Abstract
Several polymorphisms in genes related to the ubiquitin-proteasome system exhibit an association with pathogenesis and prognosis of various human autoimmune diseases. Our previous study reported the association between multiple sclerosis (MS) and the PSMA3-rs2348071 polymorphism in the Latvian population. The current study aimed to evaluate the PSMA6 and PSMC6 genetic variations, their interaction between each other and with the rs2348071, on the susceptibility to MS risk and response to therapy in the Latvian population. PSMA6-rs2277460, -rs1048990 and PSMC6-rs2295826, -rs2295827 were genotyped in the MS case/control study and analysed in terms of genotype-protein correlation network. The possible association with the disease and alleles, single- and multi-locus genotypes and haplotypes of the studied loci was assessed. Response to therapy was evaluated in terms of ‘no evidence of disease activity’. To the best of our knowledge, the present study was the first to report that single- and multi-loci variations in the PSMA6, PSMC6 and PSMA3 proteasome genes may have contributed to the risk of MS in the Latvian population. The results of the current study suggested a potential for the PSMA6-rs1048990 to be an independent marker for the prognosis of interferon-β therapy response. The genotype-phenotype network presented in the current study provided a new insight into the pathogenesis of MS and perspectives for future pharmaceutical interventions.
Keywords: multiple sclerosis, PSMC6-PSMA6-PSMA3, rs1048990, molecular biomarkers, single nucleotide polymorphisms, no evidence of disease activity, proteasome, therapy interferon-β
Introduction
Multiple sclerosis (MS) is a lifelong demyelinating disease of the central nervous system. The clinical onset of MS tends to be between the second and fourth decade of life. Similarly to other autoimmune diseases, women are affected 3-4 times more frequently than men (1). About 10% of MS patients experience a primary progressive MS form characterized by the progression of neurological disability from the onset. In about 90% of MS patients, the disease undergoes the relapse-remitting MS course (RRMS); in most of these patients, the condition acquires secondary progressive course (SPMS) (2).
The cause of MS is not clear. It is widely believed to be an autoimmune disorder triggered in genetically predisposed subjects by environmental factors such as infection, exposure to sunlight, and vitamin D deficiency (3-5). The vitamin deficiency and other genetic factors may also contribute to the augmented production of proinflammatory cytokines such as IL-12, IL-18 and macrophage migration inhibitory factor that have been found during the active phases of the disease (6-9). Recent evidence indicated that these abnormal production of proinflammatory cytokines may reside in the hyperactivation of the inflammasome (10). Simultaneously, MS patients showed reduced production of anti-inflammatory cytokines such as IL-37 as well as endogenous anti-inflammatory mediators such as IL-1 receptor antagonist, the soluble IL-1 receptor type 2 and heme oxygenase I (11-14). MS susceptible loci have been identified in regions containing genes with immune, co-stimulatory, signal transduction functions and related to vitamin D function (15-21).
Ubiquitin proteasome system (UPS) plays a vital role in immunity and its deregulation and/or modulation may influence MS development and progression. The 20S proteasome has been identified as a target of the humoral autoreactive immune response (22) and a major autoantigen in MS patients (23). Proteolytic activities of proteasomes are reduced in the brain tissue of MS patients (24). Inhibition of proteasomes and lysosomal proteases involved in major histocompatibility complex (MHC) II antigen presentation has shown to improve MS therapeutic efficacy (22,25). Disease-modifying treatments significantly decrease the frequency of relapses and impede the progression of disability during MS (26). Interferon-β-1b (IFNβ-1b) reduces relapses in MS and improves magnetic resonance imaging (MRI) outcomes. Plasma levels of ubiquitin and proteasome enzymatic activity in MS patients before IFNβ-1b therapy have been shown to decrease after six months of treatment (25). In a similar manner, treatment with IFNβ augments other endogenous anti-inflammatory mediators such as TGFβ, Heme oxygenase 1 and IL-1 receptor antagonist thus pointing out to its pleiotropic pharmacological effects (12,14,27). Preliminary studies have shown that the potency of IFNβ can be increased further by simultaneous treatment with cyclophosphamide in patients with aggressive diseases (28). In this case, studies to identify pathways genes that predict a successful response to this treatment combination appear promising. Similarly, on the basis of analysis of genes and biomarkers it was possible to predict therapeutic effects of natalizumab (6,29-31).
Genetic variations in PSMA3 and PSMB9 proteasome genes were reported to be associated with the risk of MS in Latvians (32) and Italians (33). Polymorphisms of the 14q11-24 proteasome genes PSMA6 and PSMC6 were previously involved in susceptibility to juvenile idiopathic arthritis (JIA) (34), asthma in children (BA) (35) and obesity (OB) (36), type 2 and type 1 diabetes mellitus (T1DM and T2DM) (37-39), cardiovascular disorders (39,40) and adaptation to the environment of the population (41). Taking into account that the statistically proven phenomena of the coexistence of autoimmune diseases (42) might happen that some of these polymorphisms are associated with susceptibility to MS, disease prognosis and/or response to therapy.
The aim of the current study was to evaluate the association of genetic variations in the proteasome genes PSMA6 (rs2277460 and rs1048990) and PSMC6 (rs2295826 and rs2295827) encoding regulatory α-subunit and ATPase subunit respectively with MS development and response to the therapy in Latvians. Additionally, the interaction of the PSMA6 and PSMC6 polymorphisms with the rs2348071 locus in the PSMA3 genes were analysed on MS susceptibility. Further on, using publicly available SNPexp database (43) on correlations between SNP genotype and gene expression levels in lymphoblastoid cells of individuals included in the HapMap populations, we performed an in silico analysis of the association between the SNPs analysed and the expression levels of the number of previously reported MS gene candidates and genes involved in the interferon signalling pathway. Genotype-phenotype correlations identified in the study may provide a new insight on MS pathogenesis and response to the therapy.
Materials and methods
Case-control study
The case group consisted of 280 MS patients (mean age: 41.66±12.16 years) attending the Latvian Maritime Medicine Centre, Vecmilgravis Hospital. MS patients were diagnosed according to the revised 2010 McDonald criteria (44) and assigned to RRMS (187 patients; mean age: 38.88±12.81 years) and SPMS (93 patients; mean age: 51.20±9.94 years) groups. The control group of 305 individuals representing the total Latvian population was previously described (32,38). All controls were carefully assessed to exclude the diagnosis of any inflammatory disorders. Written consent was obtained from all participants of the study. The study was performed according to the Declaration of Helsinki and the study protocol was approved by the Central Medical Ethics Committee of Latvia (Protocol Nr. 01-29.1/17).
Treatment
Three disease-modifying agents, including IFNβ, glatiramer acetate and mitoxantrone, were used for the treatment of 210 MS patients. Details on therapy are given in Table I.
Table I.
