Abstract
The objective of the present study was to investigate the effect of quercetin and evaluate its protective effect on articular cartilage in patients with osteoarthritis (OA), by intervening the p38 pathway. The target factors of quercetin protecting articular cartilage in patients with OA were predicted scientifically and analyzed to predict the possible pathways by using network pharmacology. A pathway predicted to be closely associated with osteoarthritis was chosen for experimental verification in in vitro cells. The optimal intervention drug concentrations were selected by the of Cell Cycle Kit-8 assay, osteoarthritis and inflammatory factors relevant to osteoarthritis, interleukin-1β and tumor necrosis factor-α, were tested by of enzyme-linked immunosorbent assay, and the expression of relevant proteins and mRNA of the p38 signaling pathway was tested by reverse transcription-quantitative PCR and western blotting, following quercetin intervention. It was found that quercetin, at the concentration of 100 umol/l, can decrease inflammatory factors relevant to OA, inhibit the expression of p38, matrix metalloprotease 13 and ADAMTS in the pathway, and promote the expression of collagen Ⅱ. Therefore, it is postulated that quercetin can lower the expression of inflammatory factors in cartilage for the prevention and treatment of OA, and the expression level of relevant factors can be changed positively by blocking the p38 MAPK signaling pathway. Thus, quercetin can promote the repair of degenerative chondrocytes and protect articular chondrocytes.
Keywords: quercetin, osteoarthritis, p38 MAPK signaling pathway, network pharmacology, in vitro experiment
Introduction
Osteoarthritis (OA) is a degenerative joint disease caused by changes in joint cartilage, subchondral bone, bursa, synovium, ligament and other joint structure, which can cause pain and joint stiffness. Its main pathological features include cartilage degeneration, synovial inflammation and subchondral bone changes (1). The incidence rate of OA accounts for 4 to 13% of the world's population, which seriously affects patients' quality of life, and is one of the main causes of disability (2). Since OA has a higher incidence rate in the elderly population, its impact will grow exponentially in the coming decades (3). Medications currently recommended by the guidelines include oral painkillers, non-steroidal anti-inflammatory drugs and intra-articular corticosteroids. However, these treatments only temporarily relieve symptoms and have side effects such as irritating the gastrointestinal tract and increasing the risk of cardiovascular disease (4). Therefore, exploring better OA treatment methods has been a research focus in the field of orthopedics. Although the pathogenesis of OA is unclear, it is generally believed that OA is associated with increased proinflammatory cytokines, activation of inflammation-associated signaling pathways, and the degradation of the extracellular matrix (ECM) (5-7).
Quercetin, a flavonoid compound widely found in vegetables and fruits, is an excellent free radical scavenging compound, which has many effects such as anti-oxidative stress and anti-inflammatory, and can lower the risk of OA, rheumatoid arthritis and other chronic diseases associated with oxidative stress (8). Kanzaki et al (9) tested the intake of oral quercetin (45 mg/d) combined with glucosamine and chondroitin as the experimental group, and OA pain symptoms were significantly relieved compared with the control group. Quercetin can significantly decrease the formation of inflammatory mediators in macrophages in vivo and in vitro, significantly decrease the expression and secretion of interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) (10), and downregulate the expression of matrix metalloproteinase 13 (MMP-13) (11), which has potential medicinal value in the treatment of OA. The pathogenesis of OA and the apoptosis of chondrocytes are regulated by a variety of signaling pathways, including signaling pathway of p38 MAPK, Wnt-β catenin, nuclear factor (NF)-κβ, OPG, PANK, PANKL and Hedgehog (12-15). Among them, p38 MAPK signaling pathway is relatively clear, which is involved in the degradation of the ECM of cartilage and mediating inflammatory response, and involves a variety of kinases and substrates, laying a solid foundation for further study on the pathogenesis of OA. Therefore, the mechanism that quercetin protecting articular chondrocytes may be mediated by targeting and blocking the p38 MAPK signaling pathway was tested. Based on bioinformatics, the present study used network pharmacology to explore the targets of quercetin and analyze the mechanism of action of the drug, which is of great significance for the application and promotion of quercetin in the field of OA. Experimental studies in cells in vitro were performed to detect the expression of factors of the p38 MAPK signaling pathway in articular chondrocytes and to observe the effect of quercetin on articular chondrocytes. This will help to further explore the pathogenesis of OA and the target of quercetin, in order to provide theoretical and experimental basis for the prevention and treatment of OA.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM; cat. no. CM15019) and fetal bovine serum (FBS; cat. no. 10100147) were obtained from Gibco (Thermo Fisher Scientific, Inc.). Quercetin (cat. no. HY-18085; purity >98.0%) and p38 MAPK signal pathway blocker (SB203580; cat. no. HY-10256) were purchased from MedChemExpress. Toluidine Blue (cat. no. G3660) was purchased from Beijing Solarbio Science & Technology Co., Ltd. Cell Counting Kit-8 (CCK-8; cat. no. CCK805) and Annexin V-FITC/PI Apoptosis kit (cat. no. 70-AP101-100) were purchased from MULTI SCIENCES (LIANKE) BIOTECH CO., LTD. Anti-MMP13 antibody (cat. no. ab51072), anti-p38 antibody (cat. no. ab47363), anti-ADAMTS-4 antibody (cat. no. ab185722) and anti-collagen II antibody (cat. no. ab34712) were purchased from Abcam. Anti-phosphorylated (P)-p38 antibody (cat. no. AF4001) was obtained from Affinity Biosciences. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (cat. no. GB23303), anti-β-actin antibody (cat. no. TA-09) and first Strand cDNA Synthesis kit were purchased from Wuhan Servicebio Technology Co., Ltd. Enzyme-linked immunosorbent assay (ELISA) kit for rat 1L-1β (cat. no. EK301B/3-96) and ELISA kit for rat TNF-α (cat. no. EK382/3-96) were supplied by Wuhan Servicebio Technology Co., Ltd. Specific primers were designed and synthesized by Wuhan Servicebio Technology Co., Ltd., as displayed in Table I.
Table I.
List of primer sequences of rat for reverse transcription-quantitative PCR.
| Genes | Primer sequences (forward and reverse) |
|---|---|
| p38 | Forward, 5'-GTGCCCGAACGATACCAGAAC-3' |
| Reverse, 5'-TGAATTCCTCCAGTGACCTTGC-3' | |
| MMP-13 | Forward, 5'-CTATCCCTTGATGCCATTACCAG-3' |
| Reverse, 5'-TAAGGTCACGGGATGGATGTTC-3' | |
| ADAMTS-4 | Forward, 5'-ACCGTCAAGGCTCCTTCTGG-3' |
| Reverse, 5'-ACCAAGTTGACAGGGTTTCGG-3' | |
| Collagen Ⅱ | Forward, 5'-ACGCTACACTCAAGTCACTGAACAAC-3' |
| Reverse, 5'-TCAATCCAGTAGTCTCCGCTCTTC-3' | |
| β-actin | Forward, 5'-GTGACGTTGACATCCGTAAAGA-3' |
| Reverse, 5'-GTAACAGTCCGCCTAGAAGCAC-3' |
MMP, matrix metalloprotease.
