Abstract
BACKGROUND AND PURPOSE: Substantial intraoperative bleeding during surgical removal of head and neck paragangliomas may be a major problem in the management of these highly vascularized tumors. Traditional preoperative embolization via a transarterial approach has proved beneficial but is often limited by complex vascular anatomy and unfavorable locations. We report our experience with the preoperative devascularization of head and neck paragangliomas by using direct puncture and an intralesional injection of cyanoacrylate.
METHODS: We retrospectively analyzed nine consecutive patients with head and neck paragangliomas who were referred for preoperative devascularization. Three patients were treated for carotid-body tumors; two for vagal lesions; and four, for jugular paragangliomas. Direct puncture of the lesion was performed by using roadmap fluoroscopic guidance. Acrylic glue was injected by using continuous biplane fluoroscopy. All patients underwent postembolization control angiography and immediate postoperative CT scanning.
RESULTS: Angiograms showed that complete devascularization was achieved in all cervical glomus tumors, whereas subtotal devascularization was achieved in jugular paragangliomas. In this latter location, the injection of acrylic glue was limited by the potential risk of reflux into normal brain territory via feeders from the internal carotid or vertebral artery. The tumors were surgically removed and histologically examined. No technical or clinical complications related to the embolization procedure occurred.
CONCLUSION: Percutaneous puncture of paragangliomas in the head and neck region and their preoperative devascularization by intralesional injection of acrylic glue is a feasible, safe, and effective technique.
Paragangliomas, also called glomus tumors, are highly vascularized tumors of neural crest origin that are derived from chemoreceptor organs in the walls of blood vessels or specific nerves in the head and neck area. They can develop in the middle ear (glomus tympanicum), the jugular foramen of the skull base (glomus jugulare), or the head and neck area (glomus caroticum, glomus vagale). Glomus tumors are multiple in 25% of patients and usually benign but locally destructive (1–4). They spread along paths of least resistance and cases of malignancy may occur. Glomus tumors can be treated with surgical excision, radiation therapy, surgery and irradiation, or embolization and surgery. Surgical removal is often associated with a significant intraoperative bleeding rate because of the vascular nature of the tumors (3, 5–11), especially when they are large. To reduce intraoperative blood loss, preoperative devascularization with transarterial embolization has proved beneficial (12–18), but this often remains incomplete because of the extensive angioarchitecture and substantial arteriovenous shunting of these lesions. Alternatively, direct puncture and the injection of acrylic glue have been described (19–22), but these can also be associated with complications (23). We report our experience in nine cases of head and neck paragangliomas treated by means of direct puncture and the intratumoral injection of cyanoacrylate.
Methods
Patients and Angiography
Between April 2002 and January 2004, nine patients underwent preoperative embolization of a glomus tumor by means of direct puncture. We retrospectively reviewed their clinical status and clinical outcomes, as well as the radiologic and technical aspects of the embolization. Three of the nine patients had carotid-body tumors, two had vagal tumors, and four had jugular paragangliomas. In one patient who presented with multiple head and neck paragangliomas (one jugular, two vagal, and two carotid-body tumors), only one extensive jugular paraganglioma was embolized. The patient group consisted of three men and six women aged 18–67 years (mean, 40 years). Their radiologic workup before treatment consisted of contrast-enhanced CT scanning or MR imaging, or both, in addition to selective angiography. Tables 1 and 2 show the clinical status of the patients.