Multiple sclerosis treatment and response to therapy.
| Patient number | ||||||
|---|---|---|---|---|---|---|
| Disease-modifying agent | Commercial name | Manufacturer | Formulation of treatment | Total treated | Respon-ders | Non-responders |
| IFNβ total | 148 | 37 | 111 | |||
| IFNβ-1a | Avonex® | Biogen Idec Limited | Intramuscular 30 µg once weekly | 25 | 10 | 15 |
| IFNβ-1a | Rebif® | Merck Serono; Merck KGaA | Subcutaneous 44 µg three times per week | 59 | 14 | 45 |
| IFNβ-1b | Betaseron® or Extavia® | Bayer AG | Subcutaneous 250 µg every other day | 64 | 13 | 51 |
| Glatiramer acetate | Copaxone® | TEVA Pharmaceutical Industries, Ltd | Subcutaneous 20 mg once a day | 39 | 4 | 35 |
| Mitoxan-trone | Novantrone® | Wyeth Laboratories | Intravenous once every 3 months | 23 | 0 | 23 |
Therapeutic forms of IFNβ can be produced in bacterial expression systems (IFNβ-1b) or in mammalian cells (IFNβ-1a). IFNβ, interferon β.
Response to therapy evaluation
Progress of disease and response to treatment were evaluated in terms of ‘no evidence of disease activity’ (NEDA) standard (45) measured by relapses, the progression of disability and new and/or enlarging demyelinating lesions as seen on MRI.
DNA extraction and genotyping
Genomic DNA extraction and the rs2277460, rs1048990, rs2295826 and rs2295827 genotyping technologies (primer sequences, necessary Polymerase Chain Reaction procedure and analysis of amplified and digested products) were the same as in (34,41). For quality control, 16 randomly chosen samples per each marker were genotyped in two different experiments. Genotyping data were verified by direct sequencing of the corresponding DNA fragments in both directions using Applied Biosystems 3130xl Genetic Analyzer (Fig. S1). Loci description and nucleotide numbering are given according to the recommended nomenclature system (http://www.genomic.unimelb.edu.au/mdi/mutnomen/recs.html). Chromosome 14, GRCh37.p5 assembly (NCBI reference sequence: NC_000014.8) sequence information was used for loci description.
Data analysis
Single locus genotypes' and alleles' frequencies were estimated in the MS group by direct gene counting. The genetic diversity of SNPs rs2277460, rs1048990, rs2295826 and rs2295827 was studied previously by Sjakste et al, 2016(38) in the control group. Genotyping data on the rs2348071 variations in MS patients were described by Kalnina et al, 2014(32). These primary genotyping data were used in the current case/control study to construct multi-locus genotypes, haplotypes and identify the main effects of MS in single- and multi-locus models. Deviation from the Hardy-Weinberg equilibrium (HWE) and the differences between case and control groups in allele, genotype and haplotype frequencies were evaluated by χ2 using XLSTAT software for Windows (Data Analysis and Statistical Solution for Microsoft Excel, https://www.xlstat.com/en, Addinsoft, Paris, France 2017). Dominant, recessive, over dominant and multiplicative genetic models for every individual locus were designed according to (46), and two- or one-tailed P-value (significant <0.05) was evaluated by χ2 using 2x2 contingency tables. Odds ratio (OR) higher than two (2) and lower than 0.5 was considered to be clinically significant (46). Stratification was performed by MS course, NEDA standard and response to the therapy.
Genotype-phenotype correlation analysis
Data on the correlation between SNPs' genotypes and expression levels of the number of genes potentially involved in MS pathogenesis (15-20) and/or response to the IFNβ therapy (PathCards, https://pathcards.genecards.org/card/immune_response_ifn_alphabeta_signaling_pathway) were derived using SNPexp online tool [http://tinyurl.com/snpexp., Norwegian PSC Research Center, Rikshospitalet, Oslo, Norway (43)] for lymphoblastoid cells of all unrelated individuals of the HapMap phase II release 23 data set which consist of 3.96 million SNP genotypes from 270 individuals from four populations: CEU (90 Utah residents with ancestry from northern and western Europe), HCB (45 unrelated Han Chinese in Beijing), JPT (45 unrelated Japanese in Tokyo), YRI (90 Yoruba in Ibadan, Nigeria). Dominant and additive genetic models were tested for correlation analysis. The Spearman' correlation between genotype and the gene expression level was considered to be positive if at least one probe per transcript showed a statistically significant association (P<0.05).
Taking into account that in the SNPexp platform (43) one gene can be represented by several probes, but one probe maps only one gene, the results were manually filtered: 1) To exclude possible false calls when one probe is found to belong to several genes; 2) to optimize repeated data when several probes map to one gene; 3) to exclude potentially false calls identified for the rs2295826, but not for the rs2295827. Only Spearman correlations with P-value less than 0.05 or significant correlation found for both the rs2295826 and rs2295827 were taken into account. The data obtained as a result of filtration is given in Tables SI and SII.
Results
Genotyping results and single-locus association analysis
In the current study, we have genotyped the rs2277460, rs1048990, rs2295826 and rs2295827 in 280 MS patients. In Latvia there are 2035 registered MS patients in total. The study can be considered to be a representative for the Latvian population, as 13.7% of all patients were covered by our study. Since significance was achieved and revealed the trend on the association with the disease, our results appeared to predict the common association trends for larger sample groups, with the possibility of further studies reproduced in other populations.
Data on alleles' and genotypes' distributions and single-locus associations are given in Table II. The Genotyping call rate was 100% and all genotyped markers were found to be in a perfect HWE (P>0.05). The rs2295826 and rs2295827 were observed in a complete (D'=1, r2=1) linkage disequilibrium and in further analysis, the linkage block rs2295826-rs2295827 will be represented by the rs2295826 locus. No linkage had been identified between other polymorphic loci analysed (P>0.05).
Table II.
SNP genotype and MA frequencies and data on association with MS.
| Data on association | |||||
|---|---|---|---|---|---|
| SNP ID and MA or genotype | MS (n=280) | Controls (n=305) | Genetic model | P-value | OR [95% CI] |
| rs2277460 | |||||
| A | 51 (9.11) | 44 (7.21) | A vs. C | n/s | - |
| CC | 229 (81.79) | 261 (85.57) | - | - | - |
| CA | 51 (18.21) | 44 (14.43) | CA vs. CC | n/s | - |
| rs1048990 | |||||
| G | 50 (8.93) | 57 (9.34) | G vs. C | n/s | - |
| CC | 233 (83.21) | 250 (81.97) | - | - | - |
| CG | 44 (15.72) | 53 (17.38) | CG + GG vs. CC | n/s | - |
| GG | 3 (1.07) | 2 (0.65) | - | - | - |
| rs2295826 | |||||
| G | 96 (17.14) | 69 (11.31) | G vs. A | 0.0042 | 1.62 [1.16-2.26] |
| AA | 187 (66.79) | 243 (79.67) | - | - | - |
| AG | 90 (32.14) | 55 (18.03) | AG + GG vs. AA | 0.0004 | 1.95 [1.34-2.83] |
| GG | 3 (1.07) | 7 (2.30) | - | - | - |
| rs2295827 | |||||
| T | 96 (17.14) | 69 (11.31) | T vs C | 0.0042 | 1.62 [1.16-2.26] |
| CC | 187 (66.79) | 243 (79.67) | - | - | - |
| CT | 90 (32.14) | 55 (18.03) | CT + TT vs. CC | 0.0004 | 1.95 [1.34-2.83] |
| TT | 3 (1.07) | 7 (2.30) | - | - | - |
Numbers of alleles and genotypes (in brackets, frequency in percent) in control are given according to (38). MS and Control data are presented as n (%). MA, minor allele; MS, multiple sclerosis; OR [95% CI], odds ratio with 95% confidential interval, indicated when n/s (no statistical result or P>0.05); SNP, single nucleotide polymorphism.
