were referred for CT owing to a clinical symptom presumably referable to the sinonasal region. Patient histories were not taken into account. Therefore, the data were not sorted by specific patient complaints or symptoms. A total of 1095 consecutive CT studies were identified. Of these cases, 97 studies were eliminated because either a tumor or prior surgery had destroyed some or most of the anatomic structures being studied. Of the 998 cases included in this study, the examinations were reviewed by three neuroradiologists (J.S.S., J.N.L., P.M.S.) and any differences in opinions were resolved by consensus. The left and right sides of each of the frontal, ethmoid, sphenoid, and maxillary sinuses were assessed separately for the presence of mucosal disease. This disease was evaluated as either being present or absent. A concha bullosa was defined as being present when more than 50% of the vertical height (measured from superior to inferior in the coronal plane) of the middle turbinate was pneumatized. A concha was subjectively assessed as being absent, small, moderate, or large. If bilateral concha were present, they were assessed as being equal in size or one was designated as the dominant concha. Deviation of the nasal septum was subjectively assessed as being absent, mild, moderate, or severe, and the direction of deviation was described by the convexity of the septal curvature. The preservation or obliteration of the air channel between a concha (unilateral or dominant concha) and the nasal septum was also assessed. SAS statistical software (Institute Incorporated, Cary, NC) was used to summarize the data. Analysis was performed with chi-square and Wilcoxon ranked sum techniques.
Results
The median age of the patients was 44 years (range, 0–98 years). There were 598 female (60%) and 400 male (40%) patients. Of the 998 patients, 648/998 (65%) had nasal septal deviation; 343 (51%) had a convexity to the right, 320 (49%) had the convexity to the left, and 15 (2%) had biconvex nasal septal deviation. Because these 15 patients with biconvex deviation technically had deviation in both directions, they were included in both the “left” and “right” convexity groups, leading to a total of 102% for the three groups.
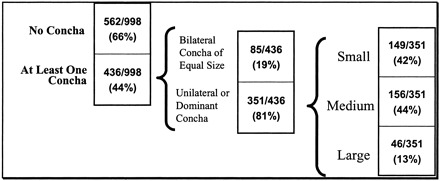
Of the 998 patients, 436 (44%) had at least one concha bullosa, 227 (23%) patients had a unilateral concha, and 209 (21%) had bilateral concha. Of the patients with bilateral concha, 85/209 (41%) had equal sized concha and 124 (59%) were judged as having a larger, dominant concha. Overall, 351/998 (35%) patients had either a unilateral or dominant (if bilateral) concha. Of these 351 patients, 149 (42%) had a small concha, 156 (44%) had a medium-sized concha, and 46 (13%) had a large concha. Of all 998 patients, 647 (65%) had either no concha or bilateral concha of equal size (Table 1).
TABLE 1:
Summary of incidence, unilateral or bilateral occurrence, and size of concha bullosa
 |
When considering patients with either a unilateral or dominant concha and the presence or absence of nasal septal deviation, 276/351 (79%) of patients with a unilateral or dominant concha had nasal septal deviation, but only 75 (21%) with a unilateral or dominant concha did not have nasal septal deviation; 357/647 (55%) of patients without a unilateral or dominant concha had nasal septal deviation, and a similar number, 275/647 (43%), of patients without a unilateral or dominant concha did not have nasal septal deviation (Table 2). In patients with a left-sided unilateral or dominant concha, 117/166 (70%) had right-sided nasal septal deviation, 11 (7%) had left-sided nasal septal deviation, and 38 (23%) had no nasal septal deviation. In patients with a right-sided unilateral or dominant concha, 127/185 (69%) had left-sided nasal septal deviation, 21 (11%) had right-sided nasal septal deviation, and 37 (20%) had no nasal septal deviation (Table 3). There was a strong association between a unilateral or dominant concha and contralateral nasal septal deviation (P < .0001).