Network pharmacology of quercetin against OA
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform database (TCMSP, https://tcmspw.com/index.php) search was used to find protein targets of quercetin, and the Uniprot database (https://www.uniprot.org/) was used to find the gene name of the associated protein. Quercetin and its corresponding target genes were introduced into Cytoscape 3.6.1 (https://apps.cytoscape.org/apps/bisogenet) to construct the information network of quercetin and gene targets. Online Mendelian Inheritance in Man (OMIM, https://omim.org/), Therapeutic Target Database (TTD, http://db.idrblab.net/ttd/) and PharmGKB databases (https://www.pharmgkb.org/) were searched for the targets associated with OA, and then the screening results were introduced into Cytoscape. Bisogenet bioinformatics plug-in (https://apps.cytoscape.org/apps/bisogenet) was used to construct a network of osteoarthritis target genes. Through Cytoscape, the aforementioned chemical components, OA and associated target gene networks were combined to screen common target genes, which were analyzed by GO (Gene Ontology, https://david.ncifcrf.gov/), KEGG (Kyoto Encyclopedia of Genes and Genomes, https://david.ncifcrf.gov/) and molecular docking technology (Autodock Vina software, http://vina.scripps.edu/download.html), respectively to further discover and elucidate the application mechanism of quercetin in the protection of articular cartilage in OA.
Primary cell extraction
All rats were used according to the national guidelines of the care and use of laboratory animals with the approval of the Animal Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (AWE-2019-043). A total of 2 Sprague-Dawley (S-D) male rats (age, 1 week) were purchased from Experimental Animal Center of Shandong University (Shandong, China). According to the method recommended by American Veterinary Medical Association (AVMA), S-D male rats were euthanized with pentobarbital sodium (100-150 mg/kg, intraperitoneally), with their hair cleaned, and disinfected with 75% alcohol. The articular cartilage of the femoral condyle and tibial plateau was extracted under aseptic conditions, washed with 1% dual-antibody PBS solution, and the tissue was cut into fragments (1 mm3 in size), digested in 0.25% trypsin, incubated at 37˚C for 30 min and digested with 0.2% collagen Ⅱ solution for 4 h. Chondrocytes were seeded at a density of 2x105/ml in a 25-cm2 culture flask and cultured in an incubator at 37˚C, in a humidified atmosphere containing 5% CO2. Then, the primary cells were isolated. When the bottom of the culture flask was covered with chondrocyte (80% confluence), the cell passage was carried out, with a ratio of 1:3. The experiment was carried out when the cells were transferred to the second generation and entered the logarithmic growth stage.
Toluidine blue staining
The chondrocytes of the knee joint (the second generation) were taken and inoculated into a 6-well plate at 5x104 cells/well. When the number of cells reached more than half of the area, 4% paraformaldehyde was used to fix them at room temperature for 30 min. After rinsing for 5 min, toluidine blue dye solution was added evenly, and after staining at room temperature for 15 min, distilled water and PBS buffer solution were rinsed until dark blue disappeared. Finally, the cells were rinsed with anhydrous ethanol until colorless and observed (10x magnification, Axiovert 40 inverted phase-contrast microscope, Zeiss AG).
Optimal intervention concentration of quercetin screened by CCK-8
Inoculation density of cells was 2,000/well, for each group of five wells. The normal complete culture medium was used in the apoptosis group and the blank group, and 10 ng/ml IL-1β was used in the drug group. After 24 h culture in the incubator, the medium was discarded. Normal medium (100 µl) was added to the apoptosis group and the blank group, and quercetin medium at different concentrations (0, 50, 100, 150, 200, 400 and 600 µmol/l) was added to the drug group, respectively. After intervention for 24 h, 10 µl CCK-8 solution was added to each well and incubated at 37˚C for 2 h. OD values of each well were detected at 450 nm wavelength.
Experimental grouping and intervention
The cells were divided into 6 groups. All groups except W1 were treated with 10 ng/ml IL-1β and cultured for 24 h at 37˚C in a humidified atmosphere containing 5% CO2. After successful intervention, normal complete medium was added to group W1 and group W2, complete medium containing 0.1% DMSO was added to group W3, complete medium containing 100 mM quercetin was added to W4 group, and complete medium containing 10 µM SB 203580 was added to W5 group. A complete culture medium containing 100 µmol/l quercetin and 10 µM SB 203580 was added to group W6 and all cells were cultured for 24 h. Supernatant was collected and stored at -80˚C.
ELISA for detecting IL-1β and TNF-α
Standard samples with 2X diluted standard was added to the standard wells in turn, cell culture medium was added to the blank wells, and buffer (1X) and samples were added to the sample wells, with three samples in each group. The treated detection antibody was added to each well, the plate was sealed, and the samples were incubated whilst shaking at room temperature for 2 h. After all the samples were washed, horseradish peroxidase-labeled streptomycin was added to each well, and the samples were incubated at room temperature for 45 min. The plate was washed. Then, after the color-rendering substrate, TMB was added to 96 orifice plate, the samples were cultured at room temperature for 20 min, avoiding light. OD value at 450 nm wavelength was measured within 30 min by an enzyme marker.
Apoptosis detected by flow cytometry
After adjusting the instrument parameters, 500 µl 1X binding buffer was added to each group of cells at room temperature. 5 µl annexin V-FITC and 10 µl PI were added to each tube. After gentle vortexing, the tubes were incubated for 5 min in dark at room temperature prior to detection with the flow cytometer used (Cyto FLEX, Beckman Coulter, Inc.).
Reverse transcription-quantitative (RT-q)PCR for detecting inflammatory factors
After the completion of cell intervention in each group, the medium was removed and the cells were washed with the PBS buffer three times. TRIzol® was added to the cells, and total RNA was extracted. After extraction, RNA concentration in each group was detected by Nanodrop 2000. cDNA was synthesized from mRNA by Revert Aid First Strand cDNA Synthesis kit at 42˚C for 60 min and at 80˚C for 5 min. The Light Cycler 480 fluorescence quantitative PCR instrument (Roche Diagnostics) and SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd.) were used for amplification and detection, respectively. A 25-µl PCR reaction mixture contained 2 µl cDNA, 12.5 µl SYBR Premix Ex Taq II, 2 µl each primer and 8 µl diethyl pyrocarbonate water. Following an initial denaturation step at 95˚C for 10 min, p38, MMP-13, ADAMTS-4, Collagen Ⅱ and ß-actin were amplified with 40 cycles at 95˚C for 15 sec, 60˚C for 30 sec and 68˚C for 30 sec. The levels of β-actin were used as an internal control. Each reaction was repeated three times. Quantitative data were calculated with 2-ΔΔCq method (16).
