Abstract
BACKGROUND AND PURPOSE: CT-guided spinal biopsy (CTGSB) is considered a safe and accurate procedure. Our goal was to determine the accuracy of a CTGSB of osseous spinal lesions in patients with known or suspected underlying malignancy in reference to major variables such as the radiographic appearance of the biopsied lesion and its location within the spinal column.
METHODS: We retrospectively reviewed results of 410 consecutive percutaneous CTGSB procedures of osseous spinal lesions. Biopsy was determined to be adequate if diagnostic tissue was obtained (n = 401) or unsatisfactory (n = 9) if only blood without cellular elements was present on final pathologic-cytologic examination.
RESULTS: The level of spinal biopsy was cervical in nine patients (2%), thoracic in 123 (31%), lumbar in 164 (42%), and sacral in 96 (25%). The overall diagnostic accuracy of CTGSB was 89%, with a false-negative rate of 11%. Biopsy of lytic lesions yielded an accurate diagnosis in 93% (220 of 236). Despite technical challenges inherent to biopsy of sclerotic lesions, diagnostic accuracy was 76% (63 of 83), although more importantly, 24% (20 of 83) of the results in sclerotic lesions were falsely negative.
CONCLUSION: CTGSB of osseous spinal lesions is an important tool in the workup of patients with known or suspected underlying neoplastic disease. However, a negative result must be confirmed with either close follow-up or, preferably, open biopsy, especially in cases of sclerotic lesions for which diagnostic accuracy is decreased and the false-negative rate is high.
Since the first descriptions of percutaneous spinal biopsy by Robertson and Ball in 1935 (1) and Valls, Ottolenghi, and Schajowicz in 1948 (2), the addition of CT has allowed safe and accurate biopsy of osseous lesions throughout the spine, obviating invasive open biopsy in most cases (3). The aim of the study was to determine the accuracy of CT-guided spinal biopsy (CTGSB) in patients with known or suspected neoplastic disease with respect to variables such as patient age, sex, radiographic appearance, and lesion location.
Methods
Patient Selection and Preparation
We retrospectively reviewed findings of 410 consecutive CTGSB of osseous lesions of the spine performed in the neuroradiology service at Memorial Sloan-Kettering Cancer Center, New York, NY. The patients had known or suspected neoplastic disease. Eighteen patients were excluded from the study: nine were lost to follow-up and nine had unsatisfactory biopsy samples (i.e., samples yielding only blood). Two biopsy procedures of the same lesion within 4 weeks (22 patients) were counted as one biopsy.
The major indication for a CTGSB at our institution was an indeterminate osseous lesion in the spine, most commonly in a patient with a documented history of cancer and less commonly in a patient with no history of cancer. Most patients were referred from the neurosurgical service or the orthopedic service. Workup consisted of MR imaging of their spine, as well as bone scanning and staging CT scanning of the chest, abdomen, and pelvis. The studies were reviewed by a neuroradiologist (E.L., G.K.), who determined whether a solitary lesion was suitable for CTGSB or which one of multiple lesions was most suitable for biopsy (i.e., the lesion likely to yield diagnostic results while affording a safe approach). Blood workup performed before all procedures included a determination of the patient’s platelet count, prothrombin time, activated partial thromboplastin time, and international normalized ratio. Coagulopathies were corrected before the procedure, often by using fresh frozen plasma or platelets during the procedure, with a platelet count generally preferred to be >100,000 K/mcL. Aspirin was withheld for 1 week before the procedure.
CTGSB Technique
At our institution, all spinal biopsies are done under CT guidance. We have a dedicated CT scanner for biopsy operated by an interventional radiology technologist and equipped with patient-monitoring devices. We prefer CT to fluoroscopy, because many lesions are difficult to identify by using fluoroscopy. CT allows better documentation of the placement of the biopsy needle within the lesion, especially when cytologic or histologic findings are negative. For lesions in which definitive resection will eventually be performed, including resection of the biopsy tract, CT better documents the path of the biopsy needle and the skin entry point for the surgeon.