TABLE 1:
Patients, pathology, and results
| Patient/Age (y)/Sex | Pathology | Glue (mL) | Punctures | Embolization | Complications |
|---|---|---|---|---|---|
| 1/52/M | Glomus vagale | 10 | 1 | Complete | No |
| 2/43/F | Glomus vagale | 10 | 1 | Complete | No |
| 3/67/F | Glomus caroticum | 3 | 1 | Complete | No |
| 4/33/M | Glomus caroticum | 13 | 2 | Complete | No |
| 5/47/F | Glomus caroticum | 4.3 | 3 | Complete | No |
| 6/54/F | Glomus jugulare | 1.3 | 1 | Almost complete | No |
| 7/26/F | Glomus jugulare | 7.2 | 5 | Almost complete | No |
| 8/18/M | Glomus jugulare | 20 | 5 | Almost complete | No |
| 9/23/F | Glomus jugulare | 4 | 2 | Almost complete | No |
TABLE 2:
Clinical findings and surgical results
| Patient | Deficit | Procedural Bleeding | RBC Transfusion | Surgical Outcome | Surgical Removal |
|---|---|---|---|---|---|
| 1 | CN X | No | No | No change | Complete |
| 2 | None | No | No | CN X deficit | Complete |
| 3 | None | No | No | No deficit | Complete |
| 4 | None | No | No | No deficit | Complete |
| 5 | None | No | No | No deficit | Complete |
| 6 | CN VIII | Yes | Yes, 1000 mL | CN VII deficit | Complete |
| 7 | CNs VII–X | Yes | Yes, 1000 mL | No change | Complete |
| 8 | CN VII, IX–XII | Yes | Yes, 1250 mL | No change | Partial |
| 9 | CN VIII | Yes | Yes, 1000 mL | No change | Complete |
Note.—CN indicates cranial nerve.
At preoperative treatment, detailed angiography was performed in each patient. The study included selective series of the internal and external carotid arteries and the vertebral arteries (VAs). All patients underwent surgery, which was performed 1 or 2 days after these procedures. The degree of devascularization and the amount of blood loss were determined intraoperatively, and material was collected for histologic examination.
The angioarchitecture of the tumors was angiographically studied, and their arterial supply was found to be similar to that described in the literature. The jugular paragangliomas were supplied by the ascending pharyngeal artery (AsphA). In one case, they were additionally supplied by the occipital artery. In three cases, they were supplied by the inferolateral trunk arising from the C4 portion of the internal carotid artery (ICA). The lesions were also supplied by the VAs in all cases. The carotid-body tumors had supply from branches of the AsphA, and the vagal paragangliomas were supplied by feeding vessels of the AsphA and occipital artery.
Procedural Technique
The embolic agent was a currently available, modified acrylic glue (Glubran 2; GEM Srl, Viareggio, Italy). The chemical structure of this liquid adhesive was similar to that of N-butyl-2-cyanoacrylate (Histoacryl; Braun, Aesculap AG, Tuttingen, Germany), but it also contains a monomer additive called MS (GEM Srl). This monomer decreases exothermic reaction during polymerization, reducing the temperature to 45°C, and slightly extending the polymerization time (24). The longer carboxylic side chain of the minor cyanoacrylate reduces the cytotoxity of this adhesive material. This feature may theoretically reduce adverse reaction sometimes seen after the injection of N-butyl-2-cyanoacrylate.
All procedures were performed with general anesthesia to provide absolute immobility of the patient and to eliminate the pain of the puncture or the glue injection. Multiple projections were acquired to obtain optimal views to control the potential reflux of the glue during injection. A 5F diagnostic catheter was placed in the common carotid artery to guide the puncture and to perform control angiography during and after the injection of glue. The anticoagulation protocol consisted of an intravenous bolus of 3000 IU of heparin at the time of femoral puncture. Each puncture was performed under high-quality biplanar roadmapping (Integris Allura; Philips Medical Systems, Best, the Netherlands) by using the previously chosen working projection. For puncture, a 20- or 22-gauge spinal needle (Terumo Corporation, Tokyo, Japan) was used. After puncture, the needle position was considered correct when blood reflux was slow but continuous. Parenchymography was performed; we assessed arterial reflux, venous drainage, potential extravasation, and the vascular compartment of the tumor to be filled with glue.
After a glucose flush was administered under a blank roadmap, we injected the glue (Glubran 2; GEM Srl) diluted 1:5 with lipiodol. The number of punctures varied from 1 to 5, and the quantity of glue injected varied from 1.3 to 20 mL (Table 1).
The procedure was stopped when complete devascularization was achieved as determined by nonvisualization of intratumoral flow or when the risk of potential arterial reflux to the intracranial circulation was considered high. After a final control angiogram was obtained, the needle was removed. Bleeding from the puncture site was easily controlled with manual compression and bandages. No profuse bleeding at the puncture site was observed.