The rs2277460 and rs1048990 exhibited similar alleles' and genotypes' frequencies in the cases and controls and appeared to be MS neutral.
The linkage block rs2295826-rs2295827 showed statistically significant MS main effect for both rare alleles [P<0.05; odds ratio, OR=1.62, 95% confidence interval (CI) (1.16-2.26)] and genotypes in the dominant model [P<0.001; OR=1.95, 95% CI (1.34-2.83)].
Identification of the risk/protective multi-loci genotypes
Using personalized data documentation, we reconstructed individual multi-loci genotypes and tested them on the association with the disease. Full-spectrum and frequencies of the rs2277460-rs1048990-rs2295826-rs2295827-rs2348071 genotypes is presented in Table SIII. Genotype having the homozygotes on major alleles simultaneously at the rs2277460 and rs1048990 and MS risk genotypes at the rs2295826-rs2295827 and rs2348071 showed a strong association with the disease [4-LG7, P=0.0001; OR=3.01, 95% CI (1.70-5.33)]. Genotypes including risk alleles at loci rs2277460, rs2295826-rs2295827 and rs2348071 (4-LG12) and risk genotypes at loci rs2277460 and rs2295826-rs2295827 (4-LG11) were found about two times more frequently in MS patients than in controls.
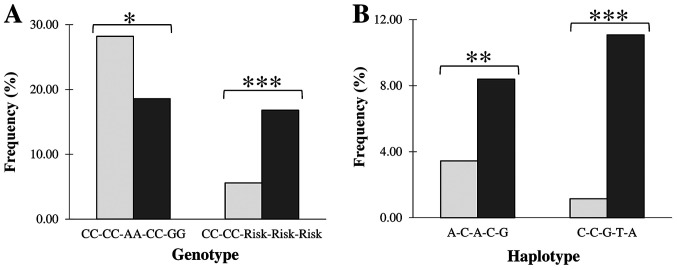
Genotype homozygous on major alleles at all five loci was the most frequent in controls (28%) and second by frequency in MS patients (~18%) showing significant disease protective effect [P<0.05; OR=0.31, 95% CI (0.66-0.96), Fig. 1A, Table SIII].
Figure 1.
Presentation of the controls and patients with multiple sclerosis of the (A) 5 loci genotypes and (B) 5 loci haplotypes associated with the disease in the Latvian population. Light and dark columns correspond to controls and cases, respectively. *P<0.05, **P<0.001 and ***P<0.0001 correspond to nominal, moderate and strong levels of statistical significance calculated by χ2 or Fisher's exact test.
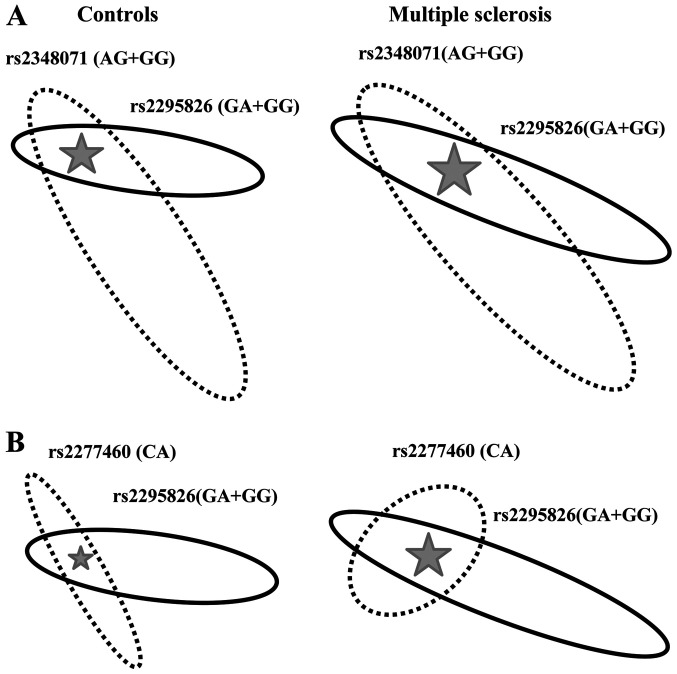
Further on, we analysed the interaction between any two loci (the rs2295826-rs2295827 linkage block was considered as one locus) on MS susceptibility. Data on statistically significant interactions are illustrated in Fig. 2.
Figure 2.
Visualization of the interaction between the components of multi-loci genotypes. (A) Interaction between the rs2295826-rs2295827 and rs2348071 MS risk genotypes. (B) Interaction between the rs2277460 rare CA genotype and MS risk genotypes at the rs2295826-rs2295827. Linkage block rs2295826-rs2295827 is represented by the rs2295826 locus. Two-loci genotypes associated with MS are localized in the cross of ellipses and marked by stars. The Square of the ellipses corresponds to the genotype frequency of the corresponding loci. MS, multiple scleroses.
The rs2295826-rs2348071 Risk-Risk genotype, was more than twice more frequent in MS patients (22.14%) than in controls (8.54%) and showed strong (P<0.0001) association with the disease [OR=3.60, 95% CI (1.60-4.22); Fig. 2A]. The rs2277460-rs2295826 genotype of the CA-Risk structure rarely observed in controls (2.63%) was more frequent in MS patients (6.43%) showing nominal (P<0.05) association with the disease [OR=2.45, 95% CI (1.05-5.73); Fig. 2B].
Haplotype analysis
Results on haplotype analysis are given in the Table SIV and illustrated in Fig. 1B.
Haplotype diversity was similar in both groups of MS patients and healthy individuals. Although, the rs2277460 locus did not show MS susceptibility in a single-locus analysis, the haplotype of the A-C-A-C-G structure (Hap6) showed a significant association with the disease [P<0.001; OR=2.44 95% CI (1.45-4.11), Fig. 1B].
The rs2277460-rs1048990-rs2295826-rs2295827-rs2348071 haplotype of the C-C-G-T-A composition (Hap8) having MS risk alleles at the rs2295826-rs2295827 and rs2348071 loci simultaneously, showed strong (P<0.0001) association with the disease [OR=9.65 95% CI (4.49-20.76), Fig. 1B] supporting the above presented data on the single- and multi-loci genotype susceptibility.
Polymorphism association with the response to therapy
Patients did not respond to mitoxantrone therapy. Among 39 patients treated by glatiramer acetate, only four were susceptible to this treatment. In the group of 148 patients treated with IFNβ, 37 responders were detected (Table I).
Genotyping data were further analysed for the association with response to IFNβ therapy (Table III). Alleles' and genotypes' distribution were found to be similar between responders and non-responders at the rs2277460, rs2295826-rs2295827 and rs2348071. However, the G allele at the rs1048990 was approximately twice more frequent in non-responders than in responders and the association of the G allele with no IFNβ therapy response was found to be close to statistical significance [Table III; OR=2.53 95% CI (0.90 7.08) in the multiplicative model].