In this study, the location of the lesion selected for biopsy dictated patient positioning on the CT table. Typically, patient positioning for thoracic, lumbar or sacral biopsy is prone, but positions for cervical spinal biopsy positioning vary as supine, prone, or oblique. To ensure patient comfort and immobilization, biopsy was performed with the patient under conscious sedation with intravenous meperidine hydrochloride (25–100 mg) or fentanyl citrate (50–200 mg) and midazolam hydrochloride (0.5–3 mg). General anesthesia could be used for pediatric patients or patients with intractable pain. All patients were monitored during the procedure with a pulse oximeter, electrocardiographic monitor, and automated blood pressure cuff.
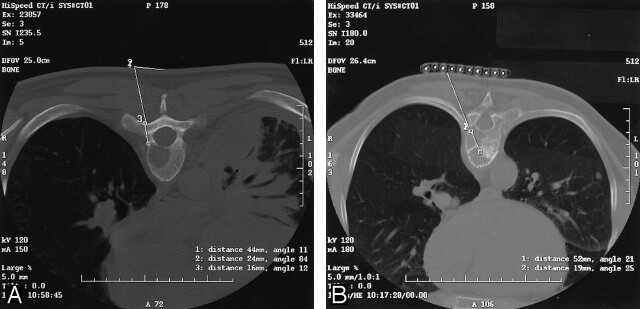
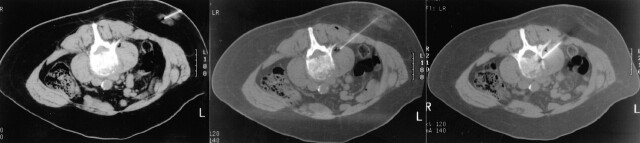
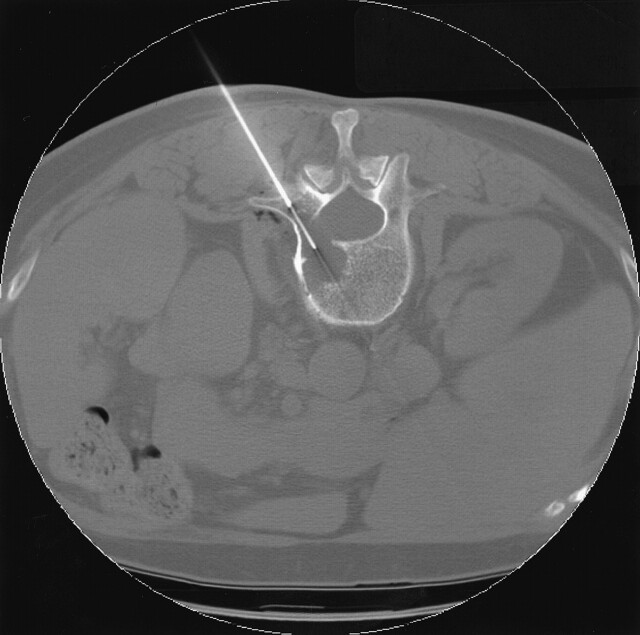
The lesion selected for biopsy was localized by using 2.5–5-mm thick axial images through the vertebral body in question. A single axial CT image depicting the lesion was selected. The best biopsy approach was determined and traced back to the skin by using the cursor on the CT console. The depth of the lesion and cutaneous entry point were determined (Fig 1A). The point of entry was then estimated on the patient’s skin. Alternatively, a radiopaque grid was placed on the patient’s skin to localize the skin point of entry (Fig 1B). The overlying skin and soft tissues were then anesthetized with a local anesthetic usually consisting of 1% lidocaine occasionally buffered with sterile sodium bicarbonate. For deep lesions, the subcutaneous tissue, paraspinal muscles, and spinal periosteum could be anesthetized with a 21-gauge vanSonneberg one-step access needle (Cook, Bloomington, IN) that had a detachable hub. The detachable hub was removed, and a 15-gauge Ostycut bone biopsy needle (C. R. Bard) was coaxially advanced over the access needle, which was then removed (Fig 2). The bone biopsy needle had the dual advantages of having the strength to be advanced through normal bone or overlying intact cortex to provide access and also the capability to obtain a core specimen. Typically, the Ostycut needle was advanced through the overlying cortex to the periphery of the lesion. The stylet was removed, and a smaller-gauge, thin-wall, cutting needle (Percucut biopsy needle; EZ-EM, Westbury, NY) was advanced into the lesion with its stylet slightly retracted. A repeat CT scan was obtained through the plane of the needle to confirm its placement in the lesion (Fig 3).
