Abstract
BACKGROUND AND PURPOSE: Children with medulloblastoma demonstrate post-treatment neurocognitive deficits in a number of areas, including memory performance. However, there is no definitive understanding of the neuropathology underlying these functional deficits. Previous literature has reported that hippocampal integrity is crucial to the acquisition of new episodic memories. Therefore, we hypothesized that longitudinal hippocampal volume measurements are abnormal in patients with medulloblastoma and thereby provide a possible substrate for explaining memory dysfunction.
METHODS: Twenty-five pediatric patients underwent 159 serial MR imaging examinations (mean = six examinations per patient) for up to 5 years after irradiation and chemotherapy treatment for medulloblastoma. Right and left hippocampal volumes were obtained by manually tracing 1.5-mm contiguous coronal sections through the structure. Random coefficient models were used to examine longitudinal change in hippocampal volume as a function of time after diagnosis.
RESULTS: Both right and left hippocampal volumes initially decreased after treatment. This abnormal volume pattern continued until approximately 2–3 years after diagnosis, when hippocampal volumes returned toward a normal positive growth pattern. Volume loss occurred predominately in the posterior regions. Female sex, low parental education, shunt placement, and positive seizure history all had a significant negative impact on hippocampal volume.
CONCLUSION: Pediatric medulloblastoma survivors demonstrate an abnormal pattern of hippocampal volume development after treatment. Radiation dose mapping may expand our understanding of region-specific changes in hippocampal volume. Further exploration of the relationships between radiation therapy, memory dysfunction, and hippocampal pathology in this population is warranted.
Medulloblastoma is the most common malignant brain tumor of childhood (1). Contemporary medical treatments strive to be efficacious and also to minimize subsequent neurocognitive impairments. Despite these efforts, patients with medulloblastoma continue to demonstrate post-treatment deficits in a variety of areas, including overall intellectual development and memory functioning (2–5) for which there is no known effective treatment. Studies have also demonstrated that cranial irradiation dose plays a critical role in moderating the severity of cognitive impairments (3, 6, 7).
Advances in neuroimaging techniques have enabled researchers to demonstrate a number of post-treatment brain changes, such as decreases in normal-appearing white matter (8, 9) and declines in corpus callosum volume among children with medulloblastoma (10). While most of these white matter abnormalities have been correlated with, and may partially account for, impairments in overall intellectual functioning in these patients (9, 11), none of these brain changes have been found to be specifically related to memory performance (12). Therefore, there is no definitive understanding of the neuropathology underlying memory deficits in children with medulloblastoma.
Hippocampal integrity plays a crucial role in intact memory functioning (13–15). Research has demonstrated that normal hippocampal development continues into early adulthood (16, 17). Investigators specifically examining the development of the hippocampus have suggested that the hippocampal formation undergoes a steep increase in volume until approximately the second year of life, then the volume slowly increases throughout adolescence (18–20). Findings from animal models have suggested that irradiation causes apoptosis and decreased neurogenesis in the hippocampus (21, 22) and that these pathologic processes limit performance on hippocampal-dependent behavioral tasks (23).
In that medulloblastoma patients undergo craniospinal irradiation (CSI) and studies have demonstrated deficits in memory functioning (3, 5), it is possible that hippocampal pathology (including failure to mature at a normally expected rate) contributes to memory impairments. The current study was designed to examine longitudinal patterns of hippocampal volume as a potential underlying neural substrate of the impaired memory function so often found in children with medulloblastoma.
Methods
Patient Characteristics
Patients (n = 33) with histologically proved medulloblastoma diagnosed before 17 years of age were considered for this study. The patients were consecutively treated between October 1996 and February 2000 and enrolled in a single institutional protocol. This study was part of a larger project examining memory performance. Patients were required to have progression-free survival of 2 or more years and also two or more psychological evaluations with the California Verbal Learning Test for Children (24). These criteria resulted in the exclusion of eight patients (one blind, one non-English speaking, six too young for testing or undergoing only one examination). The appropriate institutional review board reviewed and approved this project.