In one extensive jugular glomus tumor, the additional supply by a feeder of the VA required an adjunctive technique. In addition to the diagnostic catheter positioned in the common carotid artery, a 6F guiding catheter was placed in the VA, and a balloon catheter (Endeavor, Boston Scientific/Target, Fremont, CA) was introduced and positioned at the origin of the feeder. This allowed us to control the feeder by inflating the balloon each time the glue migrated toward the VA. In this case, embolization by direct puncture was achieved by using additional transarterial embolization with 150–250 μm polyvinyl alcohol particles (Boston Scientific/Target). Because of repeated occlusion of the VA, full anticoagulation with a 5000-IU bolus of heparin, followed by 3000 IU per hour was necessary. Each procedure was followed by immediate postembolization CT scanning.
Results
As shown by angiography, complete devascularization was achieved in all five cervical glomus tumors, two vagal glomus tumors (Fig 1), and three carotid-body tumors (Fig 2).
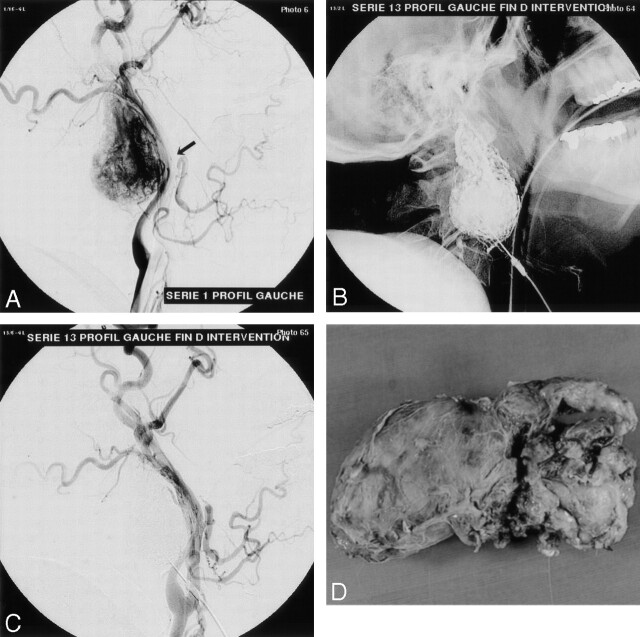
Fig 1.
Vagal glomus tumor.
A, Left common carotid arteriogram, lateral view, shows the highly vascularized tumor displacing the ICA posteriorly (arrow).
B, Nonsubtracted view shows the glue cast after embolization.
C, Control arteriogram demonstrates complete devascularization of the vascular tumor bed at the end of the procedure.
D, Macroscopic specimen of the tumor after en bloc removal.
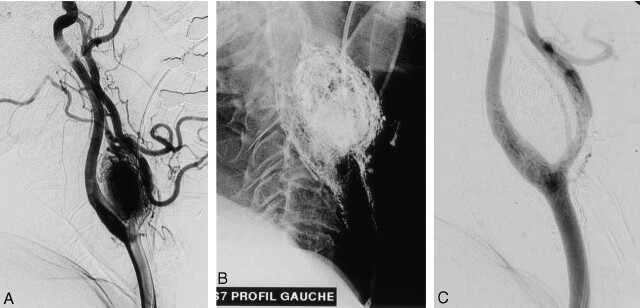
Fig 2.
Carotid-body glomus tumor.
A, Left common carotid arteriogram, lateral view, shows significant pathologic vascularization and associated mass effect of the tumor enlarging the carotid bifurcation.
B, Cast after glue injection.
C, Postembolization arteriogram demonstrates complete devascularization at the end of the procedure.
In the four jugular glomus tumors at the skull base, subtotal devascularization was achieved (Fig 3). Complete devascularization was not possible in this location because of the proximity of the ICA and VA branches and because the associated high risk of arterial reflux into normal territory. In one case of an extensive jugular glomus tumor, additional balloon protection of the normal vascular territory was necessary. In the same patient, the direct puncture technique was completed by means of transarterial particle embolization. In this patient, who presented with multiple head and neck paragangliomas (including bilateral vagal glomus and carotid-body tumors), the surgeon elected to embolize only the extensive jugular glomus tumor at the skull base.