Western blot analysis for detecting the expression levels of proteins associated with the p38 MAPK signaling pathway
Following treatment with quercetin as described for the immunofluorescent staining assay, protein was extracted from the cartilage cells using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology) containing phenylmethane sulfonyl fluoride. Protein samples (30 µg/lane) were separated by SDS-PAGE (8%) and transferred to polyvinylidene difluoride membranes. All primary antibodies were prepared to the same dilution (dilution, 1:1,000), the membranes were incubated overnight at 4˚C with primary anti-p38 antibody (cat. no. ab47363, Abcam), anti-phosphorylated (P)-p38 antibody (cat. no. AF4001, Affinity Biosciences), anti-MMP13 antibody (cat. no. ab51072, Abcam), anti-ADAMTS-4 antibody (cat. no. ab185722, Abcam) and anti-collagen II antibody (cat. no. ab34712, Abcam) followed by the HRP-conjugated goat anti-rabbit IgG secondary antibody (dilution, 1:3,000) at room temperature for 30 min. The HRP-conjugated goat anti-rabbit IgG antibodies (cat. no. GB23303) were purchased from Wuhan Servicebio Technology Co., Ltd.. The blots were then visualized using a chemiluminescent detection kit (Amersham; GE Healthcare Life Sciences). A Typhoon Phosphor Imager with Image Quant TL software version 7.0 (both GE Healthcare Life Sciences) was used to quantify the protein. The antibody against β-actin served as an internal reference. The expression level of Gray scale integration of target protein in each sample was determined as follows: Expression level of target protein-densitometric value of target protein/densitometric value of β-actin.
Statistical analysis
All data were expressed as mean ± standard deviation (X±S), and SPSS 20.0 statistical software (IBM Corp.) was used to perform a one-way analysis of variance (ANOVA) among multiple groups, Dunnett's multiple comparisons test was used as the post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Network pharmacology study of quercetin in the treatment of osteoarthritis
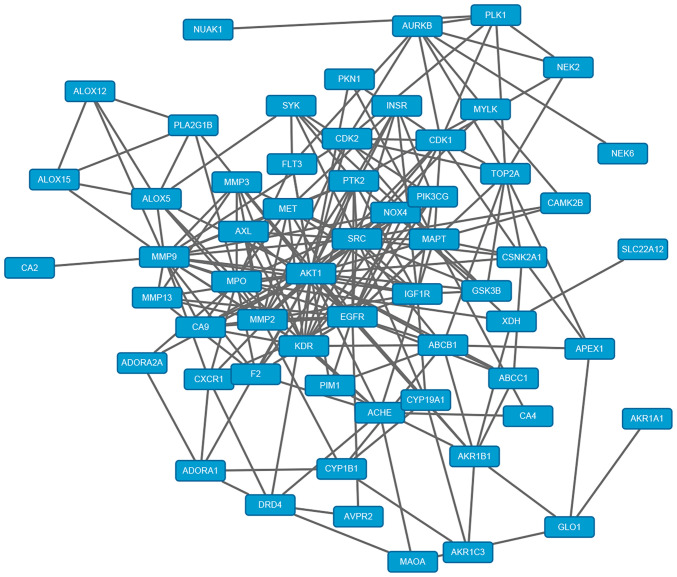
In total, there were 89 corresponding targets of quercetin. Protein names were found through Uniprot online database. Meanwhile, the target network of quercetin was constructed and analyzed by using the Cytoscape3.6.1 software. Each node represents the target gene or compound, each edge represents the association between nodes, and the degree represents the corresponding number of nodes associated with other nodes. The greater the degree is, the more likely it is to be the ‘center’ in the construction network diagram. The network has 90 nodes (1 chemical component, 89 target genes) and 89 edges, which reflects the complex network of multi-target associations of one drug and further validates the synergistic prevention and treatment effect of quercetin on multi-targets and multi-paths. Three databases (OMIM, TTD and PharmGKB) were screened to obtain the target genes associated with osteoarthritis. The duplicated genes were eliminated, and 2976 genes associated with OA remained. The Bisogenet plug-in was used to construct a network of osteoarthritis-target genes, in which the constructed network has a total of 436451 edges and 14141 nodes. Through the analysis, the complex association between the pathogenic factors and pathogenesis of osteoarthritis was demonstrated. A total of 66 co-acting target genes were screened out by combining the network of chemical component-target gene and that of osteoarthritis-target gene (Fig. 1). Autodock Vina software was used to dock quercetin with OA inflammatory factor target proteins (TNF-α, 1L-1β, MMP-13, ADAMTS-4, ADAMTS-5 and MAPKK6) under the p38 MAPK signaling pathway. Quercetin had the strongest binding energy with MMP-13 (Table II).
Figure 1.
Prediction of target factors and pathways of quercetin in the protection of articular cartilage by network pharmacology. Target factors of quercetin and osteoarthritis target docking, screening out the common target, identified 66 co-acting target genes in the network.
Table II.
Table of molecular docking between quercetin and compounds.
| Chemical compound | Binding energy, kcal/mol |
|---|---|
| TNF-α | -6.1 |
| IL-1β | -8.3 |
| MMP-13 | -9.7 |
| ADAMTS-4 | -9.3 |
| ADAMTS-5 | -8.2 |
| MAP2K6 | -9 |
Reference value of binding energy, -5 kcal/mol. IL, interleukin; TNF, tumor necrosis factor; MMP, matrix metalloprotease.
Extraction and identification of articular chondrocytes in rats
In the present study, toluidine blue staining was used to identify articular chondrocytes isolated from articular cartilage tissues in SD rats. As shown in Fig. 2, chondrocytes grew adherent to the wall, in the shape of polygonal or fusiform, with basically the same shape and size, abundant cytoplasm, clear nuclei, and good growth status (Fig. 2A). After staining with toluidine blue, the nuclei were stained dark blue and the cytoplasm and stroma were stained light blue (Fig. 2B).
Figure 2.