Mean patient age at the time of diagnosis was 8.27 years (standard deviation [SD] = 2.66, range = 4.78–12.98 years). The Table provides additional patient characteristics. Given the location of the tumor bed, children with medulloblastoma often have hydrocephalus that may or may not require shunt placement for resolution. In the current study, 16 of 25 patients required shunt placement. All patients underwent maximum surgical resection of the primary tumor followed by histologic examination to confirm the diagnosis of medulloblastoma. Those considered high-risk patients (those with residual tumor or metastatic disease) received one or two courses of topotecan therapy before irradiation. All patients received risk-adapted CSI. High-risk patients received 36–39.6 Gy, while average-risk patients (those with total tumor resection and no metastasis) received a reduced dose of 23.4 Gy. In addition, all patients received a radiation boost to the tumor bed, for a total dose of 55.8 Gy in that region. The posterior fossa boost was administered by means of individually conformed radiation beams (primarily to avoid damage to cochlear regions). Six weeks after the completion of radiation therapy, all patients received adjuvant chemotherapy consisting of four cycles of high-dose cyclophosphamide, cisplatin, and vincristine. All patients were closely monitored for neuroendocrine problems and received replacement therapy as needed.
Patient demographic and clinical characteristics
| Characteristic | Value |
|---|---|
| Sex (male:female) | 16:9 |
| Handedness (R:L) | 16:9 |
| Risk Group | |
| Average | 16 |
| High | 9 |
| Race | |
| Caucasian | 15 |
| African American | 7 |
| Latino | 2 |
| Asian | 1 |
| Shunted hydrocephalus (yes:no) | 9:16 |
| Dexamethasone use (yes:no) | 20:5 |
| Seizure history (yes:no) | 4:21 |
| Posterior fossa syndrome | 18:7 |
| Parental education (y)* | 14.8 ± 2.80 (8.00–20.00) |
| Age at diagnosis (y)* | 8.27 ± 2.66 (4.78–12.98) |
| Time since diagnosis (y)* | |
| To first MR study | 0.31 ± 0.24 (0.03–0.94) |
| To all MR studies | 1.82 ± 1.26 (0.03–4.88) |
Data are the mean ± SD (range).
MR Imaging Acquisition and Analysis
Each eligible patient received protocol-driven serial MR imaging. Mean elapsed time between diagnosis and the initial examination was 0.31 years (SD = 0.24, range = 0.03–0.94 years). Baseline MR images were obtained before radiation therapy in nine of the 25 patients. The mean time between first MR imaging examination and the initiation of radiation therapy for these nine patients was 26.22 days (SD = 25.14, range = 1.00–75.00 days); however, six patients underwent baseline MR imaging within 3 weeks before beginning treatment. These examinations were processed at an average interval of 0.56 years (SD = 0.32), resulting in 159 examinations for 25 patients (mean = 6.36 examinations per patient, SD = 1.93). The examinations were performed by using a 1.5-T whole-body unit (Magnetom; Siemens Medical Systems, Iselin, NJ) with standard circular polarized volume head coils. Volumetric studies were performed by using a radiofrequency-spoiled, fast low-angle shot, 3D sequence (TR/TE = 9.7/4, flip angle = 15°, field of view = 210 mm, acquisition time = 7 minutes 28 seconds, sagittal acquisition, 128 sections, 1.25-mm thickness).
From each volumetric MR imaging examination, coronal multiplanar reconstruction images were constructed perpendicular to the anterior commissure–posterior commissure plane. Sections were constructed with section intervals of 1.5 mm. Manual tracings of the right and left hippocampi were performed by the primary author (B.J.N.), who was blinded to the patients’ psychometric data. All tracings were performed in Photoshop (Adobe Systems, San Jose, CA) by using a pixel-count histogram. Right and left hippocampal volumes were calculated by multiplying pixel counts by known associated pixel volumes.
Boundaries of the Hippocampus
The criteria used for defining boundaries of the hippocampus were comparable to those used in previous hippocampal volumetry research (18, 25). Because of the difficulty in visually distinguishing the anterior hippocampus from the amygdala, a more stereotaxic approach was taken to define the anterior extent of the hippocampus. The most anterior section of the hippocampus was chosen by the coronal section that cut through the most medial portion of the mammillary bodies (as viewed in sagittal orientation). The anterior border of the hippocampus emerged beneath the amygdala and was demarcated by a horizontal line from the anterior recess of the temporal horn to the alveus. The ventral boundary was delineated by white matter of the parahippocampal gyrus. At the level of the hippocampal body, the alveus and the floor of the temporal horn outlined the dorsal and lateral boundaries. Medially, the hippocampal boundaries were defined by the ambient cistern (above the hippocampal sulcus) and an arbitrary line to the most medial portion of the subiculum (below the hippocampal sulcus). Posteriorly, the boundary was the last section before the crus fornix visualized in it its entirety and where the pulvinar was still visible.
