Abstract
BACKGROUND AND PURPOSE: Combined intravenous (IV) and intra-arterial (IA) thrombolytic therapy may be faster and easier to initiate than monotherapy, and its recanalization rate may be better as well. The sequential combination of recombinant tissue plasminogen activator (rTPA) and urokinase (UK) has synergistic and complementary effects on clot lysis. We prospectively evaluated the effectiveness and safety of sequential combination of IV rTPA and IA UK in acute ischemic stroke.
METHODS: IV rTPA was administered to patients with acute stroke within 3 hours of onset. Those whose condition had not improved at the end of rTPA infusion were further treated with selective IA UK. We evaluated baseline and 30-day National Institutes of Health Stroke Scale (NIHSS) scores and 90-day modified Rankin Scale scores.
RESULTS: Thirty patients were initially treated with IV rTPA; 24 were further treated with IA UK. Four patients who had rapid reocclusion following initial successful IA therapy received IV abciximab. Fourteen of 24 patients who underwent angiography had an effective perfusion state of Thrombolysis in Myocardial Infarction grade 3 flow. Median baseline and 30-day NIHSS scores were 18 and 2, respectively. Eighteen patients improved to a modified Rankin scale score of 0 or 1 after 90 days. Symptomatic hemorrhage developed in two patients.
CONCLUSION: The strategy of using conventional-dose IV rTPA and the sequential combination of IA UK in patients without an early clinical response to IV treatment was safe and feasible. This strategy achieved high complete arterial recanalization rates and good functional outcomes.
Early and complete recanalization of an occluded artery is the main therapeutic goal of acute ischemic stroke. Since the National Institute of Neurologic Disorders and Stroke (NINDS) study (1) was done, intravenous (IV) recombinant tissue plasminogen activator (rTPA) therapy within 3 hours after the onset of symptoms has been widely used. Despite the advantage of the rapid and easy initiation of thrombolytic therapy, IV rTPA treatment is limited by low recanalization rates, particularly in larger proximal arterial occlusions (2, 3). Compared with the IV approach, intra-arterial (IA) thrombolysis is reported to have higher recanalization rates and an extended therapeutic window (4–6). However, IA thrombolysis is limited by the delay to the initiation of treatment and the need for specialized angiographic techniques.
The combined IV and IA approach has been increasingly examined in pilot studies. This approach is reportedly feasible and safe, with a better recanalization rate and potentially improved patient outcomes (7–11). In most of these studies, rTPA was used for both IV and IA thrombolysis with divided doses for the IV and IA treatments. However, the combined use of thrombolytic agents with different mechanisms may have an additive and/or complementary effect. TPA and urokinase (UK) are the native plasminogen activators in the fibrinolytic system and have been used effectively in stroke patients. They are known to have synergistic and complementary effects in experimental studies in vitro and in vivo and also in patients with acute myocardial infarction when the agents are administrated sequentially (12–17). In this pilot study, we examined the efficacy and safety of a sequential combination of IV rTPA and IA UK in patients with acute ischemic stroke patients who presented within 3 hours after the onset of symptoms.
Methods
Patient Selection
We prospectively enrolled consecutive patients with acute ischemic stroke who were admitted to the neurology department during a period of 21 months and who underwent thrombolysis. The institutional review board approved this study, and informed consent was obtained from the patients or their surrogates. The inclusion criteria for thrombolysis, based on those of previous major thrombolysis trials, included ischemic stroke with the planned initiation of IV thrombolysis within 3 hours after onset, patient age of 18–85 years, a National Institutes of Health Stroke Scale (NIHSS) score >4 (except for isolated aphasia or hemianopia), normal or early signs on nonenhanced brain CT scans without evidence of hemorrhage, and obtainable informed consent.
Exclusion criteria were the following: a history of symptomatic ischemic stroke within 6 weeks; a history of head trauma within the previous 90 days; a history of surgery; biopsy involving a major organ; recent or active hemorrhage; trauma associated with internal organ injury or an ulcerative wound within the previous 30 days; a history of an arterial puncture at a noncompressible site within the previous 7 days; seizure at onset; a history of intracranial hemorrhage; a clinical presentation suggestive of subarachnoid hemorrhage or septic embolism; systolic blood pressure >185 mm Hg or diastolic blood pressure >110 mm Hg; bleeding diathesis; a history of serious, advanced, or terminal illness; rapidly improving neurologic signs; a clinical presentation of a lacunar infarction; or a comatose state in suspected carotid arterial stroke. Patients with an initial blood pressure above the specified limits were excluded if aggressive treatment was required to reduce their blood pressure below that level. Laboratory exclusion criteria included an increased international normalized ratio of >1.7, a history of heparinization within the previous 48 hours, an elevated activated partial thromboplastin time, platelet counts <100,000/mm3, a hematocrit level of <25%, or a glucose concentration <50 mg/dl (2.7 mmol/L) or >400 mg/dL (22.2 mmol/L).