Table III.
The rs1048990 alleles and genotypes presentation in responders and non-responders to IFNβ therapy.
| IFN-β (n) | Statistics | ||||
|---|---|---|---|---|---|
| Allele or genotype | R | NR | Model | P-value | OR [95% CI] |
| C | 70 (94.59) | 194 (87.39) | G vs. C | 0.059 | 2.53 [0.90-7.08] |
| G | 4 (5.41) | 28 (12.61) | G vs. CC | 0.056 | 2.69 [0.92-7.84] |
| CC | 33 (89.19) | 86 (77.48) | - | - | - |
| CG | 4 (10.81) | 22 (19.82) | CG + GG vs. CC | 0.090 | 2.40 [0.78-7.42] |
| GG | - | 3 (2.70) | - | - | - |
The numbers of alleles and genotypes (in brackets, frequency in percent) in the groups of study participants are presented as n (%). R, responders; NR, non-responders; IFN-β, interferon β; OR, odds ratio; CI, confidence interval.
Correlation between SNPs' genotype and gene expression
Suggesting the potential for genetic variations to produce a local or distant functional effect in the genome, we analysed the further correlation between genotypes of the rs1048990, rs2295826-rs2295827 and rs2348071, identified in the current and previous (32) studies as MS susceptible and expression levels of 13 well-characterized genes associated with the susceptibility to MS (15-20) and 21 genes involved in IFNα/β signalling pathway (PathCards, https://pathcards.genecards.org/card/immune_response_ifn_alphabeta_signaling_pathway). The name of the CD25 gene was not found on the expression chips. A statistically significant correlation between mRNA transcript level and genotypes of any SNP was found for six genes implicated in MS pathogenesis and nine genes involved in IFN pathways (Tables IV and SII).
Table IV.
Data on correlation between mRNA expression and the rs1048990, rs2295826-rs2295827 and rs2348071 genotypes.
| Gene group | In correlation | Without correlation | Not found on the expression chip | Total |
|---|---|---|---|---|
| MS | CD58a,d, HLA-Aa, HLA-Bb,c, IL7Ra,d, STAT3a,d, TNFRSF5 (CD40)a,d | CD6, HLA-DRB1, IL2RA, IL12A, IRF8 (ICSBP), TNFRSF1A | CD25 | 13 |
| IFN | IFI6 (G1P3)d, IFNA1 (IFNα)a, IRF1a, IRF2d, PRMT1 (HMRT1L2)d,PTP-1B (PTPN1)a,d, SHP-2 (PTPN11)d, TYK2a, USP18a,d | IFNAR1, IFNAR2, IFNB1, ISG15 (G1P2), ISG54 (IFIT2), ISGF3 (IRF9), JAK1, PML (MYL), SHP-1 (PTPN6), SOCS1, STAT1, STAT2 | - | 21 |
| Total | 15 | 19 | 1 | 34 |
MS, genes implicated in pathogenesis of multiple sclerosis (15-20); IFN, genes involved in IFNα/β signalling pathway (PathCards, https://pathcards.genecards.org/card/immune_response_ifn_alphabeta_signaling_pathway).
ars1048990,
brs2295826,
crs2295827 and
drs2348071.
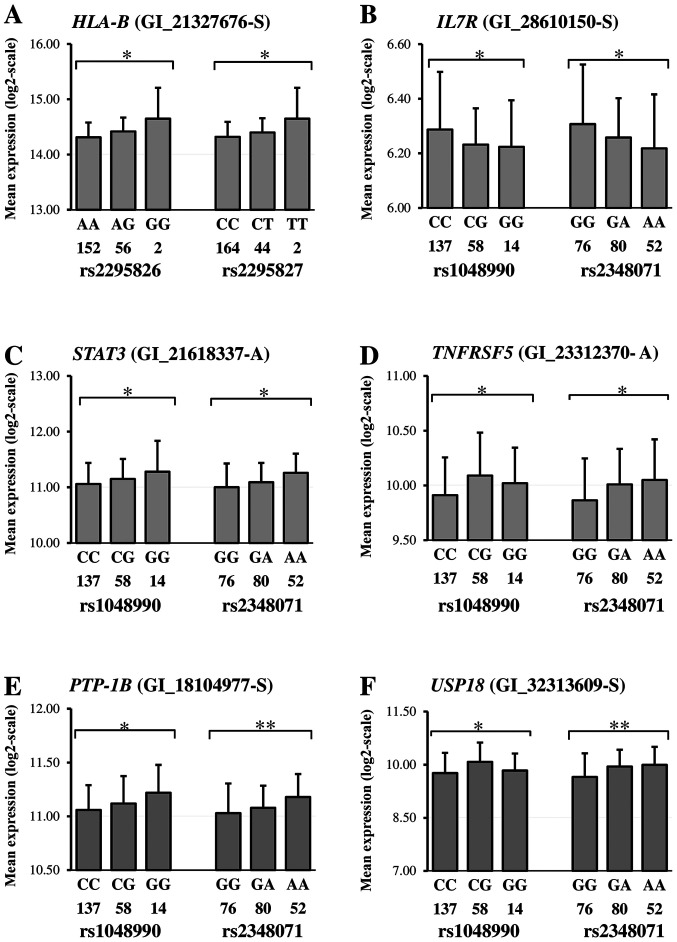
Examples of correlations revealed and details on the correlated genes are given in Fig. 3 and Table SII, respectively. Risk genotypes of the rs1048990 and rs2348071 were found in a simultaneous correlation with expression levels of the IL7R (Fig. 3B), STAT3 (Fig. 3C), TNFRSF5 (Fig. 3D), PTP-1B (Fig. 3E) and USP18 (Fig. 3F). Fig. 3A illustrated the situation when the HLA-B expression level correlates with the rs2295826 and rs2295827.
Figure 3.
Gene expression according to genotypes. Genotyping data (number under genotype) and mRNA expression levels of the (A) HLA-B, (B) IL7R, (C) STAT3, (D) TBFRSF5, (E) PTP-1B and (F) USP18 were obtained from the HapMap phase II release 23 data set for lymphoblastoid cells of all unrelated individuals using publicly available SNPexp online tool (43). Each gene symbol is followed by the probe name indicated in brackets. Information on SNP genotypes and their quantitative presentation is given in the legends for the horizontal axis. Differences in mRNA expression between genotypes are given according to the dominant model and symbols. *P<0.05 and **P<0.001. SNP, single nucleotide polymorphisms; PTP-1B, Protein tyrosine phosphatase 1B; USP18, Ubiquitin specific peptidase 18.