Validation and Reliability
Initial intrarater reliability was established by tracing five right and five left hippocampi, five times each (50 total tracings). The intraclass correlation coefficient was 0.98 for the right hippocampus and 0.99 for the left. Intrarater reliability was reassessed 5 months into the data collection process to account for possible drift in rater accuracy. At that time, reliability was maintained, with an intraclass correlation coefficient of 0.98 for the right hippocampus and 0.97 for the left. In addition to intrarater reliability, interrater reliability was established with a trained neuroradiologist (K.J.H). Effective reliability, or the aggregate reliability of both judges (26), was calculated for 20 randomly selected right and left hippocampal sections and found to be 0.99 for the right hippocampus and 0.98 for the left.
Statistical Analyses
The random coefficient model was used to assess changes in hippocampal volume over time after diagnosis, with each patient serving as his or her own control. (No separate control group was used.) This statistical model was a particular variant of a random-effects model. Random coefficient model analysis considers each subject’s slope and within-patient variability to estimate the average rate of change after the completion of treatment within the total group, as well as the differences in slopes between subgroups (ie, high- and low-risk treatment groups). Thus, each subject has his or her own longitudinal trend represented by individual intercept (baseline) and slope (rate of change). The means of these individual intercepts and the slopes are the intercept and the slope of a regression line that represents the longitudinal trend of the entire sample.
Random coefficient model analysis was also used to examine the volumes of anterior hippocampal regions versus posterior hippocampal regions. This analysis was used to detect region-specific hippocampal changes to provide a more detailed examination of potential abnormalities in volume.
In addition, random coefficient model analysis was performed to examine changes in CSF volume over time since diagnosis. Methods for segmentation of CSF volume are described in previously published work (8) but were expanded to assess a 3-cm section centered at the level of the basal ganglia. CSF volume was quantified to assess the degree of diffuse cerebral atrophy over time. Quantitative CSF volumes have been shown to be significantly correlated with radiologists’ grading of cerebral atrophy (8).
Analysis of Other Variables
Longitudinal trends in hippocampal volume over time since diagnosis were examined with the covariates of sex, handedness, study risk group (CSI dose), shunt placement, steroid administration, history of seizures, and diagnosis of posterior fossa syndrome (a constellation of symptoms including resolving mutism and ataxia) as individual parameters of this function. Shunt placement served as a marker of severe hydrocephalus, a variable shown to be associated with hippocampal pathology in animal models (27). The continuous variables of parental education (parents’ mean number of years of education) and patient age at diagnosis were evaluated for their potential influence on the growth trend of hippocampal volume by including them separately into the mixed model. Parental education was selected as an estimate of patients’ socioeconomic status (28). All medical data were gathered by means of retrospective chart review.
Results
The Table provides information about the patients’ demographic and clinical variables.
Hippocampal Volume
Mean initial right hippocampal volume was 2.5458 cm3 (±0.26), and mean initial left hippocampal volume was 2.5913 cm3 (±0.27). Although this study did not have a control group for direct comparison, previous research (18) examining hippocampal development in healthy children yielded hippocampal volumes within 2.2–3.0 cm3 of those for the age range in the present study.
Time since Diagnosis
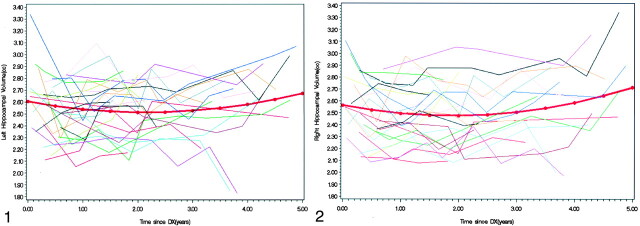
A significant quadratic function of time since diagnosis was fit for both right and left hippocampal volumes (P < .0001). The predictive model demonstrated an initial decline in volume until approximately 2 years following diagnosis. This abnormal volumetric pattern was followed by a gradual return to a normal positive growth pattern (Figs 1 and 2). The predictive models were as follows: Left hippocampal volume = 2.6037 − 0.0846t + 0.0197t2. Right hippocampal volume = 2.5643 − 0.0928t + 0.0246 t2, where t is time.
Fig 1.
Individual patients’ and predicted left hippocampal volumes over time since diagnosis (DX).