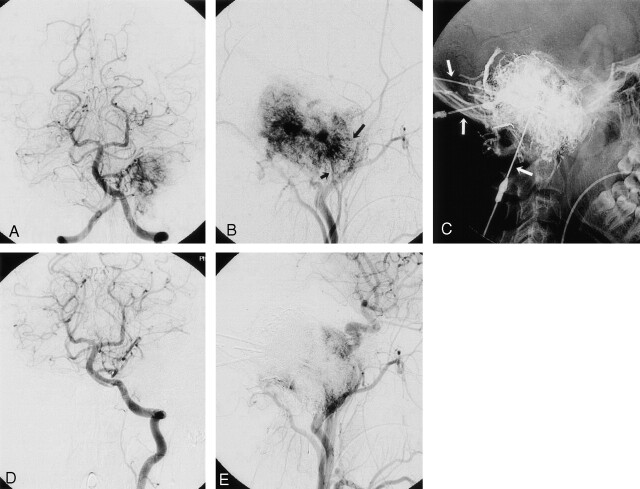
Fig 3.
Jugular glomus tumor.
A, Left vertebral arteriogram, anteroposterior view, shows tumor blush in the left posterior fossa. The tumor is supplied by the feeders of the left posterior inferior cerebellar artery.
B, Left external carotid arteriogram, lateral view, shows tumor vascularization from the posterior branch of the AsphA (short arrow) and the posterior auricular artery (long arrow).
C, Glue cast after several punctures (arrows) and glue injections.
D and E, Final control angiogram of the vertebrobasilar system and the left common carotid artery demonstrates complete devascularization of the tumor bed previously supplied by posterior inferior cerebellar artery feeders and subtotal devascularization of the ECA territory.
Subsequent surgical resections were easily performed in cervical glomus tumors, which were completely embolized. Tumors were removed en bloc; the glue cast delineating precisely their boundaries with the surrounding tissue, facilitating their removal (Fig 1). No blood transfusions were necessary. Despite subtotal devascularization achieved in the jugular paragangliomas, moderate intraoperative bleeding was observed. The average intraoperative bleeding required 4.5 red blood cell units of 250 mL each. Of the nine excised glomus tumors, all but one (a jugular paraganglioma) was completely removed (Table 2).
In this series, no unwanted glue migration via direct reflux or intracranial-extracranial anastomoses was observed. No parent-vessel occlusion occurred, and no clinical complication or adverse effects were noted in the immediate postembolization period. The postoperative clinical status was uneventful in patients with carotid-body tumors. One patient presenting with vagal paragangliomas developed a postoperative deficit of the vagal nerve. One patient with jugular paragangliomas developed additional facial nerve palsy (Table 2).
Discussion
The value of preoperative embolization of hypervascularized tumors such as paragangliomas is well established in the literature (12–18). By reducing intraoperative bleeding, it shortens the time of surgery and thus decreases morbidity and mortality. The traditional technique for preoperative devascularization performed by most interventional radiologists is superselective catheterization of the supplying branches and transarterial embolization with particulate agents; this can lead to satisfactory results in many cases. However, because of the complex angioarchitecture with multiple, small feeding branches, involvement of branches arising from the ICA and VA, and possible vasospasm, complete devascularization of the tumor bed is frequently not achieved.
Direct puncture and direct intratumoral injection of the embolic agent was initially described as an alternative method for cases in which conventional transarterial embolization was technically impossible or posed associated risks that were considered too high (19–21). The intralesional injection of a liquid adhesive such as N-butyl-2-cyanoacrylate proved reliable and permitted higher degrees of devascularization compared with transarterial particulate embolization. Although these promising results have widened the application of the technique to less complex cases in which either method could be used, few reports were published to further establish this method (22, 23). Our results underline the usefulness of this technique as a feasible, safe, and effective method for the preoperative devascularization of paragangliomas. Even in tumors in which traditional transarterial techniques can be successfully applied (eg, vagal or carotid-body glomus tumors), direct puncture with the intralesional injection of liquid adhesives can be considered superior regarding the degree of devascularization achieved. Therefore, this method may play an increasing role in the preoperative treatment of highly vascularized tumors in various locations.