Discussion
The main finding of this study is that the PSMC6-rs2295826-rs2295827 and PSMA3-rs2348071 loci showed MS susceptibility, and data on single-locus associations were confirmed by haplotype and multi-loci genotype analysis. Remarkably, MS susceptible to rs2277460-rs1048990-rs2295826-rs2348071 haplotypes of the A-C-A-C-G and C-C-G-T-A configuration is identified in this study (Fig. 1B) were found to be linked with poly-JIA and oligo-JIA, respectively (41), and T1DM (38). Similarly, multi-loci genotype CC-CC-Risk-Risk-Risk, associated with MS risk effect in this study (Fig. 1A), is also a risk factor for T1DM (38), BA (35) and JIA (41). We did not find any data about the association of the polymorphisms studied by us with MS in the literature. However, haplotypes encompassing the KIAA0391 localized in 14q13.2 and PSMA6 gene cluster confer a genetic link for myocardial infarction and coronary artery disease (47). Additionally, in a GWAS study performed by the International Multiple Sclerosis Consortium (48) on association of SNP rs12436216 of the gene KIAA0391, it was reported that 14q13.2 could be a MS susceptibility locus.
Some loci of susceptibility may be shared among many autoimmune and other immune-mediated diseases (49,50). Earlier, the pleiotropic genetic effect as a frequent phenomenon in human complex traits and diseases (51) has been reported for BA and OB (52,53) and for BA and juvenile rheumatoid arthritis (54,55). Similarly, rs2277460 associated with BA in our previous study (35) has been found to determine susceptibility in Latvians with JIA (34) and T1DM (38). This locus turned out not to be associated with MS primary cohort in a single-locus analysis in Latvians, however, it was found in the block of interaction of risk loci PSMA6-rs2277460-PSMC6-rs2295826, which was more frequent in disease's group (Fig. 2B). This data was confirmed by haplotype analysis: Haplotype A-C-A-C-G included only the rs2277460 risk allele A and showed a significant association with MS [P<0.001; OR=2.44 95% CI (1.45-4.11), Fig. 1B].
In silico analysis revealed (34,41) that the nucleotide substitutions we have studied, modified transcription factor (TFs) binding sites and miRNAs. This can significantly modulate the transcription of related genes and gene networks in response to the inflammation and other environmental stimuli.
The PSMA6-rs2277460 and PSMA3-rs2348071 rare alleles and the PSMC6-rs2295826 major allele assisted the cytoplasmic ribonucleoprotein hnRNPA1 targeting. This multifunctional protein is involved in the pathogenesis of many neurodegenerative diseases, including MS (45,56,57). It can influence protein-protein interactions, including those involving NF-κB, which are known to play a critical role in the initiation and progression of MS (58,59) and participate in the crosstalk with UPS at different levels of the NF-κB signalling pathways (60). This splicing signal, affecting splicing and post-transcriptional modification of the majority of expressed genes in mammals, was shown to directly interact with PSMA3 proteins (61) and may be involved in the regulation of the PSMA3 gene expression through the rs2348071 allele-specific targeting.
PSMA6-rs1048990 rare allele G generates binding sites for multifunctional p53 protein, overexpression of which can induce apoptosis of oligodendrocytes in MS (62). The PSMA6-rs2277460 substitution to A, produces binding sites for BARBIE box proteins, which are shown to be involved in innate immunity modulation (63). The major allele of PSMC6-rs2295826 generates sequence affinity to transcription factors of MYT1 and PARF families known to be involved in primary neurogenesis; substitution at the PSMC6-rs2295827 can bind the BRN5 and LHXF factors and replacement at the PSMA3-rs2348071 may significantly change patterns of the local targets for regulatory proteins of MEF2 and HBPX families known to mediate transcriptional control of neuronal differentiation (64-67); HOXF family NANOG.01 is involved in signal transduction pathways during development (68).
Previously, PSMA6-rs1048990 had shown to affect PSMA6 gene expression in vivo (69) and in vitro (40). However, it should be taken into account that the data on the possibility of a particular genetic variant to have multiple transcription effects are limited.
To explain the above speculation it should be kept in mind also, that changes in the expression of different proteasome subunits in response to outer factors is not co-ordinated. For example, cocaine mainly upregulates PSMB1 and PSMA5 subunits, decreases PSMA6 subunit, but does not affect PSMB2 and PSMB5 subunit expression (70). Similar ‘chaotic’ changes in proteasome gene expression can happen in pathogenesis of the diseases, including MS. General expression of the proteasomes is increased in MS and, decreased by the interferon therapy (25). At the first stage of this process, the expression of the PSMA6 gene could increase, which can further promote the formation of more proteasomes with a subsequent increase in their activity. The same might happen with immune proteasome expression. The composition of α subunits in ‘conventional’ and immune proteasomes is identical. Thus, higher accessibility of PSMA6 subunits can favour initial increase of the formation of ‘conventional’ proteasomes with the transformation to immune proteasomes.
Based on the present and previously published results (38), we propose a concept for correlations between genotypes involved in MS risk and the level of expression of MS gene candidates. We should keep in mind that the expression studies in silico using data obtained for the lymphoblastoid cell line is a standard approach used for the MS studies (16).
A group of well-known MS gene candidates (Tables IV and SII) is represented by genes encoding the lymphocyte function-associated antigen 3 (CD58), MHC antigens (HLA-A and HLA-B), interleukin-7 receptor subunit α (IL7R), signal transducer and activator of transcription 3STAT3, tumor necrosis factor receptor superfamily member 5 [TNFRSF5 (CD40)].
The gene group involved in IFNα/β signalling pathway (Tables IV and SII) includes: Genes encoding interferon-α inducible protein 6 and interferon-α1 (IFI6 (G1P3) and IFNA1 (IFNα), respectively), representatives of interferon regulatory factors (IRF1 and IRF2), proteins related to enzyme binding and protein domain specific binding (PRMT1 (HMRT1L2), PTP-1B (PTPN1), SHP-2 (PTPN11, TYK2 and USP18)).
So, the expression of MS susceptible genes coding proteins of immune function (CD58, IL7R, STAT3, TNFRSF5 (CD40)) and genes involved in the IFN pathway (PTP-1B (PTPN1) and USP18) was found to be in a statistically significant correlation with the rs1048990 and rs2348071 risk genotypes simultaneously (Fig. 3B-F). Besides, among genes involved in IFNα/β signalling pathway the expression levels of IFI6 (G1P3), IRF2, PRMT1 (HMRT1L2) and SHP-2 (PTPN11) were found to be in a correlation only with PSMA3-rs2348071, but IFNA1 (IFN-α), IRF1 and TYK2 genes correlate only with the PSMA6-rs1048990 (Table IV). Loci PCMC6-rs2295826 and rs2295827, were identified as susceptible to MS cohort in this study, are in a correlation with HLA-B encoding the MHC antigen expression level (Fig. 3A).
These results prove that the possibility of the combined functional effect of genetic risk factors on various metabolic aspects of MS pathogenesis is parallel with the potential for each SNP to have its own transcriptional effect on the genes involved in this study.
UPS components possess the potential of being a therapeutic target for the treatment of several diseases (71). IFNβ is a pleiotropic cytokine belonging to the type I interferons; it is involved in the regulation of innate and adaptive immune responses and is widely used as the first-line disease-modifying treatment for multiple sclerosis (72). Interestingly, among the studied loci, the association of the G allele at the PSMA6-rs1048990 with no IFNβ therapy response in the Latvian population in the current study was found to be close to statistical significance (Table III). It might be that with the increase in the number of patients in the studied cohorts, the significance of statistical reliability in this associative study can increase. The above data provided evidence of the cumulative effect of immune-response genes on IFNβ treatment efficacy.