Fig 2.
Individual patients’ and predicted right hippocampal volumes over time since diagnosis (DX).
Sex
The inclusion of patient sex as a covariate in the model yielded a significant quadratic function of time since diagnosis (P < .0001). Baseline hippocampal volumes did not significantly differ between boys and girls. Both boys and girls had an initial decline in right and left hippocampal volume, with an increase in volume approximately 3 years after diagnosis. The linear term of the predictive model showed that girls had a steeper slope of decline in volume for both right (P < .021) and left (P = .0086) hippocampal volumes than boys. The predictive models were as follows: Left hippocampal volume (female) = 2.5971 − 0.1094t + 0.0171t2. Left hippocampal volume (male) = 2.5971 − 0.0548t + 0.0171t2. Right hippocampal volume (female) = 2.5579 − 0.1143t + .0223t2. Right hippocampal volume (male) = 2.5579 − 0.0664t + 0.0223t2, where t is time.
Parental Education
By including parental education as a covariate of the model, a quadratic function of time since diagnosis was fit (P < .0001). This indicated that increasing levels of education were positively related to higher baseline values for right and left hippocampus (P = .044 and P = .026). Among patients whose parents achieved higher levels of education (ie, > 15 years), right and left hippocampal volumes declined for a period of approximately 2 years after treatment. However, this decline was followed by an increase comparable to the normal developmental pattern. Among patients whose parents attained fewer years of education (ie, <15 years), right and left hippocampal volumes initially declined and did not recover to a positive growth pattern. The predictive models were as follows: Left hippocampal volume = 2.0127 × parental education + (0.5041 − 0.0373 × parental education)t + (−0.1257 + 0.0093 × parental education)t2. Right hippocampal volume = 1.9802 + (0.0378 × parental education) + (0.4109 − 0.0323 × parental education)t + (−0.0834 + 0.0069 × parental education)t2, where t is time.
Shunt Placement
When shunt placement was included as a covariate of the model, it yielded a significant quadratic fit for time since diagnosis (P < .0001). Baseline right and left hippocampal volumes were not significantly different among patients who had a shunt and those who did not. The fitted model had a linear term (P = .0435) and a quadratic term (P = .0009) for the left hippocampal volume. However, the linear and quadratic terms of the model were not significant for right hippocampal volume. The analysis predicted that, in hydrocephalic children with shunts, left hippocampal volume significantly declined and was followed by an increasing pattern of growth at approximately 2 years after diagnosis. In children without shunts, left hippocampal volume demonstrated a plateau over time after diagnosis, with no significant increase or decrease. The predictive models were as follows: Left hippocampal volume (no shunt) = 2.5914 − 0.0229t + 0.0036t2. Left hippocampal volume (shunt) = 2.5914 − 0.1433t + 0.0336t2, where t is time.
Seizure History
By including the seizure history as a covariate of the model, a significant quadratic function of time since diagnosis was fit (P < .0001). The model indicated that baseline right and left hippocampal volumes were significantly different among patients with a history of seizure and those without this history (P = .016 and .049). In patients without seizures, baseline hippocampal volume was significantly greater than that of patients with seizures. Patients with seizure history were predicted to have an initial rise in left and right hippocampal volume until 2 years after diagnosis then a decline. Patients without seizures demonstrated an initial decline in right and left hippocampal volume until 2 years after diagnosis, with a return to the normal positive hippocampal growth pattern. The predictive models were as follows: Left hippocampal volume (no seizures) = 2.6146 − 0.0989t + 0.0239t2. Left hippocampal volume (seizures) = 2.3648 + 0.0558t − 0.0177t2. Right hippocampal volume (no seizures) = 2.5579 − 0.1143t + .0223t2. Right hippocampal volume (seizures) = 2.5579 − 0.0664t + 0.0223t2.
Risk Group, Age at Diagnosis, Handedness, Posterior Fossa Syndrome, and Steroid Use
Baseline estimates of hippocampal volume were not affected by risk group (which affected CSI dose), age at diagnosis, handedness, posterior fossa syndrome, or steroid (dexamethasone) administration. No statistically significant models were derived; this outcome suggested that these variables did not have a significant effect on hippocampal volume over time since diagnosis.