Easier access to the vascular tumor bed, which is not limited by arterial tortuosities, atherosclerotic disease, or induced vasospasm, is one of the main advantages of this technique. However, this does not fully overcome the complex vascular anatomy and the multiplicity of the feeders often seen in these tumors. Feeding vessels arising from the ICAs or VAs still represent a technical challenge and can potentially cause the reflux of glue into the intracranial circulation. Casasco et al (23) pointed this out. Using this technique in two cases, they observed glue migration into the normal territory that resulted in serious clinical impairments. In one of their cases, reflux occurred through collaterals, occluding the ophthalmic artery. In another case, reflux through the ICA lead to occlusion of the middle cerebral artery. Therefore, the authors suggest protecting the intracranial branches during the injection of glue by using nondetachable balloons. Although these are helpful tools, we believe balloon protection is not needed in all cases and should be reserved for complex lesions.
The development of high-quality, biplanar fluoroscopy has markedly improved visualization of liquid adhesives during their injection, and it has increased the safety and efficacy of direct percutaneous techniques. Nevertheless, supplying feeders arising from the intracranial circulation and potentially dangerous external carotid artery (ECA)/ICA and ECA/VA anastomoses should be considered to prevent adverse events due to unwanted glue migration during intralesional injection.
We believe that the low concentration of glue (20%) that we used allowed homogeneous intratumoral penetration of the embolic agent, and under continuous fluoroscopic control, it appears to be safe. The injection should be performed slowly to permit as much glue as possible to penetrate the vascular tumor bed while avoiding its passage to the venous side or its reflux into normal arterial territory. A large-caliber puncture needle and a low concentration of glue allow for prolonged injection of relatively large quantities of glue. During the glue injection, retrograde filling into the arterial tumoral feeders might be observed, or the glue may fill the venous outflow in antegrade fashion. This particularly occurs at the end of the procedure when the tumor vascular bed is already filled from previous glue injections.
Surgery of a glomus tumor after an intralesional injection of glue appears to differ from that after transarterial embolization. Resection is facilitated because intraoperative bleeding is decreased and also because the tumor is transformed into a rigid avascular mass that is well delineated against the surrounding normal tissue. These advantages allow en bloc removal of the tumor, particularly those in the cervical region.
When intralesional penetration with liquid adhesives appears too risky or technically impossible, complementary transarterial embolization with particulate agents can be used (22). In this series, we observed no adverse events related to the injection of glue, including unwanted glue migration, parent-vessel occlusion, and clinical complications or adverse effects. Although good angiographic results were also achieved in patients with jugular paragangliomas, intraoperative bleeding still occurs, although less frequently and less profusely. Even if subtotally embolized, these tumors are technically challenging to treat surgically because of their location at the skull base.
Conclusion
Direct puncture of head and neck paragangliomas with the intralesional injection of acrylic glue is a feasible, safe, and effective technique for achieving presurgical devascularization. It is not limited by the number of arterial feeders, their origins, or technical difficulties in endovascular navigation such as that caused by tortuous anatomy or vasospasm. However, this method is limited in cases involving complex angioarchitecture with feeding branches arising from the ICAs or VAs, especially in extended skull-base tumors and extracranial–intracranial anastomoses. The preliminary results of this small series regarding devascularization, surgical removal, and clinical outcomes encourage us to use this technique as a routine method in managing these challenging lesions.