TABLE 2:
Incidence of nasal septal deviation in patients with and without unilateral or dominant, if bilateral concha bullosa
| No Septal Deviation | Septal Deviation | |
|---|---|---|
| No unilateral or dominant concha | 275 /647 (43%) | 357 /647 (55%) |
| Unilateral or dominant concha present | 75 /351 (21%) | 276 /351 (79%) |
TABLE 3:
Incidence of nasal septal deviation broken down by convexity compared to the presence and location of unilateral or dominant, if bilateral concha bullosa
| No Septal Deviation | Rightward Septal Deviation | Leftward Septal Deviation | |
|---|---|---|---|
| No concha or equal bilateral concha | 275 /632 (44%) | 190 /632 (30%) | 167 /632 (26%) |
| Right unilateral or dominant concha | 37 /185 (20%) | 21 /185 (11%) | 127 /185 (69%) |
| Left unilateral or dominant concha | 38 /166 (23%) | 117 /166 (70%) | 11 /166 (7%) |
When considering patients with a unilateral or dominant concha and nasal septal deviation, the association of septal deviation was greater in patients with medium or large concha than in those with small concha. Forty-one of 46 (89%) patients with a large unilateral or dominant concha had associated septal deviation; 136/156 (87%) patients with a medium unilateral or dominant concha had associated septal deviation; and 100/149 (67%) patients with a small unilateral or dominant concha had associated septal deviation (P < 001).
When considering the relationship of sinus disease and nasal septal deviation, 504/648 (78%) of patients with nasal septal deviation had some sinus disease, and 253/350 (72%) without nasal septal deviation had some sinus disease (P = .0531) (Table 4). Of all 998 patients, 757 (76%) had some sinus disease; 318/436 (73%) of patients with a concha had sinus disease, and 439/562 (78%) of patients without a concha also had some sinus disease (P = .44). The relationship between a unilateral or dominant concha and right-sided sinus disease was not significant (P = .78), and the relationship between a unilateral or dominant concha and left-sided sinus disease was not significant (P = .53).
TABLE 4:
Distribution of nasal septal deviation and concha bullosa in the patient population studied
| Sinus Disease Present | |
|---|---|
| No septal deviation | 253 /350 (72%) |
| Septal deviation | 504 /648 (78%) |
| No concha | 439 /562 (78%) |
| Concha | 318 /436 (73%) |
There was no association between a unilateral or dominant concha and disease in any single sinus (eg, right or left frontal, right or left ethmoid, etc). P values ranged from 0.26 to 0.90.
Specifically, there was no association between the presence of a concha bullosa and an increased incidence of disease in any ipsilateral sinus. There was no relationship between a deviated septum (P = .41) or a unilateral or dominant concha (P = .72) and the side of any sinus inflammatory disease based on the Wilcoxon ranked sum test.
Discussion
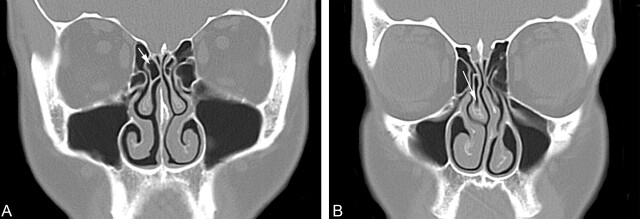
One of the explanations given for the wide reported incidence (14–53%) of concha bullosa, as assessed on the basis of CT findings, is that the definition of a concha bullosa has varied among studies. Some reports have defined a concha as any aeration of the middle turbinate, even if the aeration is restricted to the upper nonbulbous portion of the turbinate. Other reports have restricted the definition of a concha bullosa to those cases wherein the aeration extends caudally into the bulbous portion of the middle turbinate (2, 3, 5–13). Because most otolaryngologic surgeons restrict the diagnosis of a concha to those cases that have pneumatization of the bulbous portion of the middle turbinate, we adapted the definition of a concha as a middle turbinate with pneumatization extending caudally at least 50% of the vertical height of the middle turbinate. This invariably brought the aeration into the bulbous portion of the turbinate and was an easily measured criterion on coronal CT studies (Fig 1). In our study, a concha was present in 44% of the cases and was unilateral or dominant in 35% of patients. Of these conchae, 42% were small, 44% were moderate, and 13% were large.