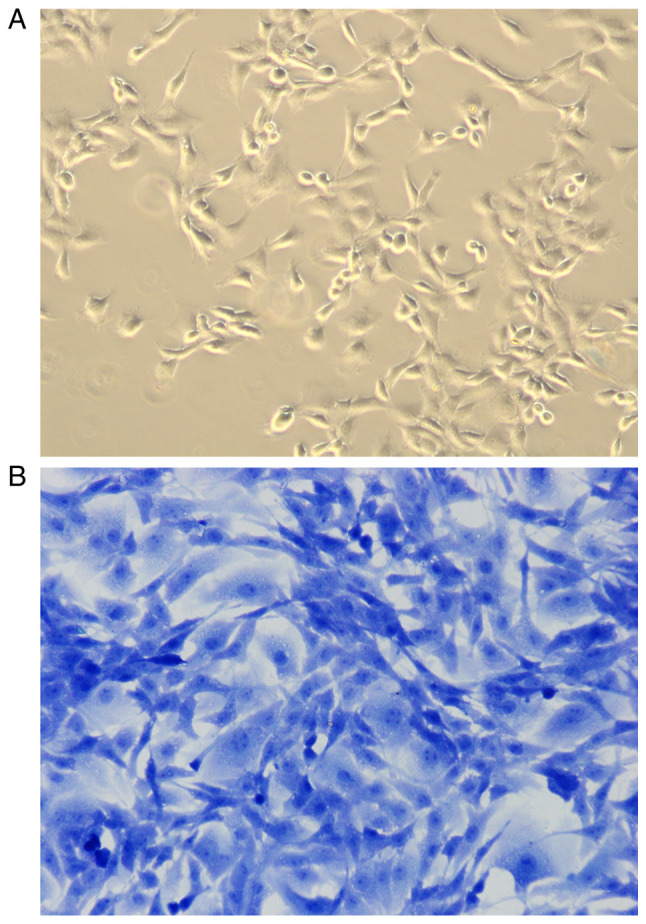
Identification of chondrocytes (magnification, x10). (A) The shape of healthy chondrocytes. (B) The healthy chondrocytes stained with toluidine blue.
Optimal concentration of quercetin to prevent IL-1β-induced inflammatory cytokines expression in chondrocytes
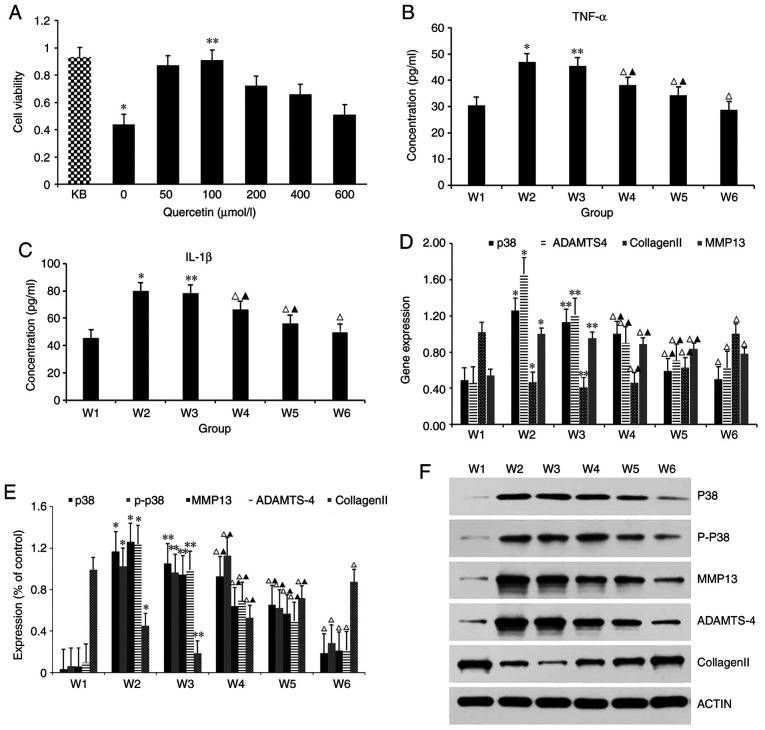
The optimal concentration of quercetin in intervening with articular chondrocytes of rats was screened by CCK-8 (Fig. 3A). The cell viability of the 0 µmol/l quercetin intervention group was significantly lower compared with that of the non-treated control after culturing in medium containing 1L-1β for 24 h, and the difference was statistically significant. Thus, IL-1β at the concentration of 10 ng/ml could induce the degeneration of rat chondrocytes, and the model was successfully established. At the same time, compared with the cell viability of degenerative articular chondrocytes of the 0 µmol/l quercetin intervention group cultured in normal complete medium, the increase in cell viability of the 100 µmol/l quercetin intervention group was significant, which shows that the optimal concentration of quercetin is 100 µmol/l. In the present study, 100 µmol/l quercetin was used for subsequent interventions.
Figure 3.
Various studies in IL-1β-induced chondrocytes treated with quercetin. (A) Cell viability was evaluated in IL-1β-induced chondrocytes treated with quercetin by Cell Cycle Kit-8. KB represents group without 10 ng/ml IL-1 β intervention. *P<0.05 vs. KB. **P<0.05 vs. 0 µmol/l. (B and C) Detection of the expression of IL-1β and TNF-α in p38 MAPK signaling pathway were measured by enzyme-linked immunosorbent assay kit. *P<0.05 vs. W1; **P>0.05 vs. W2; ΔP<0.05; ▲P<0.05 vs. W1. (D) Reverse transcription-quantitative PCR was performed to determine the expression levels of p38, ADAMTS-4, collagen Ⅱ and MMP-13 in chondrocytes under IL-1β stimulation. *P<0.05 vs. W1; **P>0.05 vs. W2; ΔP<0.05; ▲P<0.05 vs. W1. (E and F) The associated proteins (p38, P-p38, MMP-13, ADAMTS-4 and collagen Ⅱ) were evaluated by western blotting. *P<0.05 vs. W1;**P>0.05 vs. W2; ΔP1,0.05; ▲P<0.05 vs. W1. IL, interleukin; TNF, tumor necrosis factor; MMP, matrix metalloprotease; P-, phosphorylated.
Effects of quercetin on the expression levels of inflammatory factors upstream of the p38 MAPK pathway
The cell culture supernatant was collected as a specimen, and the expression levels of inflammatory factors, IL-1β and TNF-α in the p38 MAPK signaling pathway were detected by ELISA (Fig. 3B and C). The ELISA results showed that the expression levels of IL-1β and TNF-α were the lowest in the W1 group and the highest in the W2 group, and there was a significant difference between them, indicating that IL-1β at 10 ng/ml could cause chondrogenic degeneration. Compared with the inflammatory factor expression level of the W2 group, the expression level in the W3 group (DMSO) was approximately the same, and no obvious difference was found between the two groups, showing that 0.1% DMSO had no effect on the expression of inflammatory factors in cartilage cells. DMSO can be applied as a solvent to dissolve the blocker, SB2035804, when its concentration is <0.1%. For the expression levels of inflammatory factors, IL-1β and TNF-α in the p38 MAPK signaling pathway, quercetin intervention in W4 group, W5 group and W6 group can effectively decrease the expression of inflammatory factors in degenerative chondrocytes, compared with that in the W2 group inflammatory factor expression level. However, when quercetin is used in combination with the blocker, the expression level of inflammatory factors is the lowest, with a significant statistical difference, indicating that both quercetin and SB203580 can lower the expression of inflammatory factors in chondrocytes, with similar effects, but the effect of quercetin is weaker than SB203580.
