Abstract
Summary: Chordomas are the most common sacrococcygeal tumors in adults. They have a high recurrence rate, are locally aggressive, and are resistant to radiation therapy. Radio frequency is a relatively new therapeutic technique, used primarily in the treatment of liver tumors; however, its application has widened to include other neoplasms. We report its use in the palliative treatment of chordomas in two patients for debulking and control of pain, with good results and low morbidity.
Chordomas (1, 2) are the most common primary malignant tumors of the spine in adults, excluding lymphoproliferative neoplasms. Between 50–66% of chordomas occur in the sacrococcygeal region. The usual treatment is surgical resection, either radical or palliative, followed by radiation therapy. Nonetheless, the recurrence rate is high, reaching almost 100%. Radio frequency is a well-established technique used for the treatment of soft tissue tumors of the liver and kidney, (3–6) the adrenal and parathyroid glands, and the breast (7). It is also used for the treatment of bony tumors such as osteoid osteoma (8), chondroblastoma (9) and metastatic bone disease (10).
A review of the literature reveals a single case report describing the use of radio frequency in the treatment of sacral chordomas (11). We present two cases of postoperative recurrent sacral chordoma treated palliatively, by using radio-frequency ablation.
Case Reports
Case 1
A 72-year-old man presented on September 22, 2000 for a progressively worsening painful sacral mass of a few years’ duration, dysuria, and constipation (Fig 1). MR imaging showed a 15-cm mass originating from the sacrum and extending up to S3 and anteriorly involving most of the pelvis, compressing the rectum and bladder. Complete surgical excision of the tumor with free surgical margins on pathologic examination was performed. Between February and November 2001, he underwent surgery twice for local recurrence at the surgical bed and metastasis to regional inguinal and iliac lymph nodes. Follow-up MR imaging examinations showed rapid recurrences with increase in the size and extent of regional involvement. He underwent radiation therapy (40 Gy) without improvement. The decision was then taken to stop all surgical interventions. The patient sought help in Germany, where he underwent medical therapy, to no avail. Follow-up MR imaging examinations in April and October 2002 showed a remarkable increase in the number, size, and extent of the recurrent tumoral masses. His symptoms worsened progressively, with bilateral sciatic pain, heaviness in both lower extremities, and difficulty in walking. A superficial mass caused excruciating pain and the patient’s inability to sit, lie supine, or walk in spite of medical therapy, including 120 mg of Morstel (morphine) per day.
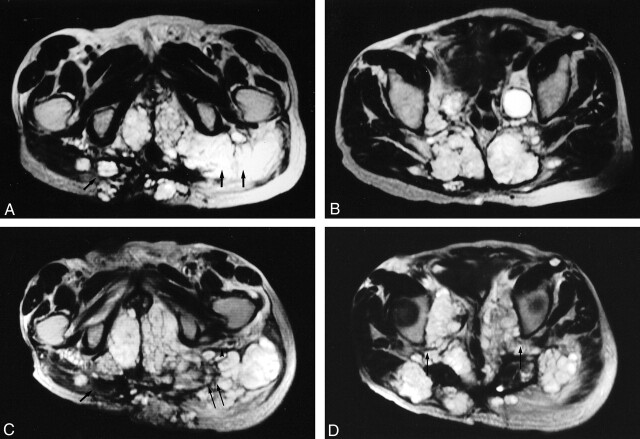
Fig 1.
A 72-year-old male patient with recurrent sacral chordoma and regional invasion. All the images are obtained in the transverse plane T2-weighted Turbo spin-echo. Images A and B were obtained in December 2002; C and D depict the same regions as those of A and B (obtained August 2003).
A, the single arrow points to invasion of the right gluteus muscle with extension into the subcutaneous tissues and the skin. The double arrow points to the involvement of the left gluteus muscle with encasement of the tract of the left sciatic nerve, which is not clearly defined and probably encased. Note the presence of invasion of the perineum bilaterally (B) shows regional invasion of the posterior pelvic wall with invasion of the sacrum, the piriformis muscle, and bilateral extension into the gluteus muscles. In addition, a metastatic deposit is seen anterior to the left sartorius muscle. Large metastatic iliac nodes are also noted.
C, obtained in August 2003 (8 months later) at approximately the same level as A, shows the first site treated devoid of tumor tissue (single arrow). The component at the skin remains intact. The third site treated (double arrows) shows debulking of the tumor. Note that the left sciatic nerve tract and the fat planes surrounding it are better visualized, because of the retraction of the tumor secondary to ablation (arrowheads). Also note the increase in the extent of the involvement of the perineum with significant compression of the structures within it. The nontreated mass in the left gluteus muscle, laterally, increased in size.