Fig 1.
Axial CT images of midthoracic lesions.
A, Image obtained in a patient with no known cancer shows the planned angle, entry point, depth to the most posterior part of the cortex, and depth to the lesion. Biopsy revealed adenocarcinoma, probably originating from the lung.
B, Image obtained in a patient with a history of breast cancer shows radiopaque skin markers in place to determine the entry point. Biopsy confirmed suspected metastatic breast cancer.
Tissue aspirates were placed on glass slides for microscopic examination by a cytotechnologist or cytopathologist, who was present in the radiology suite during the procedure to determine the adequacy of the specimen. If the tissue sample revealed only red blood cells or necrotic acellular material, additional aspirations could easily be obtained via the indwelling large-bore needle. A small core sample was often obtained with the thin-walled 18-gauge needle, although this was lesion-dependent. The core sample was rolled on a glass slide for analysis by the cytopathologist then placed in formalin or occasionally sodium chloride solution if lymphoma was suspected. More recently, if the initial samples were suggestive of lymphoma, the aspirates were the placed in a Roswell Park Memorial Institute solution that kept the cells viable for flow cytometry. If inflammatory cells were identified or if infection was clinically suspected, cultures were obtained.
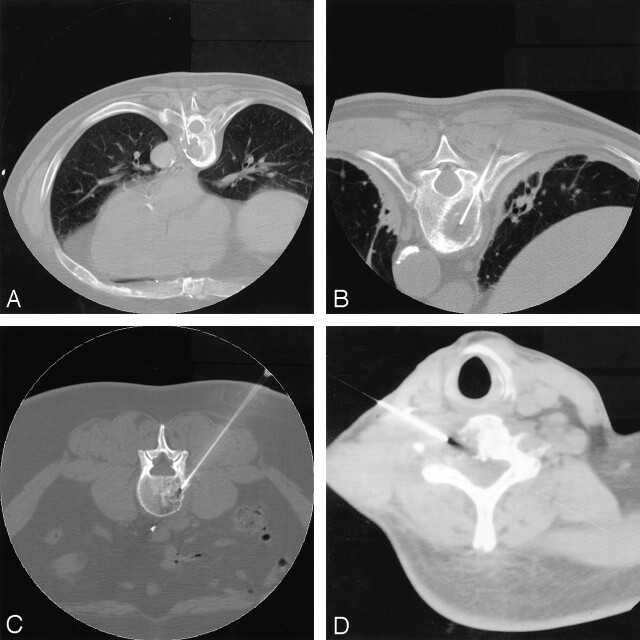
Although every case was planned individually, the techniques were similar (3). Typically, biopsy was performed in osseous lesions of the thoracic spine with either a transpedicular (Fig 4A) or a transcostovertebral approach, which allowed the needle to enter the posterolateral aspect of the vertebral body across the costotransverse ligament (Fig 4B). In the lumbar spine, a paraspinal approach was often used (Fig 4C). Cervical spine biopsy posed more challenges given the relatively small size of the vertebrae and the proximity of vital structures. A lateral or anterolateral approach was often used (Fig 4D). Transoral approaches for anterior lesions of the upper cervical spine were used as well (Fig 5).