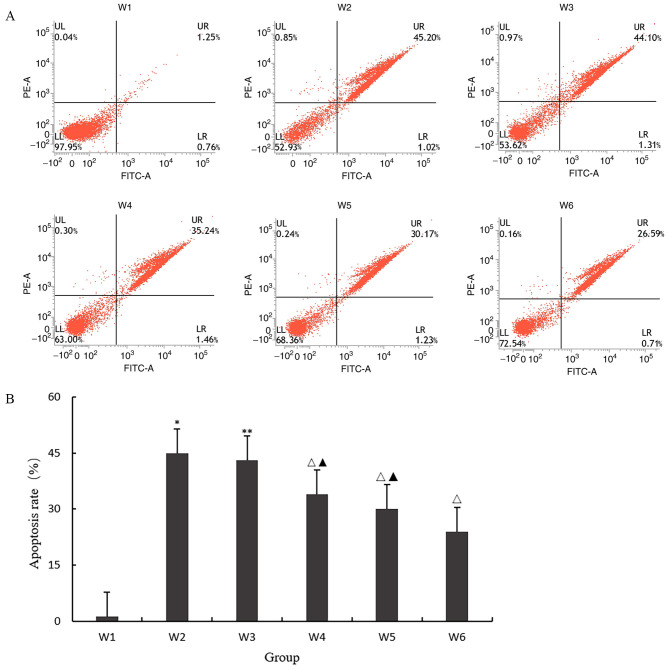
Effect of quercetin on apoptosis
As shown in Fig. 4A and B, the apoptosis rate of the W2 group was significantly higher compared with that of the W1 group (P<0.05), indicating that 10 ng/ml IL-1β could induce chondrocyte degeneration and apoptosis; the apoptosis rate of W3 group was approximately the same as that of the W2 group (P>0.05), indicating that 0.1% DMSO had no effect on the expression of inflammatory factors in chondrocytes. When the final concentration was <0.1%, DMSO solution could be used as solvent to dissolve SB203580; W4, W5 and W6 had the same effect, and the apoptosis rate of the three groups was significantly decreased. The results showed that quercetin and SB203580, a p38 MAPK signal pathway blocker, could inhibit the apoptosis of degenerative chondrocytes, but the effect of quercetin was weaker than that of the pathway blocker.
Figure 4.
(A and B) Effect of quercetin on apoptosis. *P<0.05 vs. W1; **P>0.05 vs. W2; ΔP<0.05; ▲P<0.05 vs. W1.
Expression levels of associated proteins by targeting p38 MAPK signaling pathway via quercetin
Western blot analysis was used to detect the expression levels of associated proteins p38, P-p38, MMP-13, collagen Ⅱ and ADAMTS-4 in the p38 MAPK signal pathway of cells in each group (Fig. 3E and F). Results showed that the expression level of collagen II was highest in the W1 group and lowest in the W2 group. The expression of MMP-13, p38, P-p38, and ADAMTS-4 in the W1 group was lowest but highest in the W2 group. There was significant difference between them, showing that IL-1β at 10 ng/ml can stimulate the expression of MMP-13, p38, P-p38 and ADAMTS-4 in chondrocytes, and inhibit the expression of type II collagen, causing chondrocyte degeneration. Compared with protein expression level in the W2 group, the expression level in the W3 group was about the same, and there was no significant difference between them, indicating that 0.1% DMSO had no effect on chondrocyte protein expression, and 0.1% DMSO solution can be used as a solvent to dissolve SB2035803. For expression levels of associated proteins, quercetin intervention in the W4, W5 and W6 groups can effectively lower the expression levels of MMP-13, p38, P-p38, and ADAMTS-4, compared with that in the W2 group, while increasing the expression of type II collagen. When combined, the protein expression level changed most significantly, and the difference was statistically significant, showing that quercetin and the blocking agent SB203580 can change the expression level of proteins associated with p38 MAPK signaling pathway of chondrocytes; however, the effect of quercetin is weaker than that of SB 2035803 blocking agent.
Quercetin decreases the expression of IL-1β-induced inflammatory cytokines in chondrocytes with blocking the p38 MAPK pathway
For mRNA levels, RT-qPCR results showed that the expression level of collagen II mRNA was highest in the W1 group and lowest in the W2 group. The expression of the MMP13 mRNA and p38 mRNA in the W1 group was lowest and highest in the W2 group, respectively. The significant difference shows that IL-1β at 10 ng/ml can stimulate the expression of MMP-13, p38 and ADAMTS-4 in chondrocytes, and inhibit the expression of type II collagen, causing chondrocyte degeneration. Meanwhile, compared with mRNA expression level in the W2 group, W3 expression level in the DMSO group was approximately the same, indicating that 0.1% DMSO had no effect on the expression of chondrocyte genes. Quercetin intervention in the W4, W5 and the W6 groups could effectively lower the expression levels of MMP-13 mRNA, p38 mRNA and ADAMTS-4 mRNA, compared with that in the W2 group, but it increased the expression of type II collagen in each group. However, when used in combination, the gene expression level changed more significantly and the difference was statistically significant, indicating that quercetin and the blocking agent SB203580 both have the same effect, and both can change the expression levels of genes associated with the p38 MAPK signaling pathway of chondrocytes; however, the effect of quercetin is weaker than that of the blocker (Fig. 3D).
Discussion
Network pharmacology is a new research method based on bioinformatics system and drug information system, which can use official databases to explore data on drugs or their ingredients and make scientific predictions of disease-associated target factors and pathways. Cytokines are a type of high-activity proteins secreted by cells, and are widely involved in the physiological functions and pathological changes in the body, playing an important role in the regulation of chondrocyte proliferation, apoptosis and ECM metabolism. Specific signal pathways are important for the development of diseases. Quercetin, widely found in fruits, vegetables, and Chinese herbal medicines, has anti-inflammatory and anti-oxidative stress effects. According to the existing research, quercetin may have a protective effect on the articular cartilage of osteoarthritis. Therefore, the network pharmacology technology was used in the present study to explore the targets and pathway of quercetin and analyze the mechanism of the drug in the field of OA. Cell experiments were conducted to verify that quercetin blocks the p38 signaling pathway to protect articular cartilage, which is of great significance for the development of quercetin as a therapeutic in this field.