Fig 1.
Coronal CT scans showing the definition of a concha bullosa.
A, Coronal CT scan of the paranasal sinuses shows pneumatization (arrow) of just under 50% of the vertical height of the right middle turbinate. This was not considered to be a concha bullosa in this study. There is no deviation of the nasal septum. There is inflammatory mucosal thickening obstructing the left infundibulum.
B, Coronal CT scan of the paranasal sinuses shows pneumatization (arrow) of more than 50% of the vertical height of the right middle turbinate. This pneumatization extends into the caudal bulbous portion of the turbinate. This was considered to be a small concha bullosa in this study. Also note that the nasal septum is moderately deviated convexity to the left and there is preservation of the air channel between the concha and the nasal septum. There is some mucosal thickening in both maxillary sinuses.
The population studied was referred for CT owing to a specific symptom presumably related to potential disease in the sinonasal region. Therefore, statistical inference of our results applies only to a symptomatic population. The incidence of concha bullosa, sinusitis, nasal septal deviation, and associations thereof apply only to patients who present to a healthcare provider with complaints potentially caused by sinonasal disease. No conclusion about the general population is made from the results of this study.
Some reports have suggested a relationship between the presence of a concha bullosa and sinusitis (14, 15), but other reports have found no direct relationship (16, 17). We found no correlation between the presence of a concha bullosa and sinus disease. Seventy-two percent of patients with a concha had sinus disease, but 78% of patients had sinus disease without a concha. This relationship held true for unilateral and contralateral sinusitis.
With regard to nasal septal deviation, it is reported in 20–31% of the population and severe deviation has been noted as a contributing factor for sinusitis (14, 18, 19). Another study reported a possible causal relationship between concha bullosa or septal deviation and sinus disease (15). Conversely, one study did not demonstrate a causal relationship between nasal septal deviation and sinusitis (20).
We defined nasal septal deviation as any bending of the nasal septal contour as evaluated on coronal CT studies. The direction of the deviation was defined by the side of the convexity of the curvature. Subjectively, we classified the deviation as absent, mild, moderate, or severe. We found that nasal septal deviation of some degree was present in 65% of the cases. There was no relationship found between nasal septal deviation and sinus disease.
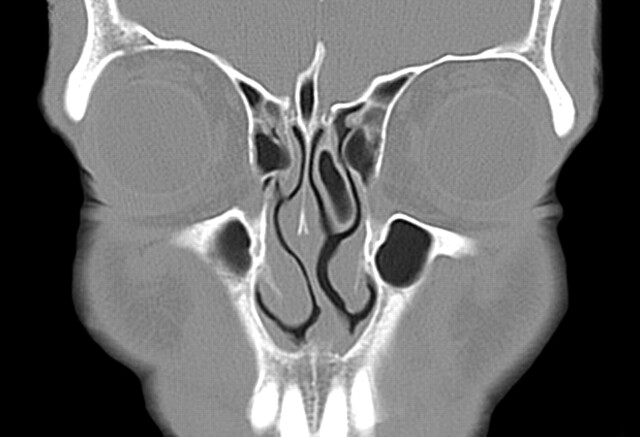
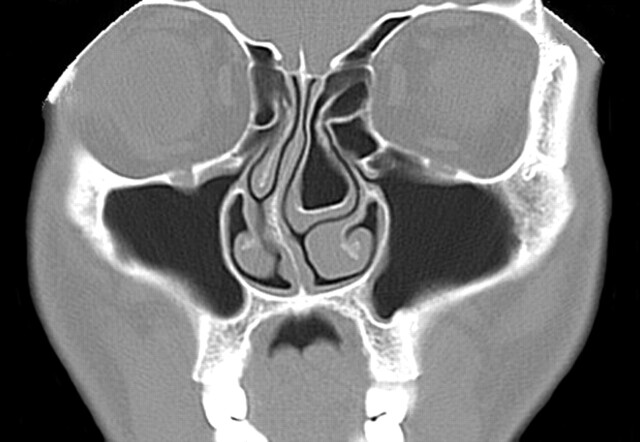
We also found that there was a strong relationship between the presence of a concha (unilateral or a dominant concha) and deviation of the nasal septal convexity away from the concha (P < .0001). We also found, however, that there was always maintenance of the nasal air channel between the medial aspect of the concha (unilateral or dominant concha) and the adjacent surface of the nasal septum (Figs 2–4). This implies that the deviation of the septum away from the concha is not the result of the concha pushing the septum. Rather, there appears to be some as yet unknown developmental relationship between a concha and the nasal septum. It is analogous to the “chicken and the egg” conundrum. We could find no information that suggested whether the concha develops first and the nasal septum somehow “senses” the mass effect of the concha and correspondingly developed away from this side, or if the septal deviation develops first and then the concha enlarges to partially fill the expanded air channel. In either case, the septal deviation is often so great that there is compromise of the contralateral nasal air channels.