The PSMA6-rs1048990-rs2277460, PSMC6-rs2295826-rs2295827 and PSMA3-rs2348071 single- and multi-loci variations may contribute to the risk of MS in Latvians. The nucleotide substitutions may either have their own functional effect on transcription of numerous genes involved in MS pathogenesis or may be linked to other MS-susceptible genetic variations.
Our results suggested the potential for the rs1048990 of the PSMA6 gene to be an independent marker for the prognosis of the IFNβ therapy response.
Supplementary Material
Acknowledgements
The authors would like to thank Ms Natalija Mjagkova (Latvian Maritime Medicine Centre, Riga, Latvia) for technical assistance.
Funding Statement
Funding: The current study was funded by the European Regional Development Fund (project no. 1.1.1.1/16/A/016; 'Identification of proteasome related genetic, epigenetic and clinical markers for multiple sclerosis.').
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TS conceived of the study and acquired the funding. KDo and KDi analyzed and interpreted the data. IT performed the formal analysis. NP, KDo and KDi performed the experiments. IT and TS conceived of the methodology. NS and NP obtained resources and were associated with the project administration. KDo and KDi ran the software. JK performed data validation. NP, JK and NS confirmed the authenticity of all the raw data. NP and TS wrote the original draft of the manuscript. NP, KD, IT and NS wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed according to the Declaration of Helsinki and the study protocol was approved by the Central Medical Ethics Committee of Latvia (protocol no. 01-29.1/17). Informed consent was obtained from all individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Compston A, Confavreux C. The distribution of multiple sclerosis. In: McAlPine's multiPle Sclerosis. 4th edition. Pioli S (ed). Churchill Livingstone/Elsevier, Philadelphia, pp69-180, 2006. [Google Scholar]
- 2.Bellavista E, Santoro A, Galimberti D, Comi C, Luciani F, Mishto M. Current understanding on the role of standard and immunoproteasomes in inflammatory/immunological pathways of multiple sclerosis. Autoimmune Dis. 2014;2014(739705) doi: 10.1155/2014/739705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AE. Multiple sclerosis: Where will we be in 2020? Mt Sinai J Med. 2011;78:268–279. doi: 10.1002/msj.20242. [DOI] [PubMed] [Google Scholar]
- 4.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 5.Song GG, Choi SJ, Ji JD, Lee YH. Genome-wide pathway analysis of a genome-wide association study on multiple sclerosis. Mol Biol Rep. 2013;40:2557–2564. doi: 10.1007/s11033-012-2341-1. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti F, Patti F, Cocuzza C, Zaccone P, Nicoletti A, Di Marco R, Reggio A. Elevated serum levels of interleukin-12 in chronic progressive multiple sclerosis. J Neuroimmunol. 1996;70:87–90. doi: 10.1016/s0165-5728(96)00101-4. [DOI] [PubMed] [Google Scholar]
- 7.Nicoletti F, Di Marco R, Mangano K, Patti F, Reggio E, Nicoletti A, Bendtzen K, Reggio A. Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology. 2001;57:342–244. doi: 10.1212/wnl.57.2.342. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli E, Mazzon E, Basile MS, Mangano K, Di Marco R, Bramanti P, Nicoletti F, Fagone P, Petralia MC. Upregulated expression of macrophage migration inhibitory factor, its analogue D-dopachrome tautomerase, and the CD44 receptor in peripheral CD4 T cells from clinically isolated syndrome patients with rapid conversion to clinical defined multiple sclerosis. Medicina (Kaunas) 2019;55(667) doi: 10.3390/medicina55100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagone P, Mazzon E, Cavalli E, Bramanti A, Petralia MC, Mangano K, Al-Abed Y, Bramati P, Nicoletti F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J Neuroimmunol. 2018;322:46–56. doi: 10.1016/j.jneuroim.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Govindarajan V, de Rivero Vaccari JP, Keane RW. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J Neuroinflammation. 2020;17(260) doi: 10.1186/s12974-020-01944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalli E, Mazzon E, Basile MS, Mammana S, Pennisi M, Fagone P, Kalfin R, Martinovic V, Ivanovic J, Andabaka M, et al. In silico and in vivo analysis of IL37 in multiple sclerosis reveals its probable homeostatic role on the clinical activity, disability, and treatment with fingolimod. Molecules. 2019;25(20) doi: 10.3390/molecules25010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicoletti F, Patti F, DiMarco R, Zaccone P, Nicoletti A, Meroni P, Reggio A. Circulating serum levels of IL-1ra in patients with relapsing remitting multiple sclerosis are normal during remission phases but significantly increased either during exacerbations or in response to IFN-beta treatment. Cytokine. 1996;8:395–400. doi: 10.1006/cyto.1996.0054. [DOI] [PubMed] [Google Scholar]
- 13.Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, Nicoletti F, Drulovic J. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: The elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. J Neuroimmunol. 2009;207:101–106. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Fagone P, Patti F, Mangano K, Mammana S, Coco M, Touil-Boukoffa C, Chikovani T, Di Marco R, Nicoletti F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J Neuroimmunol. 2013;261:82–86. doi: 10.1016/j.jneuroim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009;41:824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 16.IL12A, MPHOSPH9/CDK2AP1 and RGS1 are novel multiple sclerosis susceptibility loci. Genes Immun. 2010;11:397–405. doi: 10.1038/gene.2010.28. International Multiple Sclerosis Genetics Conssortium (IMSGC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, de Jager PL, de Bakker PI, Gabriel SB, Mirel DB, et al. Risk allels for multiple sclerosis identified by a genome wide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. International Multiple Sclerosis Genetics Consortium. [DOI] [PubMed] [Google Scholar]
- 18.De Jager PL, Jia X, Wang J, de Bakker PI, Ottoboni L, Aggarwal NT, Piccio L, Raychaudhuri S, Tran D, Aubin C, et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet. 2009;41:776–782. doi: 10.1038/ng.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kofler DM, Severson CA, Mousissian N, De Jager PL, Hafler DA. The CD6 multiple sclerosis susceptibility allele is associated with alterations in CD4+ T cell proliferation. J Immunol. 2011;187:3286–3291. doi: 10.4049/jimmunol.1100626. [DOI] [PubMed] [Google Scholar]