By using network pharmacology to predict disease targets, 66 target genes were identified, including MMP family, ALOX family, CA family, and PI3K, which are most likely potential targets for quercetin to protect articular cartilage. GO was used to analyze and process relevant data, and it was found that the predicted targets were involved in a number of body regulation, such as active oxygen metabolism, injury response regulation, vitamin metabolism, NO metabolism process, body aging, cell movement regulation, cadmium response and other processes. The aging of the body decreases the tolerance of the articular cartilage, causing wear and tear, which leads to osteoarthritis pain. Studies have shown that reactive oxygen are associated with various diseases, including cancer, inflammation of various parts of the body and cardiovascular diseases (17,18). Nitric oxide (NO) plays an important role in osteochondral repair. Studies have found that nitric oxide synthase (NOS) inhibitors have a significant effect on inhibiting the release of NO from regenerating cartilage. When NOS activity is decreased, NOS inhibitor has obvious effect in improving the quality of cartilage regeneration (19,20). Vitamin metabolism has an inseparable association with bone diseases. Vitamin D can regulate the cell activity of osteoblasts and osteoclasts, Vitamin A can inhibit osteoblast function, and Vitamin K can inhibit bone resorption activation factors (IL-1 and IL-6), and lower osteoclast activity, bone loss, and the risk of osteoarthritis (21). Furthermore, low concentrations of vitamin B may also be a trigger for bone mass reduction (22,23). The reaction process of cadmium can enhance the activity of osteoclasts, and bones are in the list of important target organs damaged by cadmium. Therefore, osteoclasts may be the target cells for bone diseases caused by changes in cadmium (24-26). Cadmium interferes bone metabolism, and its mechanism is associated with the effect on bone formation and absorption.
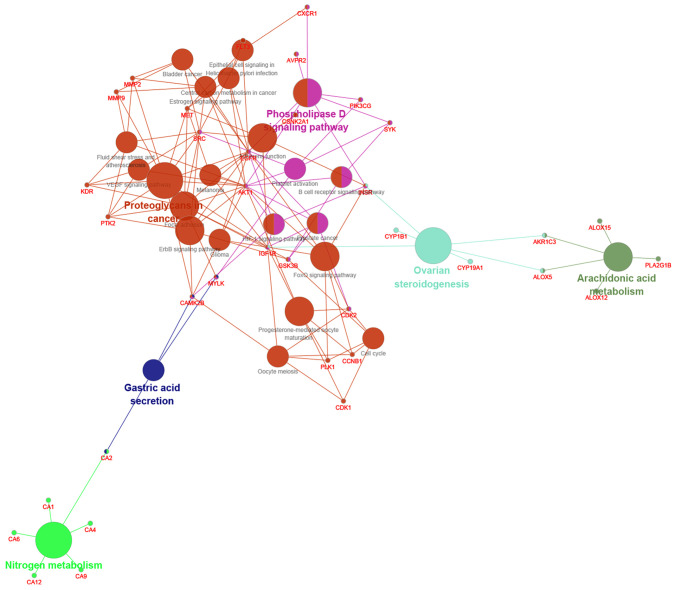
KEGG analysis for signaling pathways was used, showing that quercetin is associated with multiple signaling pathways, many of which can participate in the regulation of OA, as shown in Fig. 5. According to prediction results, there are certain associations between osteoarthritis and MAPK signal pathway, Wnt/β-catenin signal pathway, NF-κβ signal pathway, hypoxia-inducible factor (HIF)-1 signal pathway, Hedgehog signal pathway, Notch signal pathway and other associated pathways. The Wnt/β-catenin signaling pathway, which has been studied for a long time, has the function of differentiating osteoblasts and osteoclasts (27). MAPK signaling pathway is the signal transduction system that mediates and regulates degenerative changes fin cartilage in OA, and the activation of MAPK-associated signaling pathway can change the expression of matrix metalloproteinases and regulate a series of reactions, such as chondrocyte apoptosis and bone destruction (28). Pathological activation of the NF-κB signaling pathway induces a variety of inflammatory reactions in the body and affect the occurrence and development of arthritis diseases, whose role is to regulate the inflammatory response of cells (29). The Notch signal pathway is to ensure the phenotype of chondrocytes under different environments, regulate the differentiation of chondrocytes, and affects the metabolism of cartilage matrix, whose activation can affect the expression of MMP-13 and VEGFA, eventually leads to the destruction of articular cartilage (30,31).
Figure 5.
Kyoto Encyclopedia of Genes and Genomes analysis of predicted pathways. There are 29 signaling pathways that can participate in the regulation of osteoarthritis.
The p38 MAPK signaling pathway verified in the present study experiment is highly activated in OA articular chondrocytes, which is an important signaling pathway that mediates the pathological progress of OA. This signal pathway belongs to the MAPK signal pathway that was predicted through network pharmacology technology. Fan et al (32) found that the expression of phosphorylated p38 in the articular cartilage of patients with OA was significantly higher compared with that in normal articular cartilage tissue, which was consistent with the expression trend of ERK. Rasheed et al (33) detected the expression of p38α, p38γ and p38δ in OA chondrocytes, but did not detect the expression of p38β using RT-PCR, western blotting and other methods. Meanwhile, IL-1β could significantly increase p38α and p38γ phosphorylation, but did not have a significant effect on p38δ phosphorylation. Xu et al (34) clarified that the p38 MAPK signaling pathway plays a very important role in OA chondrocyte apoptosis using western blotting and other methods. At the same time, in the inflammatory cartilage matrix, activation of the p38 MAPK signaling pathway can induce the secretion of inflammatory cytokines (TNF-α, IL-1β, NO, SOD) and the expression of matrix metalloproteinases (MMP-3, MMP-9, MMP-13), depolymerization with platelet thrombin-sensitive protein domain and metal proteins (ADAMTS-4, ADAMTS-5), leading to the degradation of type II collagen, which in turn triggers chondrocyte apoptosis. Map2k3 and map2k6 were the main targets of p38 MAPK signaling pathway. The activation reaction showed that the three intracellular protein kinases were activated in turn, and MAPK was finally activated. Therefore, it is believed that the intervention of quercetin on the highly activated p38MAPK signaling pathway of OA articular cartilage can achieve the prevention and treatment of articular cartilage, based on predictions and molecular docking technology.