Region-Specific Volume Loss
A random coefficient model was used to predict change in hippocampal midline as a function of time since diagnosis. A significant quadratic function was found for hippocampal midline examination and demonstrated a significant initial anterior shift (P < .0001) with a gradual return to baseline midline. This change was consistent with the overall quadratic volume change for both the right and left hippocampus and determined that volume loss occurred predominately in the posterior regions of both sides of the hippocampus.
Estimate of Cerebral Atrophy (CSF Volume)
A random coefficient model was used to predict change in CSF volume as a function of time since diagnosis. A significant quadratic function was found and demonstrated a decline in CSF volume after diagnosis (P < .005), with a gradual leveling off around 4–5 years after diagnosis. This change suggested no evidence of diffuse cerebral atrophy in this population.
Discussion
To our knowledge, the present study is the first of its kind to evaluate patterns of hippocampal volume development among children treated for medulloblastoma with radiation therapy and chemotherapy. The hypothesis that children treated for medulloblastoma have abnormal patterns of hippocampal growth was confirmed. Specifically, without consideration of additional parameters, longitudinal analysis of 159 MR imaging studies in this population revealed that right and left hippocampal volumes significantly declined for the first 2 years after diagnosis and/or treatment and then gradually returned toward a positive growth curve. The combining of baseline data for patients who had received the full extent of their therapy and those who had not potentially confounded baseline hippocampal volumes; however, findings regarding the slope of change appeared robust to potential confounding, as hippocampal volume continued to decline after all patients had completed treatment (2–3 years after diagnosis). In addition, examination of longitudinal CSF volume suggests that hippocampal volume loss cannot be explained by diffuse cerebral atrophy. This pattern of growth is contrary to previous reports suggesting that, in healthy children of similar age, normal hippocampus slowly increases in volume until adolescence (18–20). Despite an eventual return to increasing volume, whether this period of abnormal volume development ultimately results in overall hippocampal volume deficits compared with age-matched values is unclear.
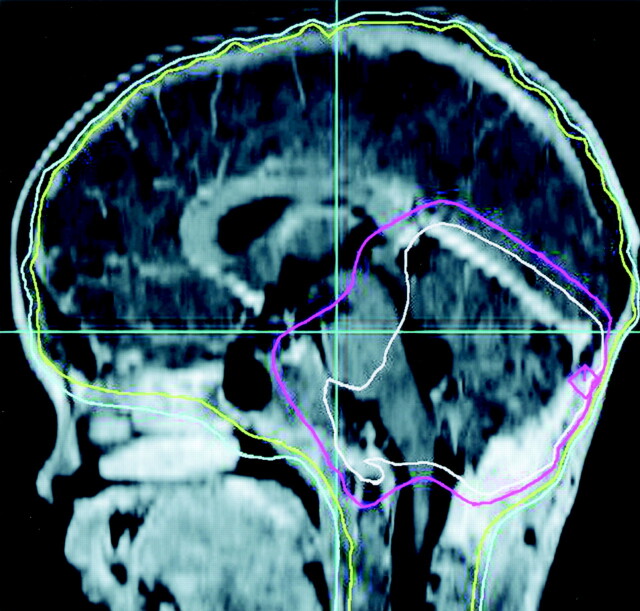
While several hypotheses may be put forth to explain this aberrant pattern of hippocampal growth, it is surprising that total irradiation dose (as tested by risk group) did not significantly affect overall volume change. However, further examination of region-specific volume change demonstrated that right and left volume loss occurred predominately in the posterior hippocampal regions. Examination of radiation dosimetry mapping for a sample case was completed (Fig 3) and showed that posterior hippocampal regions are closer to higher-dose fields of the posterior fossa. This finding suggested that, while cranial radiation dose did not differentially affect total hippocampal volumes, region-specific increases in cumulative tissue-dose exposure may explain volume loss. Further examination of dosimetry mapping and specific tissue-dose exposure is necessary to draw firm conclusions.
Fig 3.
T1-weighted MR image represents the midsagittal orientation of a dosimetry map for a high-risk patient. Small dose-map area (white outlined posterior fossa area) receives the highest total CSI dose (up to 60Gy).
If differential posterior volume loss is due to increased tissue-dose exposure, radiation is likely an agent for abnormal hippocampal volume patterns in this population. As Abayomi (29) suggested, radiation-induced vascular compromise that results in hypoxic-ischemic injury to the hippocampus may explain the decline in hippocampal volume. However, more recent research in animal models, has more specifically demonstrated that therapeutic doses of cranial radiation lead to greater apoptosis, yielding a decrease in cell proliferation and a decrease or delay in neural stem cell or precursor cell proliferation in hippocampal regions (30). Results of the current study suggest that, in pediatric medulloblastoma patients treated with radiation, hippocampal growth returns approximately 2–3 years after diagnosis. Given this, we speculate that the ability of neural stem cells or precursor cells to effectively produce hippocampal neurons is disrupted rather than destroyed.