References
- 1.Erickson D, Kudva YC, Ebersold MJ, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 2001;86:5210–5216 [DOI] [PubMed] [Google Scholar]
- 2.Bishop GB Jr, Urist MM, el Gammal T, et al. Paragangliomas of the neck. Arch Surg 1992;127:1441–1445 [DOI] [PubMed] [Google Scholar]
- 3.Van Den Berg G, Rodesch G, Lasjaunias P. Management of paragangliomas: clinical and angiographic Aspects. Intervent Neuroradiol 2002;8:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somasundar P, Krouse R, Hostetter R, et al. Paragangliomas: a decade of clinical experience. J Surg Oncol 2000;74:286–290 [DOI] [PubMed] [Google Scholar]
- 5.Patetsios P, Gable DR, Garrett WV, et al. Management of carotid body paragangliomas and review of a 30-year experience. Ann Vasc Surg 2002;16:331–338 [DOI] [PubMed] [Google Scholar]
- 6.Muhm M, Polterauer P, Gstottner W, et al. Diagnostic and therapeutic approaches to carotid body tumors. Review of 24 patients. Arch Surg 1997;132:279–284 [DOI] [PubMed] [Google Scholar]
- 7.Marchesi M, Biffoni M, Nobili-Benedetti R, et al. Surgical treatment of paragangliomas of the neck. Int Surg 1997;82:394–397 [PubMed] [Google Scholar]
- 8.Leonetti JP, Donzelli JJ, Littooy FN, et al. Perioperative strategies in the management of carotid body tumors. Otolaryngol Head Neck Surg 1997;117:111–115 [DOI] [PubMed] [Google Scholar]
- 9.Green JD Jr, Brackmann DE, Nguyen CD, et al. Surgical management of previously untreated glomus jugulare tumors. Laryngoscope 1994;104:917–921 [DOI] [PubMed] [Google Scholar]
- 10.Brown JS. Glomus jugulare tumors revisited: a ten-year statistical follow-up of 231 cases. Laryngoscope 1985;95:284–288 [DOI] [PubMed] [Google Scholar]
- 11.Sniezek JC, Sabri AN, Netterville JL. Paraganglioma surgery: complications and treatment. Otolaryngol Clin North Am 2001;34:993–1006 [DOI] [PubMed] [Google Scholar]
- 12.Persky MS, Setton A, Niimi Y, et al. Combined endovascular and surgical treatment of head and neck paragangliomas–a team approach. Head Neck 2002;24:423–431 [DOI] [PubMed] [Google Scholar]
- 13.Gruber A, Bavinzski G, Killer M, et al. Preoperative embolization of hypervascular skull base tumors. Min Invasive Neurosurg 2000;43:62–71 [DOI] [PubMed] [Google Scholar]
- 14.Gheyi VK, Dross P, Flores C, et al. Endovascular therapy in the treatment of head and neck lesions. Del Med J 2000;72:391–395 [PubMed] [Google Scholar]
- 15.Tikkakoski T, Luotonen J, Leinonen S, et al. Preoperative embolization in the management of neck paragangliomas. Laryngoscope 1997;107:821—826 [DOI] [PubMed] [Google Scholar]
- 16.Murphy TP, Brackmann DE. Effects of preoperative embolization on glomus jugulare tumors. Laryngoscope 1989;99:1244–1247 [DOI] [PubMed] [Google Scholar]
- 17.Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol 1986;7:943–952 [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Li B, Zhou D. Preoperative embolization of paraganglioma in head and neck. Zhonghua Wai Ke Za Zhi 1995;33:675–676 [PubMed] [Google Scholar]
- 19.Pierot L, Boulin A, Castaings L, et al. Embolization by direct puncture of hypervascularized ORL tumors. Ann Otolaryngol Chir Cervicofac 1994;111:403–409 [PubMed] [Google Scholar]
- 20.George B, Casasco A, Deffrennes D, et al. Intratumoral embolization of intracranial and extracranial tumors: technical note. Neurosurgery 1994;35:771–773; discussion 773–774 [DOI] [PubMed] [Google Scholar]
- 21.Casasco A, Herbreteau D, Houdart E, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. AJNR Am J Neuroradiol 1994;15:1233–1239 [PMC free article] [PubMed] [Google Scholar]
- 22.Chaloupka JC, Mangla S, Huddle DC, et al. Evolving experience with direct puncture therapeutic embolization for adjunctive and palliative management of head and neck hypervascular neoplasms. Laryngoscope 1999;109:1864–1872 [DOI] [PubMed] [Google Scholar]
- 23.Casasco A, Houdart E, Biondi A, et al. Major Complications of Percutaneous Embolization of Skull-Base Tumors. AJNR Am J Neuroradiol 1999;20:179–181 [PubMed] [Google Scholar]
- 24.Leonardi M, Barbara C, Simmonetti L, et al. Glubran 2: a new acrylic glue for neuroradiological endovascular use. Intervent Neuroradiol 2002;8:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]