Related literatures demonstrated that there are many commonly used methods for establishing in vitro degenerative joint chondrocyte models. Qin et al (35) applied 10 ng/ml lipopolysaccharide (LPS) to interfere with normal chondrocytes for 8 h, and successfully obtained degenerative joint chondrocytes. LPS is composed of lipid A, specific polysaccharides and non-specific core polysaccharides, which can induce the expression of inflammatory factors such as IL-1β and TNF-α, but this method acts on the NF-κB pathway (36). Therefore, this method does not meet the requirements because the signal pathway target of the present study is the p38 MAPK pathway. There have been many studies using 10 ng ml IL-1β for the induction of normal chondrocytes for 24 h to successfully obtain degenerated chondrocytes (37,38). This method uses inflammatory factors to directly stimulate the cells to undergo inflammatory changes, which is similar to the pathogenesis of OA with high modeling success rate, and it is also one of the most widely used methods in research. In addition, there are also modeling methods using 10 µM trans-retinoic acid for 24 h or using NO to induce normal chondrocytes, but the use of these methods is still in the preliminary exploration stage, and they are not commonly used and have not been widely promoted (39). Thus, the most recommended methods are 10 ng ml IL-1β induction of normal chondrocytes for 24 h, 1 mM sodium nitroprusside intervention for 24 h, and 10 ng/ml LPS intervention for 8 h (40). The purpose of the study was to verify that quercetin protects articular cartilage through targeted regulation of the p38 MAPK signaling pathway, and IL-1β induction is more direct and rapid, which is closest to the pathogenesis of OA and has been used most frequently in the literature with higher rate of success.
Experiments on IL-1β-induced degeneration of cartilage cells showed that the expression level of TNF-α and IL-1β was significantly increased. The expression quantity of p38 was sharply increased and phosphorylated, generating P-p38. Then, the expression of type II collagen declined, while the expression of MMP-13 increased. It is postulated that TNF-α and IL-1β, as start factors in the p38 MAPK inflammatory signaling pathways, are increased in the degeneration of articular cartilage cells, and then p38 MAPK signaling pathways is activated, leading to its phosphorylation. As a result, phosphorylation levels increase after p38 MAPK signaling pathways are activated, which in turn will increase the secretion of TNF-α and IL-1β, inhibit the expression of type II collagen, and activate the expression of MMP-13, leading to further degeneration of articular cartilage cells. The degradation of type II collagen fibers plays a major role in the degeneration of cartilage. MMP-13 is 10 to 30 times as capable of degrading type II collagen fibers as other MMPs, and MMP-13 is also required for the degradation of other collagen fiber enzymes. MMPs are a family of zinc-dependent proteinases that are characterized by promoting the turnover of various ECM proteins, including collagens and proteoglycans. MMP13 is the major enzyme involved in OA cartilage erosion, due to its potent proteolytic effects on type II collagen (41). Thus, the inhibition of MMP-13 expression may impede type II collagen degradation. The target gene of quercetin protecting articular cartilage predicted by GO analysis is involved in the metabolism of NO, which also indicates that quercetin could protect articular cartilage. The ADAMTS protein family is involved in pathogenic cartilage degradation. The most efficient aggrecanases relevant to joint disease are ADAMTS-4 and ADAMTS-5(42). As for factors associated with the signal pathway, the expression level of TNF-α and IL-1β was significantly decreased with the intervention of quercetin, MAPK signaling pathway blocker SB 203580, or the two mixed; the expression of type II collagen rises, and that of p38, MMP-13, or ADAMTS-4 drops. Thus, it can be seen that p38 MAPK signaling pathway plays an important role in regulating the pathological progress of OA and is one of the important targets for the prevention and control of OA. Quercetin and blockers SB203580 both can inhibit the expression of inflammatory factor, MMPs, TNF, IL-1β, improve collagen Ⅱ expression to accelerate the degeneration of cartilage cell repair, and protect the articular cartilage cells. However, according to the experimental results, it is found that its effect is weaker compared with that of the blocker SB203580, which blocks the p38 MAPK signaling pathway but not completely, and its specificity needs to be further explored.
Based on the data from network pharmacology and in vitro experiments, it was demonstrated that quercetin could lower the expression of inflammatory factors of cartilage for the prevention and treatment of OA. By blocking the p38 MAPK signaling pathway, the expression level of associated factors can be improved. Quercetin can promote the repair of degenerative chondrocytes and protect articular chondrocytes.
Acknowledgements
Not applicable.
Funding Statement
Funding: Funding was provided by Jinan Science and Technology plan project (grant no. 201805044).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XPW and RXB contributed to the conception and design of the study, and the acquisition, analysis and interpretation of the data of the study. They also contributed to the drafting of the work and its critical revision for important intellectual content. WPX and SLW contributed to the conception and design of the study, the acquisition, analysis and interpretation of the data of the study, contributed to the drafting of the work and its critical revision for important intellectual content. BAW, YFB and HBS contributed to the acquisition, analysis and interpretation of data of the study, contributed to the drafting of the work and its critical revision for important intellectual content. XPW and RXB confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript and agreed to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All rats were used according to the national guidelines of the care and use of laboratory animals with the approval of the Animal Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine (approval no. AWE-2019-043).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lane NE, Shidara K, Wise BL. Osteoarthritis year in review 2016: Clinical. Osteoarthritis Cartilage. 2017;25:209–215. doi: 10.1016/j.joca.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Frank M, Bwemero J, Kalunga D, Sangu W, Semeni S, Hamisi M, Julius M. OA60 Public health and palliative care mix; a ccpmedicine approach to reverse the overgrowing burden of non-communicable diseases in tanzania. BMJ Support Palliat Care. 2015;5 (Suppl 1)(A19) doi: 10.1136/bmjspcare-2015-000906.60. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Kang D, Cho Y, Kim JH. Epigenetic regulation of chondrocyte catabolism and anabolism in osteoarthritis. Mol Cells. 2015;38:677–684. doi: 10.14348/molcells.2015.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mc Alindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker GA, Henrotin Y, Hunter DJ, Kawaguchi H, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu J, Zuo B, Zhao C, Wang C, Zhang X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8(e3140) doi: 10.1038/cddis.2017.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang X, Zhou Y, Sun H, Tan S, Lu Z, Huang L, Wang W. Ivabradine abrogates TNF-α-induced degradation of articular cartilage matrix. Int Immunopharmacol. 2019;66:347–353. doi: 10.1016/j.intimp.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Favero M, Belluzzi E, Trisolino G, Goldring MB, Goldring SR, Cigolotti A, Pozzuoli A, Ruggieri P, Ramonda R, Grigolo B, et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: A coculture study. J Cell Physiol. 2019;234:11176–11187. doi: 10.1002/jcp.27766. [DOI] [PubMed] [Google Scholar]