- 20.Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang K, Chang S, Cui S, Guo L, Zhang L, Wang J. ICSNPathway: Identify candidate causal SNPs and pathways from genome-wide association study by one analytical framework. Nucleic Acids Res. 2011;39:W437–W443. doi: 10.1093/nar/gkr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fissolo N, Kraus M, Reich M, Ayturan M, Overkleeft H, Driessen C, Weissert R. Dual inhibition of proteasomal and lysosomal proteolysis ameliorates autoimmune central nervous system inflammation. Eur J Immunol. 2008;38:2401–2411. doi: 10.1002/eji.200838413. [DOI] [PubMed] [Google Scholar]
- 23.Mayo I, Arribas J, Villoslada P, Alvarez DoForno R, Rodríguez-Vilariño S, Montalban X, De Sagarra MR, Castaño JG. The proteasome is a major autoantigen in multiple sclerosis. Brain. 2002;125:2658–2667. doi: 10.1093/brain/awf274. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Bizzozero OA. Decreased activity of the 20S proteasome in the brain white matter and gray matter of patients with multiple sclerosis. J Neurochem. 2011;117:143–153. doi: 10.1111/j.1471-4159.2011.07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minagar A, Ma W, Zhang X, Wang X, Zhang K, Alexander JS, Gonzalez-Toledo E, Albitar M. Plasma ubiquitin-proteasome system profile in patients with multiple sclerosis: Correlation with clinical features, neuroimaging, and treatment with interferon-beta-1b. Neurol Res. 2012;34:611–618. doi: 10.1179/1743132812Y.0000000055. [DOI] [PubMed] [Google Scholar]
- 26.Pardo G, Jones DE. The sequence of disease-modifying therapies in relapsing multiple sclerosis: Safety and immunologic considerations. J Neurol. 2017;264:2351–2374. doi: 10.1007/s00415-017-8594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicoletti F, Di Marco R, Patti F, Reggio E, Nicoletti A, Zaccone P, Stivala F, Meroni PL, Reggio A. Blood levels of transforming growth factor-beta 1 (TGF-beta1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-beta1b) Clin Exp Immunol. 1998;113:96–99. doi: 10.1046/j.1365-2249.1998.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patti F, Cataldi ML, Nicoletti F, Reggio E, Nicoletti A, Reggio A. Combination of cyclophosphamide and interferon-beta halts progression in patients with rapidly transitional multiple sclerosis. J Neurol Neurosurg Psychiatry. 2001;71:404–407. doi: 10.1136/jnnp.71.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagone P, Mazzon E, Mammana S, Di Marco R, Spinasanta F, Basile MS, Petralia MC, Bramanti P, Nicoletti F, Mangano K. Identification of CD4+ T cell biomarkers for predicting the response of patients with relapsing-remitting multiple sclerosis to natalizumab treatment. Mol Med Rep. 2019;20:678–684. doi: 10.3892/mmr.2019.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derfuss T, Mehling M, Papadopoulou A, Bar-Or A, Cohen JA, Kappos L. Advances in oral immunomodulating therapies in relapsing multiple sclerosis. Lancet Neurol. 2020;19:336–347. doi: 10.1016/S1474-4422(19)30391-6. [DOI] [PubMed] [Google Scholar]
- 31.Kulakova OG, Tsareva EY, Boyko AN, Shchur SG, Gusev EI, Lvovs D, Favorov AV, Vandenbroeck K, Favorova OO. Allelic combinations of immune-response genes as possible composite markers of IFN-β efficacy in multiple sclerosis patients. Pharmacogenomics. 2012;13:1689–1700. doi: 10.2217/pgs.12.161. [DOI] [PubMed] [Google Scholar]
- 32.Kalnina J, Paramonova N, Sjakste N, Sjakste T. Study of association between polymorphisms in the PSMB5 (rs11543947) and PSMA3 (rs2348071) genes and multiple sclerosis in Latvians. Biopolymers Cell. 2014;30:305–309. [Google Scholar]
- 33.Mishto M, Bellavista E, Ligorio C, Textoris-Taube K, Santoro A, Giordano M, D'Alfonso S, Listì F, Nacmias B, Cellini E, et al. Immunoproteasome LMP2 60HH variant alters MBP epitope generation and reduces the risk to develop multiple sclerosis in Italian female population. PLoS One. 2010;5(e9287) doi: 10.1371/journal.pone.0009287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sjakste T, Paramonova N, Rumba-Rozenfelde I, Trapina I, Sugoka O, Sjakste N. Juvenile idiopathic arthritis subtype- and sex-specific associations with genetic variants in the PSMA6/PSMC6/PSMA3 gene cluster. Pediatr Neonatol. 2014;55:393–403. doi: 10.1016/j.pedneo.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Paramonova N, Wu LS, Rumba-Rozenfelde I, Wang JY, Sjakste N, Sjakste T. Genetic variants in the PSMA6, PSMC6 and PSMA3 genes associated with childhood asthma in Latvian and Taiwanese populations. Biopolymers Cell. 2014;30:377–387. [Google Scholar]
- 36.Paramonova N, Kupca S, Rumba-Rozenfelde I, Sjakste N, Sjakste T. Association between the PSMB5 and PSMC6 genetic variations and children obesity in the Latvian population. Biopolymers Cell. 2014;30:477–480. [Google Scholar]
- 37.Sjakste T, Kalis M, Poudziunas I, Pirags V, Lazdins M, Groop L, Sjakste N. Association of microsatellite polymorphisms of the human 14q13.2 region with type 2 diabetes mellitus in Latvian and Finnish populations. Ann Hum Genet. 2007;71:772–776. doi: 10.1111/j.1469-1809.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- 38.Sjakste T, Paramonova N, Osina K, Dokane K, Sokolovska J, Sjakste N. Genetic variations in the PSMA3, PSMA6 and PSMC6 genes are associated with type 1 diabetes in Latvians and with expression level of number of UPS-related and T1DM-susceptible genes in HapMap individuals. Mol Genet Genomics. 2016;291:891–903. doi: 10.1007/s00438-015-1153-0. [DOI] [PubMed] [Google Scholar]
- 39.Sjakste T, Poudziunas I, Ninio E, Perret C, Pirags V, Nicaud V, Lazdins M, Evanss A, Morrison C, Cambien F, Sjakste N. SNPs of PSMA6 gene-investigation of possible association with myocardial infarction and type 2 diabetes mellitus. Genetika. 2007;43:553–559. [PubMed] [Google Scholar]
- 40.Wang H, Jiang M, Zhu H, Chen Q, Gong P, Lin J, Lu J, Qiu J. Quantitative assessment of the influence of PSMA6 variant (rs1048990) on coronary artery disease risk. Mol Biol Rep. 2013;40:1035–1041. doi: 10.1007/s11033-012-2146-2. [DOI] [PubMed] [Google Scholar]
- 41.Sjakste T, Paramonova N, Wu LS, Zemeckiene Z, Sitkauskiene B, Sakalauskas R, Wang JY, Sjakste N. PSMA6 (rs2277460, rs1048990), PSMC6 (rs2295826, rs2295827) and PSMA3 (rs2348071) genetic diversity in Latvians, lithuanians and taiwanese. Meta Gene. 2014;2:283–298. doi: 10.1016/j.mgene.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas-Villarraga A, Amaya-Amaya J, Rodriguez-Rodriguez A, Mantilla RD, Anaya JM. Introducing polyautoimmunity: Secondary autoimmune diseases no longer exist. Autoimmune Dis. 2012;2012(254319) doi: 10.1155/2012/254319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holm K, Melum E, Franke A, Karlsen TH. SNPexp-A web tool for calculating and visualizing correlation between HapMap genotypes and gene expression levels. BMC Bioinformatics. 2010;11(600) doi: 10.1186/1471-2105-11-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stangel M, Penner IK, Kallmann BA, Lukas C, Kieseier BC. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: The multiple sclerosis decision model. Ther Adv Neurol Disord. 2015;8:3–13. doi: 10.1177/1756285614560733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis CM. Genetic association studies: Design, analysis and interpretation. Brief Bioinform. 2002;3:146–153. doi: 10.1093/bib/3.2.146. [DOI] [PubMed] [Google Scholar]