Thrombolytic Therapy
V thrombolysis was performed by using rTPA (Actilyse; Boehringer Ingelheim, Germany). A total dose of 0.9 mg/kg was given, with a 10% loading bolus injection followed by a continuous infusion over 60 minutes. Patients showing no early clinical responses to IV rTPA at the end of rTPA infusion (i.e., improvement of NIHSS score of <4) received IA thrombolysis. When an occlusion of the intracranial arteries was documented on diagnostic cerebral angiograms obtained by using a 5F catheter (Headhunter cerebral catheter; Cook, Bloomington, IN) via a femoral approach, super-selective angiography was performed through a microcatheter. The tip of the microcatheter was imbedded in the face of the thrombus by using an end-hole microcatheter (Excelsior; Target Therapeutics, Fremont, CA) or through the thrombus by using a side-hole microcatheter (Micro-Soft Stream; Target Therapeutics). UK (Yuhan, Seoul, Korea) 100,000 U was reconstituted in 5 mL of 0.9% normal sodium chloride solution. This was manually infused in a pulsatile injection manner at a rate of 15,000 U/min by using a 1-mL syringe. UK 300,000 U was given initially, and angiography was then performed. If recanalization was not achieved, an additional 200,000 U was infused until recanalization occurred. The maximum doses of UK were limited to 1,000,000 U. The recanalized arteries were reevaluated 20 minutes later by means of repeat angiography to determine if the arteries had maintained their patency. When a reocclusion occurred, IV abciximab was administrated as a bolus at 0.2 mg/kg, and a subsequent infusion was given for 12 hours at rate of 0.125 μg/kg/min.
Assessment of Hemorrhagic Transformation and Clinical Outcome
Nonenhanced brain CT scanning or MR imaging was performed 24 hours after rTPA infusion and at any time when clinical deterioration was noted or hemorrhage was suspected. Hemorrhagic transformation (HT) was assessed by means of CT and defined as areas of increased attenuation on nonenhanced scans. Symptomatic hemorrhage was defined as any increase in the NIHSS score attributable to intracranial hemorrhage. Clinical assessments included baseline and 30-day determinations of NIHSS scores, as well as modified Rankin scale (mRS) scores at 90 days. One of two neurologists (K.Y.L., S.H.K.) who were not blinded to the angiographic findings performed the assessments.
Statistical Analysis
The chi-square test was used to define the difference of HT rate between the successful and unsuccessful recanalization groups. P value <.05 was considered significant.
Results
Demographic Data
Thirty consecutive patients (18 men, 12 women) with a median age of 63 years (range, 47–82 years), were enrolled. Twenty-five patients were admitted via the emergency room, whereas five developed an acute stroke during their admission for systemic illnesses other than cerebrovascular disease. The median time from onset to visit or discovery was 45 minutes. Nine patients had cardioembolic sources, such as an atrial fibrillation or a sick sinus syndrome.