In the present study, several additional variables were found to be associated with abnormal hippocampal growth patterns over time since diagnosis. One of these parameters was parental education. Hippocampal volume increased more among children whose parents achieved higher levels of education than in children whose parents completed fewer years of schooling. Although many hypotheses could explain these findings, one of the most striking is that greater levels of parental education may imply a more enriched environment for the patient. Studies have demonstrated that an enriched environment may lead to the preservation or formation of increased dendritic spines in the hippocampus (31). In addition, it may act as a neuroprotective mechanism against excitotoxic injury and reduce spontaneous apoptosis in animal models (32). One study in twins highlighted the genetic heritability of gray matter and white matter volumes, as well as the significant relationships between these brain volumes and general intelligence (33). These findings may also help to explain the role of parental education on hippocampal growth. If greater parental education implies a more enriched environment for the patient, this study provides further replication of the positive effect of an enriched environment on hippocampal development. This finding may have specific implications in terms of rehabilitation and remediation for patients, both in the acute phase of neurologic compromise and also in long-term recovery.
The present results also suggest that girls are more susceptible to the negative effects of radiation therapy on hippocampal volume than boys. This finding is consistent with that of previous studies in which female sex was identified as a significant risk factor for CNS toxicity resulting from radiation and chemotherapy for treatment of malignant brain tumors (34) and acute lymphoblastic leukemia (35). While known sex differences in neuroendocrinology (eg, hormonal and receptor differences) may explain these findings, research to substantiate this hypothesis has not be done, to our knowledge. It is also possible that the hippocampi of girls might have been exposed to higher radiation dose fields, as girls’ brains are generally smaller than boys’ (20). Again, examination of individual dosimetry mapping and specific tissue-dose exposure may be helpful in explaining this finding. Girls demonstrate a steeper slope of hippocampal volume growth during the first 2 years of life than do age-matched boys (19). This known sex difference in rate of development might also have influenced the steeper rate of hippocampal volume decline observed in the girls in this study.
Our study is consistent with previous studies demonstrating the deleterious effects of seizures on hippocampal volume (36, 37) and its related cognitive functions. Interestingly, medulloblastoma survivors with history of seizure activity did not show the initial decline in hippocampal volume that their counterparts without seizure history demonstrated. Instead, they demonstrated a slight increase in hippocampal volume until about 2 years after diagnosis that is difficult to explain. This was followed by a more expected steady decline with time since diagnosis.
Surprisingly, children with shunted hydrocephalus demonstrated no significant change in hippocampal volume over time. These children may not experience the same hippocampal volume loss as that of patients without shunt surgery. However, they also do not have normal hippocampal growth. During the acute period of volume loss experienced by most patients (within the first 2 years), patients with shunts may be demonstrating the benefits of resolving hydrocephalus, such as the decompression of brain structures including the hippocampus. The opposing forces of volume loss and resolving hydrocephalus may result in less observable initial changes in hippocampal volume. Longstanding neural compromise secondary to hydrocephalus may explain the lack of hippocampal growth 3–5 years past treatment in patients with shunts.
While this study provides important information regarding neuroanatomic changes in pediatric patients treated for medulloblastoma, it is restricted by several methodologic limitations. Perhaps the most salient limitation of this study and one that plagues much of clinical research (particularly in the use of neuroimaging techniques) is small sample size. Although the current study had the benefits of a longitudinal design, the small patient sample directly affected not only the generalizability of the findings but also the types of statistical analyses to which the data are amenable (resulting, for example, in an inability to look at multiple variable interactions). In addition, at the time of the current analyses, not all patients had been followed up for 5 years after diagnosis. Data drop-off at 2–3 years after diagnosis could have produced bias in the nonlinear models. Thus, it is possible that the trend toward an increase in volume at 2–3 years post diagnosis might have been a function of the reduced dataset rather than a true increase in volume. Continued long-term follow-up will serve to confirm the present findings.