Fig 2.
Coronal CT scan of the paranasal sinuses shows a moderate-sized left concha bullosa with moderate deviation of the nasal septum convexity to the right. Note that there is preservation of the air channel between the concha and the nasal septum. There is mucosal disease in both ethmoid sinuses.
Fig 3.
Coronal CT scan of the paranasal sinuses shows a large left concha bullosa with severe deviation of the nasal septum convexity to the right. Note that there is preservation of the air channel between the concha and the nasal septum. There is mucosal disease in both maxillary sinuses.
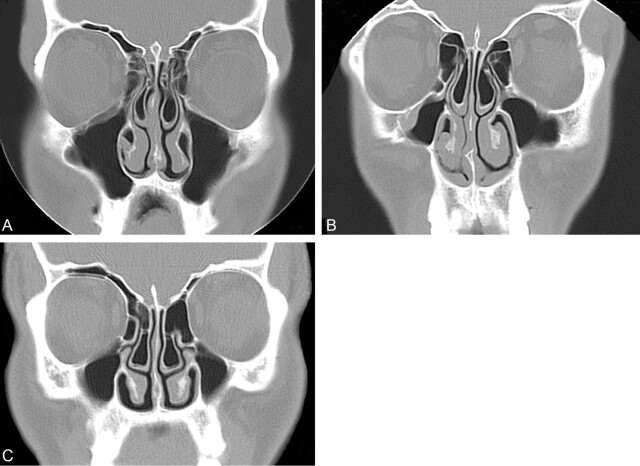
Fig 4.
Coronal CT scans showing variation in concha size wth preservation of nasal air channels.
A, Coronal CT scan of the paranasal sinuses shows moderate-sized concha bullosa bilaterally, with the one on the left side being slightly larger, or dominant. There is mild deviation of the nasal septum convexity to the right. Note that there is preservation of the air channel between the dominant concha and the nasal septum. There is, however, some loss of the air channel between the nasal septum and the right concha. There is mucosal disease in both ethmoid and maxillary sinuses.
B, Coronal CT scan of the paranasal sinuses shows moderate-sized concha bullosa bilaterally, with the one on the left side being slightly larger, or dominant. There is mild deviation of the nasal septum convexity to the right. Note that there is preservation of the air channels between each concha and the nasal septum. There is mucosal disease in both maxillary sinuses.
C, Coronal CT scan of the paranasal sinuses shows a moderate-sized bilateral concha bullosa with mild deviation of the nasal septum convexity to the right. Neither concha was considered to be dominant. Note that there is preservation of the air channels between each concha and the nasal septum. There is mucosal disease in both maxillary sinuses.
Conclusion
When a unilateral or dominant concha bullosa is present, there is no statistical relationship with any sinus disease (on either side). There is, however, a strong relationship between the presence of a unilateral or dominant concha and contralateral nasal septal deviation while the air channel between the concha and the nasal septum is preserved. There is no statistical relationship between nasal septal deviation and the presence of any sinus disease.