Thrombolytic Treatment
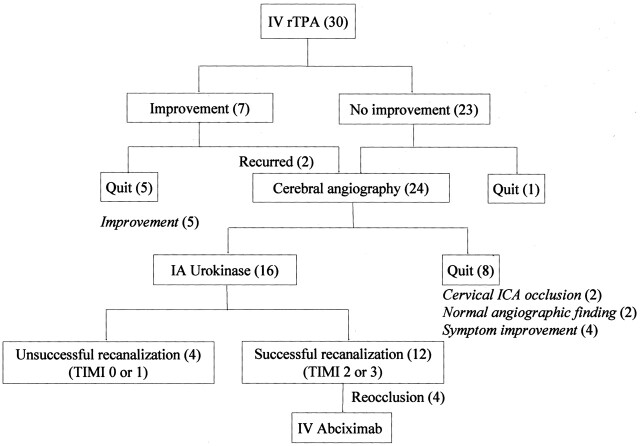
IV rTPA was initiated at a median time of 2 hours 15 minutes (range, 60–210 minutes) from the onset of stroke. All but five patients received a full dose of rTPA (0.9 mg/kg). Early clinical improvement (NIHSS score ≥4) at the end of the rTPA infusion was observed in seven patients. However, the symptoms recurred in two of these patients at 7 and 2 hours after their initial improvement. Cerebral angiography was performed immediately after IA thrombolysis in 24 patients and not in five patients who had improved or in one patient in whom occlusion of a cortical branch was suggested. The median time from onset to the femoral arterial puncture was 3 hours 50 minutes (range, 60–310 minutes). Cerebral angiography demonstrated occlusions of the proximal middle cerebral artery (MCA) in nine patients, the distal internal carotid artery (ICA) in four, the cervical ICA in two, the basilar artery (BA) in two, the distal MCA in one, and the posterior cerebral artery (PCA) in one (Table 1). Occluding thrombi were not found in five patients (three with stenosis of the clinically relevant arteries and two with normal angiographic findings). Finally, 16 patients were further treated with IA UK. Those not treated with IA UK were two patients with a cervical ICA occlusion, two with normal angiographic findings, and four with marked clinical improvement (stenosis of the relevant arteries in three and distal branch occlusion in one) during the diagnostic angiographic procedures (Fig 1). The total dose of UK ranged from 300,000 to 1,000,000 U (median, 600,000 U). IV abciximab was administrated in four patients (patients 3, 7, 10, and 28) who had a reocclusion immediately after initially successful thrombolysis (Table 1). The reoccluded arteries regained full reperfusion within 60 minutes after abciximab infusion.
TABLE 1:
Baseline characteristics, time to intervention, angiographic findings, and outcomes
| Patient/Age/Sex | Onset to (min) |
UK Dose (U) | Arterial lesion on DSA | Final TIMI Grade | NIHSS Score |
mRS Score 90 Day | HT | ||
|---|---|---|---|---|---|---|---|---|---|
| rTPA | DSA | Baseline | 30 Day | ||||||
| 1/59/F | 120 | 270 | 800,000 | Distal ICA | 2 | 9 | 5 | 2 | No |
| 2/61/M | 210 | 290 | 800,000 | BA | 3 | 28 | 1 | 0 | No |
| 3/53/F | 60 | 120 | 500,000 | M1 | 3 | 14 | 2 | 1 | No |
| 4/74/F | 195 | 300 | 300,000 | M2 | 0 | 22 | 0 | 0 | Asym |
| 5/76/F | 170 | 260 | 500,000 | M1 | 3 | 13 | 1 | 0 | No |
| 6/82/M | 100 | NA | NA | NA | NA | 30 | 0 | 0 | No |
| 7/62/M | 75 | 110 | 700,000 | M1 | 3 | 25 | 3 | 1 | No |
| 8/60/F | 90 | 160 | 600,000 | M1 | 2 | 19 | 10 | 4 | Asym |
| 9/52/M | 90 | 160 | NA | M1 stenosis | 3 | 8 | 0 | 0 | No |
| 10/47/M | 90 | 60* | 800,000 | BA | 3 | 15 | 0 | 0 | No |
| 11/48/M | 130 | 210* | NA | Normal | 3 | 7 | 2 | 0 | No |
| 12/78/M | 175 | NA | NA | NA | NA | 9 | NA | 6 | No |
| 13/74/F | 120 | 240 | 800,000 | M1 | 3 | 17 | 7 | 3 | Asym |
| 14/65/F | 150 | 210 | 400,000 | Distal ICA | 0 | 22 | 25 | 5 | Asym |
| 15/63/M | 145 | 210 | 800,000 | M1 | 3 | 22 | 7 | 2 | No |
| 16/61/M | 140 | NA | NA | NA | NA | 7 | 1 | 1 | No |
| 17/78/M | 105 | 205 | NA | Cervical ICA | 0 | 19 | NA | 5 | No |
| 18/70/M | 150 | 270 | 600,000 | M1 | 2 | 23 | 9 | 2 | No |
| 19/81/M | 130 | 230 | NA | Distal ICA stenosis | 3 | 23 | 2 | 1 | No |
| 20/67/M | 130 | NA | NA | NA | NA | 4 | 30 | 6 | Sym |
| 21/71/F | 70 | 160 | 1,000,000 | Distal ICA | 1 | 23 | NA | 6 | Sym |
| 22/59/F | 170 | 290 | NA | Normal | 3 | 21 | 1 | 1 | No |
| 23/80/M | 160 | NA | NA | NA | NA | 16 | 4 | 1 | No |
| 24/60/F | 170 | 310 | 500,000 | M1 | 0 | 17 | 15 | 4 | Asym |
| 25/59/M | 110 | 230 | NA | Cervical ICA | 0 | 22 | NA | 2 | No |
| 26/54/M | 150 | 275 | 500,000 | Distal ICA | 3 | 20 | 4 | 1 | No |
| 27/72/F | 150 | 255 | NA | PCA | 0 | 17 | 3 | 1 | No |
| 28/58/F | 180 | 265 | 500,000 | M1 | 3 | 13 | 0 | 0 | No |
| 29/57/M | 135 | NA | NA | NA | NA | 8 | 1 | 1 | No |
| 30/81/M | 150 | 250 | NA | BA stenosis | 3 | 24 | 1 | 1 | No |
Note.—Asym indicates asymptomatic hemorrhagic transformation; DSA, digital subtraction angiography; M1, M1 segment of the MCA; M2, M2 segment of the MCA; NA, not applicable; and Sym, symptomatic hemorrhagic transformation.