No additional precautions were taken for potentially hypervascular lesions such as suspected metastatic renal cell carcinoma (n = 9) or metastatic thyroid cancer (n = 2). Because they are lytic, these lesions could be entered with an 18-gauge biopsy needle. Although the aspirations were often hemorrhagic, in our experience any bleeding was self limited. We never encountered a hematoma from direct bleeding from these tumors.
The average time for CTGSB depended on the type of the lesion and, more importantly, the location of the lesion. Typically, the procedure was completed in about 1 hour, with the shortest time being about 25 minutes and difficult procedures taking up to 2 hours. Most of the CTGSBs were performed on an outpatient basis with the patient recovering in the outpatient procedure department. Most patients were discharged after a 3-hour observation period.
Data Analysis
The lesions were grouped by their location in the spine and by their radiographic appearance. They were divided into five categories according to their radiographic features: 1) lytic lesions, 2) sclerotic lesions, 3) mixed lytic and sclerotic lesions, 4) compression or burst fractures with no demonstrable lytic or blastic features, and 5) lesions identified on T1-weighted or T2-weighted short-tau inversions recovery MR images but with a normal appearance on CT scans.
The accuracy of CTGSB was determined. Diagnostically accurate biopsy results included: 1) those that demonstrated histologic evidence of neoplastic tissue (true-positive finding) and 2) those showing no evidence of tumor, which was confirmed on subsequent open biopsy or a combination of clinical and radiographic follow-up demonstrating either stability or regression of the imaging findings in the absence of ongoing treatment for a minimum of 6 months (true-negative finding). Inaccurate biopsy results were those demonstrating no evidence of tumor but subsequently determined to be falsely negative for tumor on the basis of open biopsy findings, lesion progression, or the development of new osseous lesions.
Results
Over 8 years, 410 patients underwent CTGSB at our center. Eighteen patients were excluded because they had negative biopsy results and were lost to follow-up (n = 9) or because biopsy findings had been unsatisfactory (n = 9). Of the remaining 392 patients, 226 (58%) were female and 166 (42%) were male. Their median age was 61 years (range, 7–90 years). The site of biopsy was the cervical spine in nine patients (2%), the thoracic spine in 123 (31%), the lumbar spine in 164 (42%), and the sacrum in 96 (25%). The CT appearances of the lesions were lytic in 236 (60%), sclerotic in 83 (22%), and mixed lytic and sclerotic in 13 (3%). Compression fractures were present in 44 patients (11%), and no CT evidence of tumor was found in 16 (4%).
The overall accuracy was 89%, with no significant difference between men and women. Diagnostic accuracy was similar between lesions in the thoracic (88%) and lumbar spine (85%) and slightly higher for lesions in the sacrum (96%) and cervical spine (100%). The radiographic appearance of the lesions corroborated the accuracy rate: sclerotic lesions had an accuracy of 76%, which was significantly lower than that of either lytic (93%), mixed lytic-sclerotic lesions (92%), or compression fractures (93%). A lower accuracy rate (81%) was also noted when the lesion was identified on the basis of MR imaging findings, although no abnormalities were appreciated on CT scans at the time of biopsy.
There was no significant difference in the accuracy rate of biopsy of spinal lesions in patients with a documented cancer history (n = 298, 90%) and those with no known cancer history at the time of biopsy (n = 94, 87%).
The most common diagnosis was metastatic breast carcinoma in women (64 [28%] of 226), followed by metastatic lung cancer, (15 [7%] of 226). Metastatic lung cancer was the most common diagnosis in men (20 [12%] of 166). The second most common diagnosis in men was metastatic prostate carcinoma, (12 [7%] of 166). Primary osseous tumors of the spine accounted for only 26 (6%) of 392 lesions in this study. The most common primary osseous lesion diagnosed was multiple myeloma-plasmacytoma (n = 14, 54%).
Five patients had lesions that were negative for tumor but determined to be infectious with culture or histologic findings. Two were bacterial and two fungal, with one lesion revealing tuberculosis. One patient had a lesion that revealed metastatic esophageal carcinoma and a superimposed bacterial infection.