D, obtained in August 2003 at approximately the same level as B, shows the sacral masses, which were treated with significant loss of substance and volume. Note the reexpansion of the spaces at both sciatic notches with resultant decompression of both sciatic nerves tracts (single arrow). Also note the remarkable increase in the involvement of the pelvic cavity with compression of the pelvic structures along the midline. There are also now more tumoral masses in the nontreated areas at the gluteus muscles bilaterally with atrophy of the left gluteus muscle.
In December 2002, we suggested radio-frequency treatment for palliative pain control, and the patient gave informed consent. In the first session, we treated the painful superficial lesion. Under general anesthesia and CT guidance, the patient prone, a single pass was performed with the electrode needle (Berchtold, Tuttlingen, Germany) placed in the central aspect of the tumor. Calculations were made to leave a 1-cm area of preserved tumoral tissues between the planned area of necrosis and the skin (safety zone), to avoid the possible formation of a fistulous tract. Two sessions of 50 and 55 kW, each for about 10 minutes, were applied. Following that, the patient was admitted for observation and was discharged after 48 hours. The pain due to the superficial mass completely disappeared, with significant reduction in the use of pain medication (down to 30 mg).
He was admitted a second time on February 7, 2003 for the treatment of the persisting right sciatic pain. Under general anesthesia, the needle was placed in a sacral mass adjacent to the sacral notch, leaving also a 1-cm area of tumor intact between the zone of necrosis and the presumed tract of the sciatic nerve. Following this, the patient’s right sciatica disappeared. No complications were noted.
On February 27, 2003, two sites in the left buttock region were treated for decompression of the left sciatic nerve. Each site was treated as follows: 5 minutes at 45 kW, 5 minutes at 50 kW, and 10 minutes at 55 kW. This achieved a 10-cm area of necrosis. The patient then complained of general malaise, fatigue, and oliguria for a few days’ duration and was treated symptomatically with complete recovery. To date, the patient is still symptom free and on one tab of Tramal (Grunenthal, Aachen, Germany) per day.
Case 2
A 65-year-old woman complained of intolerable sacral pain not responding to medical therapy. In December 1996, MR imaging showed a 10-cm mass in the sacrum extending up to S2, with bilateral invasion of the gluteus muscles. The same double surgical approach as in the previous patient was performed. Four months later, she presented with a large left buttock mass. Surgery was performed again on July 27, 1997. Control MR imaging every 6 months showed progressive recurrences bilaterally, larger on the left side, where the tumoral masses reached the left S1 nerve root tract. A third palliative surgical intervention was performed on January 20, 2000, for pain reduction. A fourth surgery was performed in June 2002 for the resection of a large painful left buttock mass, and a terminal left colostomy was made to correct a megarectum. A rapid recurrence of the deepest tumoral masses occurred, with remarkable increase in size over 5 months, resulting in uncontrollable pain and difficulty in sleeping and lying supine.
In January 2003, the patient’s symptoms worsened, with the appearance of severe pain along the left S1 nerve root territory. Unable to walk, she was completely bedridden. She was admitted on March 3, 2003. MR imaging was performed, and only a transverse T2 series could be obtained. It showed a 22-cm mass completely invading the pelvis and the region of the left buttock with lack of visualization of the left S1 nerve root tract. Few small masses were seen in the right gluteus muscles.
We performed radio-frequency ablation on March 4, 2003, under general anesthesia and CT guidance. Two adjacent sites were treated at the same time, with the goal of reducing the bulk of the left buttock mass. Each site was treated by two sessions of 10 minutes each, the first session using 50 kW and the second session using 55 kW. A 20-cm Berchtold electrode needle was used. The amount of saline injected was computer-controlled by the unit according to the voltage level chosen. A control CT scan showed large areas of necrosis with numerous pockets of air, measuring a total span of 10 cm for both sites. The pain subsided without the need for medical therapy, and the patient was able to walk with the help of a walker. She was discharged 1 day later. No complications are known to date.
Control MR imaging was performed in both patients on August 14, 2003. It showed an absence of recurrence at the treated sites while the residual tumoral masses elsewhere kept growing. We plan to treat other areas only when either patient becomes symptomatic.