Time from recurrent symptoms after IV thrombolysis to angiography.
Fig 1.
Flowchart shows the sequential combination of thrombolysis. Numbers in parentheses are numbers of patients. TIMI = Thrombolysis in Myocardial Infarction.
Arterial Recanalization
In 24 patients who underwent cerebral angiography, TIMI grade 3 flow was observed in 14 (58%), and TIMI grade 2 flow was observed in three (12%). The MCA had a successful recanalization of TIMI grade 3 or 2 flow in 11 of 13 patients. The BA was recanalized in all three patients, and the ICA was recanalized in three of seven patients (Table 2). In 16 patients treated with combined thrombolysis, TIMI grade 3 flow was achieved in nine (56%), and TIMI grade 2 flow was achieved in three (18%).
TABLE 2:
Final angiographic findings in 24 patients undergoing cerebral angiography
| Involved Artery | Patients |
TIMI Grade |
||||
|---|---|---|---|---|---|---|
| rTPA | rTPA + UK | 0 | 1 | 2 | 3 | |
| ICA (n = 7) | 3 | 4 | 3 | 1 | 1 | 2 |
| MCA (n = 13) | 3 | 10 | 2 | 0 | 2 | 9 |
| BA (n = 3) | 1 | 2 | 0 | 0 | 0 | 3 |
| PCA (n = 1) | 1 | 0 | 1 | 0 | 0 | 0 |
| Total (n = 24) | 8 | 16 | 6 (25%) | 1 (4%) | 3 (12%) | 14 (58%) |
Clinical Outcomes
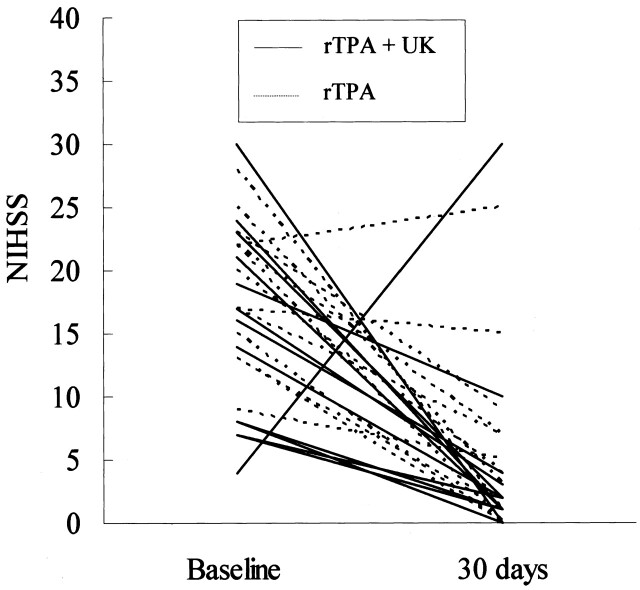
The median baseline NIHSS score was 18 (range, 4–30) in 30 patients. At 30 days, the score could be assessed in 26 patients; the median was 2 (range, 0–30) (Fig 2). An improvement in the NIHSS score to ≥4 was observed in 23 patients. About 60% (18 patients) had a good functional outcome defined as an mRS score of 0 or 1 at 90 days. Twelve (70%) of 17 patients with a successful recanalization (TIMI grade 2 or 3) and two (28%) of seven with an unsuccessful recanalization (TIMI 0 or 1) had an mRS score of 0 or 1 at 90 days. The mortality rate after 90 days of follow-up was 10% (three of 30 patients). One patient died from an intracranial hemorrhage, and the other two died after discharge due to undefined causes.