Two major (0.5%) and one minor (0.25%) complications occurred in this series. One patient with partially corrected coagulopathy developed a postprocedural posterior paraspinal hematoma requiring transfusion, but no other intervention was needed. A second major complication occurred after biopsy of a midthoracic lesion associated with epidural disease and moderate spinal canal compromise. The patient’s neurologic condition declined, and she underwent acute surgical intervention. She subsequently recovered with no permanent neurologic sequelae. A minor complication consisted of a small needle fragment that broke off during biopsy and remained in a cervical vertebral body.
Discussion
It has been almost 70 years since Robertson and Ball (1) first described percutaneous biopsy of the lumbar spine in 1935. Initial biopsy is often limited to the lower thoracic or lumbrosacral spine with radiographic-fluoroscopic guidance. The development of CT and the more-recent addition of faster image acquisition and multiplanar reconstruction capabilities have allowed safe and accurate biopsy at virtually all segments of the spine (3–23).
CTGSB is an important tool in the treatment of patients with known or suspected malignancy and osseous spinal lesions. The procedure improves accuracy for staging or initial diagnosis in patients with known or suspected underlying malignancy while offering minimal risk and discomfort.
Our results are comparable to other accuracy rates described in the literature (4–6, 8, 11, 14, 21). Interestingly, our diagnostic accuracy rates were somewhat higher in the cervical spine and sacrum compared with the thoracic and lumbar segments. Certainly, the number of cervical biopsy procedures in our study group was less than that for other segments of the spine, and in large part the high accuracy was related to lesion location. Increased accuracy rate in the sacrum was likely due to easier access and the absence of vital structures (e.g., spinal cord or major vessels), which allowed more aggressive sampling of a lesion. Although Kornblum et al (14) have reported decreased diagnostic accuracy rates in the thoracic spine, we did not find this to be the case in our series.
Lytic, mixed lytic and sclerotic lesions, and compression fractures had the highest accuracy rate (93%). Sclerotic lesions and those with no CT evidence of tumor had statistically lower accuracies of 76% and 81%, respectively. Several groups have reported lower accuracy rates in sclerotic lesions (6, 11, 21). Despite modifications of technique, such as trying larger-bore needles or sampling the periphery of the lesion, results have not improved. Typically, large core samples from needles on the order of 15 gauge or less can easily be obtained from a sclerotic lesion, although reactive change and sclerosis is often the only abnormality identified. This outcome is probably secondary to a small amount of tumor, causing the formation of extensive bone or sclerosis. We suspect that the lower accuracy associated with the lesions without no identifiable tumor on CT scans (MR imaging abnormality only) was due to the lack of a clear target.
Twenty-two patients underwent biopsy twice, although for this study these procedures were counted once, as they involved the same lesion and were generally performed within 4 weeks. The most common reason for second biopsy was a technically adequate, although negative, initial biopsy finding (n = 14, 64%). Of the 14 patients who initially had a negative biopsy result, seven had a negative second biopsy finding, which was confirmed to be truly negative at long-term follow-up or open biopsy. Four of the second biopsy procedures resulted in a change in the diagnosis, in that tumor was identified. Three of the initially negative results remained negative on second biopsy and were found to be falsely negative at long-term follow-up. In four patients who had positive results the first time, additional material was required, usually for marker or genetic studies. Four patients underwent repeat biopsy, because the initial specimen was unsatisfactory.
Conclusion
CTGSB of osseous lesions of the spine is an important tool in the care of patients with known or suspected malignancy. These findings confirm that the procedure is safe and accurate, although negative results—particularly with sclerotic lesions—should be viewed with caution, as accuracy is decreased and the false-negative rate is high for these lesions.
Fig 2.