- 8.Basu A, Schell J, Scofield RH. Dietary fruits and arthritis. Food Funct. 2018;9:70–77. doi: 10.1039/c7fo01435j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanzaki N, Saito K, Maeda A, Kitagawa Y, Kiso Y, Watanabe K, Tomonaga A, Nagaoka I, Yamaguchi H. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: A randomized, double-blind, placebo-controlled study. J Sci Food Agric. 2012;92:862–869. doi: 10.1002/jsfa.4660. [DOI] [PubMed] [Google Scholar]
- 10.Leyva-López N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int J Mol Sci. 2016;17(921) doi: 10.3390/ijms17060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad R, Sylvester J, Ahmad M, Zafarullah M. Involvement of H-Ras and reactive oxygen species in proinflammatory cytokine-induced matrix metalloproteinase-13 expression in human articular chondrocytes. Arch Biochem Biophys. 2011;507:350–355. doi: 10.1016/j.abb.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang XY, Geng Q, Cui YH, Wang XH. lncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharmacol. 2017;50:283–290. doi: 10.1016/j.intimp.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Yuan X, Liu H, Huang H, Liu H, Li L, Yang J, Shi W, Liu W, Wu L. The key role of canonical Wnt/β-catenin signaling in cartilage chondrocytes. Curr Drug Targets. 2016;17:475–484. doi: 10.2174/1389450116666150825112623. [DOI] [PubMed] [Google Scholar]
- 14.Saito T, Tanaka S. Molecular mechanisms underlying osteoarthritis development: Notch and NF-κB. Arthritis Res Ther. 2017;19(94) doi: 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tat SK, Pelletier JP, Velasco CR, Padrines M, Martel-Pelletier J. New perspective in osteoarthritis: The OPG and RANKL system as a potential therapeutic target? Keio J Med. 2009;58:29–40. doi: 10.2302/kjm.58.29. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Daina A, Michielin O, Zoete V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47 (W1):W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid MedCell Longev. 2016;2016(1245049) doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanzaki H, Shinohara F, Kajiya M, Kodama T. The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem. 2013;288:23009–23020. doi: 10.1074/jbc.M113.478545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SW, Song YS, Shin SH, Kim KT, Park YC, Park BS, Yun I, Kim K, Lee SY, Chung WT, et al. Cilostazol protects rat chondrocytes against nitric oxide-induced apoptosis in vitro and prevents cartilage destruction in a rat model of osteoarthritis. Arthritis Rheum. 2008;58:790–800. doi: 10.1002/art.23220. [DOI] [PubMed] [Google Scholar]
- 21.Yasuhara R, Miyamoto Y, Akaike T, Akuta T, Nakamura M, Takami M, Morimura N, Yasu K, Kamijo R. Interleukin-1beta induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005;389:315–323. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–4909. doi: 10.1210/jc.2003-031673. [DOI] [PubMed] [Google Scholar]
- 23.McLean RR, Jacques PF, Selhub J, Fredman L, Tucker KL, Samelson EJ, Kiel DP, Cupples LA, Hannan MT. Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. J Clin Endocrinol Metab. 2008;93:2206–2212. doi: 10.1210/jc.2007-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: Role of distal sequences in the 3'-untranslated region of COX-2 mRNA. J Biol Chem. 2003;278:26897–26907. doi: 10.1074/jbc.M212790200. [DOI] [PubMed] [Google Scholar]
- 25.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Brzoska MM, Moniuszko-Jakoniuk J. Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: Studies on a rat model of human environmental exposure. Toxicol Sci. 2004;82:468–477. doi: 10.1093/toxsci/kfh275. [DOI] [PubMed] [Google Scholar]
- 27.Brzóska MM, Majewska K, Moniuszko-Jakoniuk J. Mineral status and mechanical properties of lumbar spine of female rats chronically exposed to various levels of cadmium. Bone. 2004;34:517–526. doi: 10.1016/j.bone.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, Amling M, Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;200:537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biniecka M, Connolly M, Gao W, Ng CT, Balogh E, Gogarty M, Santos L, Murphy E, Brayden D, Veale DJ, Fearon U. Redox-mediated angiogenesis in the hypoxic joint of inflammatory arthritis. Arthritis Rheumatol. 2014;66:3300–3310. doi: 10.1002/art.38822. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Chun JS. Protein kinase C alpha and zeta regulate nitric oxide-induced NF-kappa B activation that mediates cyclooxygenase-2 expression and apoptosis but not dedifferentiation in articular chondrocytes. Biochem Biophys Res Commun. 2003;303:206–211. doi: 10.1016/s0006-291x(03)00305-x. [DOI] [PubMed] [Google Scholar]
- 31.Hosaka Y, Saito T, Sugita S, Hikata T, Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI, Kawaguchi H. Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci USA. 2013;110:1875–1880. doi: 10.1073/pnas.1207458110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Z, Söder S, Oehler S, Fundel K, Aigner T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol. 2007;171:938–946. doi: 10.2353/ajpath.2007.061083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed Z, Akhtar N, Haqqi T. Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res Ther. 2010;12(R195) doi: 10.1186/ar3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L, Zhai L, Ge Q, Liu Z, Tao R. Vacuolar protein sorting 4B (VPS4B) regulates apoptosis of chondrocytes via p38 mitogen-activated protein kinases (MAPK) in osteoarthritis. Inflammation. 2017;40:1924–1932. doi: 10.1007/s10753-017-0633-2. [DOI] [PubMed] [Google Scholar]
- 35.Qin Y, Chen Y, Wang W, Wang Z, Tang G, Zhang P, He Z, Liu Y, Dai SM, Shen Q. HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death Dis. 2014;5(e1077) doi: 10.1038/cddis.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu XF, Zhou QG, Chen YY, Guo AZ, Hu CM. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-κB and MAPK signaling pathways. Inflammation. 2017;40:1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- 37.Lu Z, Liu Q, Liu L, Wu H, Zheng L, Zhao JM. A novel synthesized sulfonamido-based gallate-JEZTC blocks cartilage degradation on rabbit model of osteoarthritis: An in vitro and in vivo study. Cell Physiol Biochem. 2018;49:2304–2319. doi: 10.1159/000493832. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Zhu Z, Hu N, Liang X, Huang W. Leonurine hydrochloride suppresses inflammatory responses and ameliorates cartilage degradation in osteoarthritis via NF-κB signaling pathway. Inflammation. 2020;43:146–154. doi: 10.1007/s10753-019-01104-z. [DOI] [PubMed] [Google Scholar]
- 39.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- 40.Wu TJ, Lin CY, Tsai CH, Huang YL, Tang CH. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ Toxicol. 2018;33:1061–1068. doi: 10.1002/tox.22618. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Cao L, Gao X, Chen Z, Guo S, He Z, Qian Y, Yu Y, Wang G. Ghrelin prevents articular cartilage matrix destruction in human chondrocytes. Biomed Pharmacother. 2018;98:651–655. doi: 10.1016/j.biopha.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Gu Y, Zhao H, Zhang S. Loganin attenuates osteoarthritis in rats by inhibiting IL-1β-induced catabolism and apoptosis in chondrocytes via regulation of phosphatidylinositol 3-kinases (PI3K)/akt. Med Sci Monit. 2019;25:4159–4168. doi: 10.12659/MSM.915064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.