Fig 2.
Baseline and 30-day NIHSS scores. Median scores were 18 and 2, respectively
Hemorrhagic Transformation
HT was observed in seven patients (23%). Six developed in those receiving combined IV rTPA and IA UK, and one affected a patient receiving IV rTPA. No HT was observed in those treated with abciximab. Clinical deterioration due to HT occurred in two patients (one from the IV rTPA group and the other from the combined IV rTPA and IA UK group) and led to death in one patient. Six patients who underwent angiography during thrombolysis had HT: four (57%, TIMI grade 0 in three and TIMI grade 1 in one) of seven patients with unsuccessful recanalization and two (11%; TIMI grade 2 in one and TIMI grade 3 in one) of 17 patients with successful recanalization (P < .05).
Discussion
Although thrombolytic therapy is effective in reducing the neurologic deficits in acute ischemic stroke, thrombolysis fails in a considerable proportion of patients. Combined IV and IA thrombolysis may have benefits because a high recanalization rate is expected with IA thrombolysis, in addition to the fast and easy introduction of the thrombolytic agent via the IV route. Although different thrombolytic regimens and strategies for combined thrombolysis have been examined and are under investigation, this study differs from previous or ongoing studies in several points. In this study, the UK was combined with rTPA, whereas others used rTPA for both the IV and the IA approaches. The sequential combination of rTPA and UK/pro-UK has complementary and synergistic effects on clot lysis (17). The fibrin selectivity of UK/pro-UK is attributed to its selective activation of fibrin-bound plasminogen, whereas TPA directly binds strongly to fibrin. Predigestion of a thrombus by TPA exposes the plasminogen binding sites, which are required for the action of UK/pro-UK. Therefore, thrombolysis may be more efficient when TPA is followed by a UK/pro-UK infusion, as was shown in experimental studies and in patients with acute coronary artery disease (14–16).
IA thrombolysis was attempted in patients who did not have an early clinical response to a full dose of rTPA. In the Emergency Management of Stroke (EMS) bridging trial (7), two-thirds (0.6 mg/kg) of the dose used in the NINDS trial for IV rTPA (0.9 mg/kg) was used for the IV thrombolysis portion, and a maximum dose of 20 mg rTPA was used for subsequent IA thrombolysis. The IV infusion time was also shortened (from 60 to 30 minutes). A similar dose regimen has been used in other trials (8, 10). In the ongoing Interventional Management of Stroke (IMS) study (18), the IV thrombolysis by using the same dose and infusion time of rTPA as in the EMS bridging trial was followed as soon as possible by cerebral angiography. The indication for the IA approach was the presence of thrombus. In this study, patients who did not have an early clinical response to a full dose of IV rTPA were given further treatment with IA thrombolysis. With this approach, some patients who improve early after IV rTPA therapy may avoid an unnecessary IA procedure. In this study, five patients had so improved after rTPA infusion that angiography for IA thrombolysis was not performed. Furthermore, IA UK infusion was unnecessary in four patients because of marked improvement in the angiographic suite. Therefore, angiography or IA UK infusion, which can cause angiographic complications or additional risks of hemorrhage, were avoidable in 30% of patients. However, in this study, the median delay from the IV rTPA bolus to angiography was 1 hour 35 minutes. In the IMS trial (18), the delay was approximately 40 minutes. In this regard, the strategy of fast angiography to identify a thrombus as a therapeutic target in the IMS study may shorten the access time to additional thrombolytic therapy.
Finally, the study protocol included evaluation and treatment of reocclusion, which can occur during or immediately after an initially successful thrombolysis (19). Abciximab, a potent and specific platelet glycoprotein IIb/IIIa receptor inhibitor, has been effective in resolving recurrent thrombosis after thrombolytic-induced thrombolysis in individuals with acute myocardial infarction or ischemic stroke, as this type of rethrombosis has platelet-mediated thrombotic mechanisms (20–23). As was shown in this study and a previous report (19), reocclusion can be completely and safely resolved with abciximab. Although the safety and efficacy of widespread use of abciximab in acute stroke awaits the final results of ongoing studies (24), its selective use may be beneficial in patients with reocclusion.