Axial CT images from biopsy of a L3 collapse deformity revealing metastatic non–small cell lung carcinoma. Left, Image through the 25-gauge anesthetic needle used to anesthetize the skin and subcutaneous soft tissues. Middle, Image shows the 21-gauge vanSonneberg one-step access needle (Cook) allowing anesthetization of the deep paraspinal soft tissues and adjacent periosteum. Right, Image shows a 15-gauge Ostycut needle (C. R. Bard, Covington, GA) in good position and ready to be advanced through the adjacent cortex.
Fig 3.
Axial CT image shows the larger-bore Ostycut needle (C. R. Bard) providing a tunnel through the posterior cortex of L2 and allowing the narrower 18-gauge, thin-walled, cutting needle to be advanced coaxially into the lytic lesion in this patient with a history of colon cancer. Biopsy showed adenocarcinoma consistent with a colonic origin.
Fig 4.
Axial CT images. A, Transpedicular approach to a T6 lytic lesion in a patient without a prior cancer history. Cytologic and histologic findings revealed numerous plasma cells compatible with plasma cell neoplasm-plasmacytoma.
B, Transcostovertebral approach to a T8 lytic lesion in a patient with a history papillary thyroid cancer. Biopsy confirmed metastatic thyroid cancer.
C, Paraspinal approach to a mixed L3 lytic-sclerotic lesion in a patient with breast cancer. Biopsy showed adenocarcinoma consistent with a mammary origin.
D, Anterolateral approach to a C5 lytic lesion in a patient with a history of gastric cancer. Cytology was compatible with metastatic gastric carcinoma.
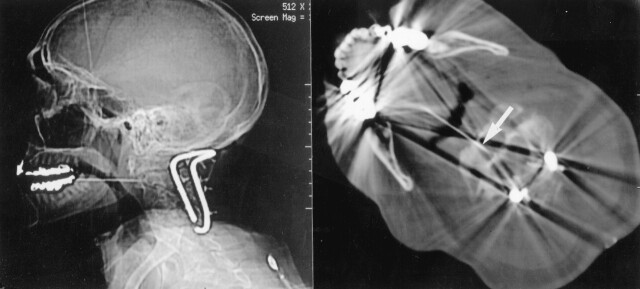
Fig 5.
Lateral scout and axial CT images through C2 show a transoropharyngeal approach to a lesion in the body of C2 in a patient without a history of cancer but who was first stabilized posteriorly. Arrow shows the needle tip in the C2 lytic lesion. Biopsy showed squamous cell carcinoma, possibly from the lung or upper aerodigestive tract. The primary site was never determined.
References
- 1.Robertson RC, Ball RP. Destructive spinal lesions: Diagnosis by needle biopsy. J Bone Joint Surg 1935;17:749–758 [Google Scholar]
- 2.Valls J, Ottolenghi CE, Schajowicz F. Aspiration biopsy in the diagnosis of lesions of vertebral bodies. JAMA 1948;136:376–382 [DOI] [PubMed] [Google Scholar]
- 3.Davis TM. Spinal biopsy techniques. In: McGraw JK, ed. Interventional Radiology of the Spine: Image-Guided Pain Therapy. Totowa, NJ: Humana Press;2003. :181–196
- 4.Babu NV, Titus VT, Chittaranjan S, Abraham G, Prem H, Korula RJ. Computed tomographically guided biopsy of the spine. Spine 1994;19:2436–2442 [DOI] [PubMed] [Google Scholar]
- 5.Brugieres P, Gaston A, Voisin MC, Ricolfi F, Chakir N. CT-guided percutaneous biopsy of the cervical spine: a series of 12 cases. Neuroradiology 1992;34:358–360 [DOI] [PubMed] [Google Scholar]
- 6.Ghelman B, Lospinuso MF, Levine DB, O’Leary PF, Burke SW. Percutaneous computed-tomography-guided biopsy of the thoracic and lumbar spine. Spine 1991;16:736–739 [DOI] [PubMed] [Google Scholar]