Although the previous literature provides an estimate of normal expectations for hippocampal growth based on cross-sectional designs, the current study could have been improved with the inclusion of a healthy matched control group to specifically compare hippocampal volume over time. The inclusion of such a control group would have allowed for more specific comparisons of both absolute volumes and volumetric changes in the hippocampus throughout development while decreasing variability associated with measurement differences and influences by demographic variables (e.g., sex, socioeconomic status).
While the current study demonstrated abnormal patterns of hippocampal volume development in pediatric patients treated for medulloblastoma, it did not provide insight into the specific hippocampal pathology that might have been present. In vivo techniques such as MR spectroscopy may provide more information about the type and degree of hippocampal injury. Future investigators should consider this methodologic advantage.
Despite its methodologic limitations, the current study provides important implications for children with medulloblastoma. The volumetric findings suggest that, in addition to previously demonstrated abnormal white matter processes, medulloblastoma patients treated with radiation therapy have deviant development in hippocampal volume. While previous studies have demonstrated the integral role of hippocampal integrity in the acquisition of new memories (13–15), the specific relationship between hippocampal volume and memory functioning remains controversial, particularly among developing populations. Future studies must be performed to adequately examine the relationship between longitudinal hippocampal volume and memory performance. MR imaging and cognitive assessment can be performed on the same date, and memory assessments can be designed for repeated administration. Given that hippocampal regions (i.e., posterior regions) receiving higher doses of radiation demonstrated the greatest volume loss, there may be important clinical implications for radiation oncology and conformal radiation techniques (e.g., avoiding hippocampal regions) if it can be demonstrated that this degree of volume loss (particularly in posterior hippocampal regions) has significant functional implications for patients with medulloblastoma.
In addition to implications for treatment, the current volumetric findings may play an important role in post-treatment memory functioning and learning abilities. Given that memory and learning impairments have been demonstrated in this population, deviant hippocampal development must still be considered a potential underlying contributor. It should also be highlighted that, in most cases, hippocampal volume development does ultimately resume a more normal course. However, the functional implications of such halted normal development may have a notable effect on age-appropriate academic achievement and learning. This possibility may hold further importance for defining a timeline on which to expect functional improvements as well as for explaining delays in academic achievement.
Conclusion
Pediatric survivors of medulloblastoma demonstrate abnormal patterns of hippocampal volume development after irradiation. Female sex, low parental education, shunt placement, and seizure history further affect these patterns. Volume loss occurred predominately in the posterior regions of the hippocampus. Gross examination of radiation dose mapping may be used to explain the region-specific changes in hippocampal volume, as posterior regions of the hippocampus are in fields receiving higher radiation doses and demonstrate greater deviance from expected normal development. These observations provide a rationale for further investigation of the relationship between memory dysfunction and hippocampal integrity in this population.
Footnotes
Supported in part by the National Cancer Institute through a Cancer Center Support (CORE) grant (P30-CA21765) and by the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children’s Research Hospital, Memphis, TN.
Presented in part at the 31st annual meeting of the International Neuropsychological Society, Honolulu, HI; February, 2003.
References
- 1.Habrand JL, De Crevoisier R. Radiation therapy in the management of childhood brain tumors. Childs Nerv Syst 2001;17:121–133 [DOI] [PubMed] [Google Scholar]
- 2.Dennis M, Hetherington CR, Spiegler BJ. Memory and attention after childhood brain tumors. Med Pediatr Oncol Suppl 1998;1:25–33 [DOI] [PubMed] [Google Scholar]
- 3.Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospinal irradiation doses. Dev Med Child Neurol 2000;42:741–745 [DOI] [PubMed] [Google Scholar]
- 4.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol 2001;19:2302–2308 [DOI] [PubMed] [Google Scholar]
- 5.George AP, Kuehn SM, Vassilyadi M, et al. Cognitive sequelae in children with posterior fossa tumors. Pediatr Neurol 2003;28:42–47 [DOI] [PubMed] [Google Scholar]