Combined thrombolysis may have the benefits of high complete recanalization rate and improved patient outcomes. Recanalization to TIMI grade 3 flow was achieved in more than one-half of patients in this study; this rate was similar to those of previous studies of combined IV/IA thrombolysis. In the present study, favorable functional outcomes of 90-day mRS scores of 0 or 1 were achieved in 60% of patients. This rate was higher than those observed in studies examining the effect of pure IA or IV thrombolysis: for IA therapy: 26% in Prolyse in Acute Cerebral Thromboembolism (PROACT) II study for IA therapy and for IV therapy, 39% in the NINDS rTPA study, and 35.7% in the European Cooperative Acute Stroke Study (ECASS) (1, 5, 25). Reported rates of favorable outcomes, 25–77% in previous combined IV/IA thrombolysis trials, are comparable to ours (7–11).
Hemorrhage is one of major negating factors of thrombolytic therapy, as it diminished the effect of thrombolysis and limits the enrollment of patients because of the risk of hemorrhage. HT was a major concern in this study because UK was administrated in addition to a conventional dose of rTPA. Although six of the seven patients with HT were treated with combined thrombolysis, the HT rate was similar to those in the previous studies or trials. In comparison data from a single center, no significant difference was found between the agents (rTPA vs UK) and the route of thrombolytics (IA vs combined IV/IA) (26). An increased dose of IV rTPA was suggested to increase the risk of hemorrhage in a small pilot study performed before the larger NINDS rTPA study (27); however, the effect of sequentially administrated UK (which reacts to a receptor different than that targeted by rTPA) on the development of HT is unclear.
Several studies have identified factors that may lead to HT after thrombolysis. In an analysis of the data from the European Cooperative Acute Stroke Study and the NINDS Stroke Study, several factors were associated with HT: severe neurologic deficits; increasing age; and early ischemic changes, brain edema, or mass effect at CT (28, 29). Increased glucose level, high NIHSS score, prolonged time to recanalization, less recanalization, and low platelet counts were independent predictors of HT in IA thrombolysis trials (26). Although these results suggest that no single clinical factor predicts the development of HT, vessel-wall damage that leads to HT is mediated by matrix metalloproteinase-9, a proteolytic enzyme that degrades the major extracellular matrix components of the vessel wall (30). Increased baseline plasma levels of this metalloproteinase before thrombolysis are associated with the development of HT and a failure of thrombolysis (31, 32). This observation may partly account for the increased rate of HT in our patients with poor recanalization.
This study had several limitations. First, a small number of patients enrolled, and there was no control group. Second, the sizes and sites of the occluded arteries were heterogeneous. However, this study showed that UK, which is known to be synergistic with and complementary to rTPA, may be safely and effectively administrated by the IA route in acute ischemic stroke without an early clinical response to conventional IV rTPA treatment.
Conclusion
In this pilot study, a full dose of IV rTPA and a sequential combination of IA UK for patients without an early clinical response to the IV approach was feasible and safe. This approach had high recanalization rates, good functional outcomes, and acceptable mortality and hemorrhage rates. However, because of the small number of patients, the lack of a control group, and the presence of heterogeneous arterial lesions, further large-scale studies are needed to confirm these results.