- 7.Kattapuram SV, Khurana JS, Rosenthal DI. Percutaneous needle biopsy of the spine. Spine 1992;17:561–564 [DOI] [PubMed] [Google Scholar]
- 8.Omarini LP, Garcia J. CT-guided percutaneous puncture-biopsy of the spine. Review of 104 cases. Schweiz Med Wochenschr 1993;123:2191–2197 [PubMed] [Google Scholar]
- 9.Kattapuram SV, Rosenthal DI. Percutaneous biopsy of the cervical spine using CT guidance. AJR Am J Roentgenol 1987;149:539–541 [DOI] [PubMed] [Google Scholar]
- 10.Schratter M. CT-guided percutaneous biopsy in orthopedics. Indications–planning–technic–personal experiences, with special reference to the spine. Radiologe 1990;30:201–213 [PubMed] [Google Scholar]
- 11.Stoker DJ, Kissin CM. Percutaneous vertebral biopsy: a review of 135 cases. Clin Radiol 1985;36:569–577 [DOI] [PubMed] [Google Scholar]
- 12.Olscamp A, Rollins J, Tao SS, Ebraheim NA. Complications of CT-guided biopsy of the spine and sacrum. Orthopedics 1997;20:1149–1152 [DOI] [PubMed] [Google Scholar]
- 13.Mick CA, Zinreich J. Percutaneous trephine bone biopsy of the thoracic spine. Spine 1985;10:737–740 [DOI] [PubMed] [Google Scholar]
- 14.Kornblum MB, Wesolowski DP, Fischgrund JS, Herkowitz HN. Computed tomography-guided biopsy of the spine. A review of 103 patients. Spine 1998;23:81–85 [DOI] [PubMed] [Google Scholar]
- 15.Kang M, Gupta S, Khandelwal N, Shankar S, Gulati M, Suri S. CT-guided fine-needle aspiration biopsy of spinal lesions. Acta Radiol 1999;40:474–478 [DOI] [PubMed] [Google Scholar]
- 16.Ippolito V, Saccalani M, Pavia M, Brembilla R, Motta C. CT-guided percutaneous trochar-biopsy of the spine. Chir Organi Mov 1998;83:7–13 [PubMed] [Google Scholar]
- 17.Gupta RK, Cheung YK, Al Ansari AG, Naran S, Lallu S, Fauck R. Diagnostic value of image-guided needle aspiration cytology in the assessment of vertebral and intervertebral lesions. Diagn Cytopathol 2002;27:191–196 [DOI] [PubMed] [Google Scholar]
- 18.Geremia G, Joglekar S. Percutaneous needle biopsy of the spine. Neuroimaging Clin N Am 2000;10:503–533 [PubMed] [Google Scholar]
- 19.Garces J, Hidalgo G. Lateral access for CT-guided percutaneous biopsy of the lumbar spine. AJR Am J Roentgenol 2000;174:425–426 [DOI] [PubMed] [Google Scholar]
- 20.Carson HJ, Castelli MJ, Reyes CV, Gattuso P. Fine-needle aspiration biopsy of vertebral body lesions: cytologic, pathologic, and clinical correlations of 57 cases. Diagn Cytopathol 1994;11:348–351 [DOI] [PubMed] [Google Scholar]
- 21.Brugieres P, Revel MP, Dumas JL, Heran F, Voisin MC, Gaston A. CT-guided vertebral biopsy: a report of 89 cases. J Neuroradiol 1991;18:351–359 [PubMed] [Google Scholar]
- 22.Adapon BD, Legada BD Jr, Lim EV, Silao JV Jr, Dalmacio-Cruz A. CT-guided closed biopsy of the spine. J Comput Assist Tomogr 1981;5:73–78 [DOI] [PubMed] [Google Scholar]
- 23.Brugieres P, Gaston A, Heran F, Voisin MC, Marsault C. Percutaneous biopsies of the thoracic spine under CT guidance: transcostovertebral approach. J Comput Assist Tomogr 1990;14:446–448 [DOI] [PubMed] [Google Scholar]