- 6.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: A pediatric oncology group study. J Clin Oncol 1998;16:1723–1728 [DOI] [PubMed] [Google Scholar]
- 7.Grill J, Kieffer Renaux V, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys 1999;45:137–145 [DOI] [PubMed] [Google Scholar]
- 8.Reddick WE, Mulhern RK, Elkin TD, Glass JO, Merchant TE, Langston JW. A hybrid neural network analysis of subtle brain volume differences in children surviving brain tumors. Magn Res Imaging 1998;16:413–421 [DOI] [PubMed] [Google Scholar]
- 9.Mulhern RK, Reddick WE, Palmer SL, et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol 1999;46:1–8 [DOI] [PubMed] [Google Scholar]
- 10.Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, Mulhern RK. Decline in corpus callosum volume among pediatric patients with medulloblastoma: longitudinal MR imaging study. AJNR Am J Neuroradiol 2002;23:1088–1094 [PMC free article] [PubMed] [Google Scholar]
- 11.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol 2001;19:472–479 [DOI] [PubMed] [Google Scholar]
- 12.Reddick WE, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer 2003;97:2512–2519 [DOI] [PubMed] [Google Scholar]
- 13.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 1992;99:195–231 [DOI] [PubMed] [Google Scholar]
- 14.Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res 1999;103:123–133 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki WA, Clayton NS. The hippocampus and memory: a comparative and ethological perspective. Curr Opin Neurobiol 2000;10:768–773 [DOI] [PubMed] [Google Scholar]
- 16.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 1994;51:477–484 [DOI] [PubMed] [Google Scholar]
- 17.Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: CA Nelson, M Luciana, eds. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: Bradford;2001. :45–58
- 18.Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol 1999;20:717–723 [PMC free article] [PubMed] [Google Scholar]
- 19.Pfluger T, Weil S, Weis S, et al. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia 1999;40:414–423 [DOI] [PubMed] [Google Scholar]
- 20.Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van England H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry 2001;40:1012–1020 [DOI] [PubMed] [Google Scholar]
- 21.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor cell dysfunction. Nat Med 2002;8:955–962 [DOI] [PubMed] [Google Scholar]
- 22.Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 2000;99:33–41 [DOI] [PubMed] [Google Scholar]
- 23.Hodges H, Katzung N, Sowinski P, et al. Late behavioral and neuropathological effects of local brain irradiation in the rat. Behav Brain Res 1998;91:99–114 [DOI] [PubMed] [Google Scholar]
- 24.Delis DC, Kramer J, Kaplan E, Ober BA. California Verbal Learning Test for Children. New York: The Psychological Corporation;1994
- 25.Szabo CA, Xiong J, Lancaster JL, Rainey L, Fox P. Amygdalar and hippocampal volumetry in control participants: differences regarding handedness. AJNR Am J Neuroradiol 2001;22:1342–1345 [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenthal R, Rosnow RL. Essentials of Behavioral Research: Methods and Data Analysis. 2nd ed. New York: McGraw-Hill;1991. :51–55
- 27.Kriebel RM, McAllister JP. Pathology of the hippocampus in experimental feline infantile hydrocephalus. Neurol Res 2000;22:29–36 [DOI] [PubMed] [Google Scholar]
- 28.Bornstein MH, Hahn CS, Suwalsky JTD, Haynes OM. Socioeconomic status, parenting, and child development: the Hollingshead Four-Factor Index of Social Status and the Socioeconomic Index of Occupations. In: Bornstein MH, Bradley RH, eds. Socioeconomic Status, Parenting, and Child Development: Monographs in Parenting Series. Mahwah, NJ: Lawrence Erlbaum Associates;2003. :29–82
- 29.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 1996;35:659–663 [DOI] [PubMed] [Google Scholar]
- 30.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol 2003;16:129–134 [DOI] [PubMed] [Google Scholar]
- 31.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci 2000;3:238–244 [DOI] [PubMed] [Google Scholar]
- 32.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med 1999;5:448–453 [DOI] [PubMed] [Google Scholar]
- 33.Posthuma D, De Geus EJC, Baare WFC, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nat Neurosci 2002;5:83–84 [DOI] [PubMed] [Google Scholar]
- 34.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J Clin Oncol 2001;19:3470–3476 [DOI] [PubMed] [Google Scholar]
- 35.Waber DP, Urion DK, Tarbell NJ, Niemeyer C, Gelber R, Sallan SE. Late effects of central nervous system treatment of acute lymphoblastic leukemia in childhood are sex-dependent. Dev Med Child Neurol 1990;32:238–248 [DOI] [PubMed] [Google Scholar]
- 36.Briellmann RS, Berkovic SF, Syngeniotis A, King MA, Jackson GD. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Annals Neurol 2002;51:641–644 [DOI] [PubMed] [Google Scholar]
- 37.Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res 2002;135:95–110 [DOI] [PubMed] [Google Scholar]