Footnotes
Supported by a grant (02-PJ1-PG3–21301–0012) of the Korean Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group: tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78–86 [DOI] [PubMed] [Google Scholar]
- 3.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol 1993;14:3–13 [PMC free article] [PubMed] [Google Scholar]
- 4.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 6.Jahan R, Duckwiler GR, Kidwell CS, et al. Intraarterial thrombolysis for treatment of acute stroke: experience in 26 patients with long-term follow-up. AJNR Am J Neuroradiol 1999;20:1291–1299 [PMC free article] [PubMed] [Google Scholar]
- 7.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–2605 [DOI] [PubMed] [Google Scholar]
- 8.Ernst R, Pancioli A, Tomsick T, et al. Combined intravenous and intra-arterial recombinant tissue plasminogen activator in acute ischemic stroke. Stroke 2000;31:2552–2557 [DOI] [PubMed] [Google Scholar]
- 9.Keris V, Rudnicka S, Vorona V, Enina G, Tilgale B, Fricbergs J Combined intraarterial/intravenous thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2001;22:352–358 [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez JI, Zaidat OO, Sunshine JL, Tarr R, Selman WR, Landis DM. Endovascular administration after intravenous infusion of thrombolytic agents for the treatment of patients with acute ischemic strokes. Neurosurgery 2002;50:251–260 [DOI] [PubMed] [Google Scholar]
- 11.Hill MD, Barber PA, Demchuk AM, et al. Acute intravenous-intra-arterial revascularization therapy for severe ischemic stroke. Stroke 2002;33:279–282 [DOI] [PubMed] [Google Scholar]
- 12.Maksimenko AV, Tischenko EG. New thrombolytic strategy: bolus administration of tPA and urokinase-fibrinogen conjugate. J Thromb Thrombolysis 1999;7:307–312 [DOI] [PubMed] [Google Scholar]
- 13.Popma JJ, Califf RM, Ellis SG, et al. Mechanism of benefit of combination thrombolytic therapy for acute myocardial infarction: a quantitative angiographic and hematologic study. J Am Coll Cardiol 1992;20:1305–1312 [DOI] [PubMed] [Google Scholar]
- 14.Califf RM, Topol EJ, Stack RS, et al. Evaluation of combination thrombolytic therapy and timing of cardiac catheterization in acute myocardial infarction: results of thrombolysis and angioplasty in myocardial infarction - phase 5 randomized trial. Circulation 1991;83:1543–1556 [DOI] [PubMed] [Google Scholar]
- 15.Morris JA, Muller DW, Topol EJ. Combination thrombolytic therapy: a comparison of simultaneous and sequential regimens of tissue plasminogen activator and urokinase. Am Heart J 1991;122:375–380 [DOI] [PubMed] [Google Scholar]
- 16.Collen D, Stassen JM, De Cock F. Synergistic effect of thrombolysis of sequential infusion of tissue-type plasminogen activator (t-PA) single-chain urokinase-type plasminogen activator (scu-PA) and urokinase in the rabbit jugular vein thrombosis model. Thromb Haemost 1987;58:943–946 [PubMed] [Google Scholar]
- 17.Pannell R, Black J, Gurewich V. Complementary modes of action of tissue-type plasminogen activator and pro-urokinase by which their synergistic effect on clot lysis may be explained. J Clin Invest 1988;81:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the interventional management of stroke study. Stroke 2004;35:904–911 [DOI] [PubMed] [Google Scholar]
- 19.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology 2002;59:862–867 [DOI] [PubMed] [Google Scholar]
- 20.Heo JH, Lee KY, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology 2003;60:1684–1687 [DOI] [PubMed] [Google Scholar]
- 21.Lee KY, Heo JH, Lee SI, Yoon PY. Rescue treatment with abciximab in acute ischemic stroke. Neurology 2001;56:1585–1587 [DOI] [PubMed] [Google Scholar]
- 22.Kwon OK, Lee KJ, Han MH, Oh CW, Han DH, Koh YC. Intraarterially administered abciximab as an adjuvant thrombolytic therapy: report of three cases. AJNR Am J Neuroradiol 2002;23:447–451 [PMC free article] [PubMed] [Google Scholar]
- 23.Vivekananthan DP, Moliterno DJ. Glycoprotein IIb/IIIa combination therapy in acute myocardial infarction: tailoring therapies to optimize outcome. J Thromb Thrombolysis 2002;13:35–39 [DOI] [PubMed] [Google Scholar]
- 24.AbESTT Investigators. Effect of abciximab for acute ischemic stroke: final results of abciximab in emergent stroke treatment trial (AbESTT). Stroke 2003;34:253 [Google Scholar]
- 25.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025 [PubMed] [Google Scholar]
- 26.Kidwell CS, Saver JL, Carneado J, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke 2002;33:717–724 [DOI] [PubMed] [Google Scholar]
- 27.Levy DE, Brott TG, Haley EC Jr, et al. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke 1994;25:291–297 [DOI] [PubMed] [Google Scholar]
- 28.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109–2118 [DOI] [PubMed] [Google Scholar]
- 29.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke: potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957–960 [DOI] [PubMed] [Google Scholar]
- 30.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab 1999;19:624–633 [DOI] [PubMed] [Google Scholar]
- 31.Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation 2003;107:598–603 [DOI] [PubMed] [Google Scholar]
- 32.Heo JH, Kim SH, Lee KY, Kim EH, Chu CK, Nam JM. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke 2003;34:48–50 [DOI] [PubMed] [Google Scholar]