- 47.Alsmadi O, Muiya P, Khalak H, Al-Saud H, Meyer BF, Al-Mohanna F, Alshahid M, Dzimiri N. Haplotypes encompassing the KIAA0391 and PSMA6 gene cluster confer a genetic link for myocardial infarction and coronary artery disease. Ann Hum Genet. 2009;73:475–483. doi: 10.1111/j.1469-1809.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 48.Andreassen OA, Harbo HF, Wang Y, Thompson WK, Schork AJ, Mattingsdal M, Zuber V, Bettella F, Ripke S, Kelsoe JR, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: Differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20:207–214. doi: 10.1038/mp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S, Xu L, Shin Y, Gardner L, Hartzes A, Dohan FC, Raine C, Homayouni R, Levin MC. A potential link between autoimmunity and neurodegeneration in immune-mediated neurological disease. J Neuroimmunol. 2011;235:56–69. doi: 10.1016/j.jneuroim.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Thompson SD, Barnes MG, Griffin TA, Grom AA, Glass DN. Heterogeneity in juvenile idiopathic arthritis: Impact of molecular profiling based on DNA polymorphism and gene expression patterns. Arthritis Rheum. 2010;62:2611–2615. doi: 10.1002/art.27561. [DOI] [PubMed] [Google Scholar]
- 51.Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, Rudan I, McKeigue P, Wilson JF, Campbell H. Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet. 2011;89:607–618. doi: 10.1016/j.ajhg.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hallstrand TS, Fischer ME, Wurfel MM, Afari N, Buchwald D, Goldberg J. Genetic pleiotropy between asthma and obesity in a community-based sample of twins. J Allergy Clin Immunol. 2005;116:1235–1241. doi: 10.1016/j.jaci.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy A, Tantisira KG, Soto-Quirós ME, Avila L, Klanderman BJ, Lake S, Weiss ST, Celedón JC. PRKCA: A positional candidate gene for body mass index and asthma. Am J Hum Genet. 2009;85:87–96. doi: 10.1016/j.ajhg.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SH, Lee EB, Shin ES, Lee JE, Cho SH, Min KU, Park HW. The interaction between allelic variants of CD86 and CD40LG: A common risk factor of allergic asthma and rheumatoid arthritis. Allergy Asthma Immunol Res. 2014;6:137–141. doi: 10.4168/aair.2014.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramírez-Bello J, Jiménez-Morales S, Espinosa-Rosales F, Gómez-Vera J, Gutiérrez A, Velázquez Cruz R, Baca V, Orozco L. Juvenile rheumatoid arthritis and asthma, but not childhood-onset systemic lupus erythematosus are associated with FCRL3 polymorphisms in Mexicans. Mol Immunol. 2013;53:374–378. doi: 10.1016/j.molimm.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114:237–246. doi: 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bekenstein U, Soreq H. Heterogeneous nuclear ribonucleoprotein A1 in health and neurodegenerative disease: From structural insights to post-transcriptional regulatory roles. Mol Cell Neurosci. 2013;56:436–446. doi: 10.1016/j.mcn.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Leibowitz SM, Yan J. NF-κB pathways in the pathogenesis of multiple sclerosis and the therapeutic implications. Front Mol Neurosci. 2016;9(84) doi: 10.3389/fnmol.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yue Y, Stone S, Lin W. Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen Res. 2018;13:1507–1515. doi: 10.4103/1673-5374.237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu ZH, Shi Y. When ubiquitin meets NF-κB: A trove for anti-cancer drug development. Curr Pharm Des. 2013;19:3263–3275. doi: 10.2174/1381612811319180010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fedorova OA, Moiseeva TN, Nikiforov AA, Tsimokha AS, Livinskaya VA, Hodson M, Bottrill A, Evteeva IN, Ermolayeva JB, Kuznetzova IM, et al. Proteomic analysis of the 20S proteasome (PSMA3)-interacting proteins reveals a functional link between the proteasome and mRNA metabolism. Biochem Biophys Res Commun. 2011;416:258–265. doi: 10.1016/j.bbrc.2011.10.126. [DOI] [PubMed] [Google Scholar]
- 62.Wosik K, Antel J, Kuhlmann T, Brück W, Massie B, Nalbantoglu J. Oligodendrocyte injury in multiple sclerosis: A role for p53. J Neurochem. 2003;85:635–644. doi: 10.1046/j.1471-4159.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- 63.Dozmorov M, Wu W, Chakrabarty K, Booth JL, Hurst RE, Coggeshall KM, Metcalf JP. Gene expression profiling of human alveolar macrophages infected by B. anthracis spores demonstrates TNF-alpha and NF-kappab are key components of the innate immune response to the pathogen. BMC Infect Dis. 2009;9(152) doi: 10.1186/1471-2334-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gill GN. Decoding the LIM development code. Trans Am Clin Climatol Assoc. 2003;114:179–189. [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips K, Luisi B. The virtuoso of versatility: POU proteins that flex to fit. J Mol Biol. 2000;302:1023–1039. doi: 10.1006/jmbi.2000.4107. [DOI] [PubMed] [Google Scholar]
- 66.She H, Mao Z. Regulation of myocyte enhancer factor-2 transcription factors by neurotoxins. Neurotoxicology. 2011;32:563–566. doi: 10.1016/j.neuro.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uzumcu A, Karaman B, Toksoy G, Uyguner ZO, Candan S, Eris H, Tatli B, Geckinli B, Yuksel A, Kayserili H, Basaran S. Molecular genetic screening of MBS1 locus on chromosome 13 for microdeletions and exclusion of FGF9, GSH1 and CDX2 as causative genes in patients with Moebius syndrome. Eur J Med Genet. 2009;52:315–320. doi: 10.1016/j.ejmg.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Ho B, Olson G, Figel S, Gelman I, Cance WG, Golubovskaya VM. Nanog increases focal adhesion kinase (FAK) promoter activity and expression and directly binds to FAK protein to be phosphorylated. J Biol Chem. 2012;287:18656–18673. doi: 10.1074/jbc.M111.322883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozaki K, Sato H, Iida A, Mizuno H, Nakamura T, Miyamoto Y, Takahashi A, Tsunoda T, Ikegawa S, Kamatani N, et al. A functional SNP in PSMA6 confers risk of myocardial infarction in the Japanese population. Nat Genet. 2006;38:921–925. doi: 10.1038/ng1846. [DOI] [PubMed] [Google Scholar]
- 70.Caputi FF, Carboni L, Mazza D, Candeletti S, Romualdi P. Cocaine and ethanol target 26S proteasome activity and gene expression in neuroblastoma cells. Drug Alcohol Depend. 2016;161:265–275. doi: 10.1016/j.drugalcdep.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torkildsen Ø, Myhr KM, Bø L. Disease-modifying treatments for multiple sclerosis-a review of approved medications. Eur J Neurol. 2016;23 (Suppl 1):S18–S27. doi: 10.1111/ene.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.