References
- 1.Stackpole SA, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol 1997;11:219–223 [DOI] [PubMed] [Google Scholar]
- 2.Zinreich S, Albayram S, Benson M, Oliverio P. The ostiomeatal complex and functional endoscopic surgery. In: Som P, ed. Head and neck imaging. 4th ed. St Louis: Mosby;2003;149–173
- 3.Bolger W, Butzin C, Parsons D. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope 1991;101:56–64 [DOI] [PubMed] [Google Scholar]
- 4.Weinberger D, Anand V, Al-Rawi M, Cheng H, Messina A. Surgical anatomy and variations of the onodi cell. Am J Rhinol 1996;10:365–370 [Google Scholar]
- 5.Lloyd GA. CT of the paranasal sinuses: study of a control series in relation to endoscopic sinus surgery. J Laryngol Otol 1990;104:477–81 [DOI] [PubMed] [Google Scholar]
- 6.Lloyd G, Lund V, Scadding G. CT of the paranasal sinuses and functional endoscopic surgery: a critical analysis of 100 symptomatic patients. J Laryngol Otol 1991;105:181–185 [DOI] [PubMed] [Google Scholar]
- 7.Arslan H, Aydinlioglu A, Bozkurt M, Egeli E. Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx 1999;26:39–48 [DOI] [PubMed] [Google Scholar]
- 8.Basic N, Basic V, Jelic M, et al. Pneumatization of the middle nasal turbinate: a CT study. Lijec Vjesn 1998;120:200–201 [PubMed] [Google Scholar]
- 9.Basic N, Basic V, Jukic T, et al. Computed tomographic imaging to determine the frequency of anatomical variations in pneumatization of the ethmoid bone. Eur Arch Otorhinolaryngol 1999;256:69–71 [DOI] [PubMed] [Google Scholar]
- 10.Scribano E, Ascenti G, Loria G, et al. The role of the ostiomeatal unit anatomic variations in inflammatory disease of the maxillary sinuses. Eur J Radiol 1997;24:172–174 [DOI] [PubMed] [Google Scholar]
- 11.Perez P, Sabate J, Carmona A, et al. Anatomical variations in the human paranasal sinus region studied by CT. J Anat 2000;197:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinreich SJ, Mattox DE, Kennedy DW, et al. Concha bullosa: CT evaluation. J Comput Assist Tomogr 1988;12:778–784 [DOI] [PubMed] [Google Scholar]
- 13.Zinreich S, Abidin M, Kennedy D. Cross-sectional imaging of the nasal cavity and paranasal sinuses. Operative Techniques Otolaryngol Head Neck Surg 1990;1:93–99 [Google Scholar]
- 14.Shin HS. Clinical significance of unilateral sinusitis. J Korean Med Sci 1986;1:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calhoun KH, Waggenspack GA, Simpson CB, et al. CT evaluation of the paranasal sinuses in symptomatic and asymptomatic populations. Otolaryngol Head Neck Surg 1991;104:480–483 [DOI] [PubMed] [Google Scholar]
- 16.Danese M, Duvoisin B, Agrifoglio A, et al. Influence of naso-sinusal anatomic variants on recurrent, persistent or chronic sinusitis. X-ray computed tomographic evaluation in 112 patients. J Radiol 1997;78:651–657 [PubMed] [Google Scholar]
- 17.Lam WW, Liang EY, Woo JK, et al. The etiological role of concha bullosa in chronic sinusitis. Eur Radiol 1996;6:550–552 [DOI] [PubMed] [Google Scholar]
- 18.Lebowitz RA, Brunner E, Jacobs JB. The agger nasi cell: radiological evaluation and endoscopic management in chronic frontal sinusitis. Operative techniques. Otolaryngol Head Neck Surg 1995;6:171–175 [Google Scholar]
- 19.Wanamaker H. Role of Haller’s cell in headache and sinus disease: a case report. Otolaryngol Head Neck Surg 1996;114:324–327 [DOI] [PubMed] [Google Scholar]
- 20.Hamdan AL, Bizri AR, Jaber M, et al. Nasoseptal variation in relation to sinusitis: a computerized tomographic evaluation. J Med Liban 2001;49:2–5 [PubMed] [Google Scholar]