Discussion
Chordomas originate from embryonic remnants of the notocord-ectopic cordal foci; hence, they may occur anywhere between the clivus and coccyx. They are the most common primary malignant tumors of the spine in the adult, excluding lymphoproliferative neoplasms. They present between the ages of 30–70 years, with a male-female ratio of 2:1. They represent 1–4% of all primary malignant spinal tumors. Between 50–66% of chordomas occur in the sacrococcygeal region, usually in the 4th or 5th sacral segments, 35% at the base of the skull, and around 15% in the spinal axis. In addition, 5% occur at other sites such as the mandible, maxilla, and scapula.
The metastatic rate varies between 5–43%, with a predilection for the liver, lung, regional lymph nodes, and rarely, the skin, peritoneum, and heart. Patients usually present with low back pain and symptoms of mass effect such as sciatica, constipation, or fecal incontinence, frequency, urgency, and dysuria. They may present with rectal bleeding in 32% of cases and a palpable sacral mass in around 20%. On diagnosis, the average size is around 10 cm. Despite a 100% recurrence rate, surgery is still used with the goal of radical resection. This is difficult to achieve in most of the cases because of high morbidity and the presence of adjacent important structures, including the sciatic nerves. Only 8% of patients show a disease-free survival rate. Chordomas are radioresistant. Therefore, in the presence of aggressive recurrences, only palliative treatment could be proposed.
Radio frequency is a well-established technique, used initially for unresectable liver tumors, whether primary or secondary. The indications for its use were extended to tumors of the adrenal and parathyroid glands, kidneys, and breasts and osteoid osteoma and chondroblastoma. To the best of our knowledge, only one case report has been published describing the use of radio frequency in the treatment of sacral chordoma (11).
Radio-frequency ablation consists of applying energy from a generator to an electrode needle, which is inserted within the tumor, generating heat at the tip of the needle, with temperature reaching around 100°C, leading to thermal destruction of tumor cells. It is a simple procedure, which can be performed on an outpatient basis, is minimally invasive, and leaves no scars. As in the treatment of liver cancer, it can be performed under local anesthesia. The total procedure time is around 1 hour.
The limits of the areas of necrosis can be well controlled by sonography or CT and determined by the power used, time in which radio frequency is applied, and the diameter of the needle used: in the first session of our first patient, we used the same parameters as in the treatment of liver cancer, with close monitoring by CT scanning. As predicted, chordomas responded like liver tumors with similar areas of tumoral ablation obtained, by using similar parameters. This may be due to the fact that chordomas consist of large vacuolated cells containing intracytoplasmic mucus and an abundant amount of extracellular mucus; hence, theoretically, they have good conductivity. In fact, the impedence, which indicates tissue resistance, did not increase during the different sessions, further confirming the good conductivity of the chordoma tissues.
Conclusion
The morbidity associated with this procedure was low, consisting of transient malaise and oliguria in one patient. Furthermore, repeated MR imaging controls over a 7-month period showed absence of recurrence at the treated sites, as opposed to continued growth at the nontreated sites. The cost of a radio-frequency needle in our country and, presumably abroad as well, varies between $US 400 and $US 600 (including the use of the machine and depending on needle size).
Footnotes
There were no grants or financial support for this study.
References
- 1.Sze G, Vichanco LS II, Brant-Sawadzki MN, et al. Chordomas: MR imaging, Radiology 1988;166:187–191 [DOI] [PubMed] [Google Scholar]
- 2.Reznick D, Greenway GD. Bone and joint imaging. 2nd ed. Philadelphia: WB Saunders;1996. :1046–1048
- 3.Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of liver cancer. AJR Am J Roentgenol 1996;167:759–768 [DOI] [PubMed] [Google Scholar]
- 4.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastasis: percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology 1997;205:367–373 [DOI] [PubMed] [Google Scholar]
- 5.Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastasis. Radiology 1997;202:205–210 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SN, Tanabe KK, Solbiati L, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation in 16 patients. AJR Am J Roentgenol 1998;168:121A. [PubMed] [Google Scholar]
- 7.Bohm T, Hilger I, Muller W, et al. Saline-enhanced radiofrequency ablation of breast tissue. Invest Radiol 2000;35:149–157 [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal DI, Hornicek FJ, Torriani M, et al. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 2003;229:171–175 [DOI] [PubMed] [Google Scholar]
- 9.Erickson JK, Rosenthal DI, Zaleske DJ, et al. Primary treatment of chondroblastoma with percutaneous heat ablation: report of three cases. Radiology 2001;221:463–468 [DOI] [PubMed] [Google Scholar]
- 10.Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology 2002;224:87–97 [DOI] [PubMed] [Google Scholar]
- 11.Neeman Z, Patty JW, Wood BJ. Percutaneous radiofrequency ablation of chordoma. AJR Am J Roentgenol 2002;179:1330–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]