Abstract
Diabetes disrupts organs throughout the body including the brain. Evidence suggests diabetes is a risk factor for Alzheimer’s disease and neurodegeneration. In this review we focus on understanding how diabetes contributes to the progression of neurodegeneration by influencing several aspects of the disease process. We emphasize the potential roles of brain insulin resistance, as well as cholesterol and lipid disruption, as factors which worsen AD.
Keywords: Diabetes, Alzheimer’s, cholesterol, oxidized lipids, insulin resistance
Introduction - Diabetes and Alzheimer’s Disease
Alzheimer’s disease (AD) is a prevalent, progressive neurodegenerative disease notorious for its devastating effects on memory and cognition. The pathology of AD has been well-documented and includes abundant extracellular plaque deposits of the amyloid β (Aβ) peptide in the brain, as well as hyper-phosphorylated intracellular tangles of the protein tau. A small subset of AD is driven by familial mutations in genes involving Aβ production, however the majority of cases have an uncertain etiology. A prominent theory behind the cause of AD is the Amyloid Hypothesis, which posits that aggregated assemblies of Aβ peptides in the brain are a primary cause of AD neurodegeneration (Selkoe & Hardy, 2016). Many people develop Aβ plaque deposits as they age and are not symptomatic for AD (Brayne et al., 2009), thus amyloid alone cannot explain the AD phenomenon. Over the past two decades, attempts to develop new therapeutic compounds, many of which have progressed through phase 3 clinical trials, have failed to produce effective treatments for AD (Cummings, Morstorf, & Zhong, 2014; Loera-Valencia et al., 2019). These failed efforts, largely based on the amyloid hypothesis, demonstrate the necessity to pursue additional avenues in the quest to develop therapeutics for AD-associated neurodegeneration.
It is increasingly evident that metabolic dysfunction plays a role in promoting cognitive dysfunction and the pathogenesis of Alzheimer’s Disease (AD). Cognitive decline is accelerated in patients with both type 1 and type 2 diabetes (T2DM)(Biessels, Deary, & Ryan, 2008). Furthermore, epidemiological evidence suggests T2DM and insulin resistance are risk factors for developing AD and increase the likelihood of developing late onset AD by approximately two-fold (Mittal & Katare, 2016; Ott et al., 1999; Vagelatos & Eslick, 2013). Obesity in general may also accelerate the progression of cognitive decline and dementia (Beydoun, Beydoun, & Wang, 2008). It is important to note that people with diabetes do not show increased Aβ plaque burden (Arvanitakis et al., 2006; Roberts et al., 2014). It appears that metabolic dysfunction potentiates AD neurodegeneration by mechanisms other than enhanced amyloidosis, including, but not limited to, vascular changes (Biessels & Despa, 2018). This review is focused on understanding how diabetes may contribute to the progression of AD by influencing numerous aspects of the disease process. We emphasize the potential roles of brain insulin resistance, as well as cholesterol and lipid disruption, as factors which worsen AD.
Brain Insulin Receptor and Insulin Resistance in Diabetes and Alzheimer’s Disease
Originally it was believed that insulin did not play a significant role in the physiology of the brain. This assumption was based on the observation that brain glucose uptake is not stimulated by, or dependent on, insulin in most regions of the brain (Best, Taborsky, Halter, & Porte, 1981; Goodner, Hom, & Berrie, 1980). However, several findings have done much to change this perspective and it is now accepted that insulin and the insulin receptor (IR) play a role in normal brain function. The insulin receptor is distributed throughout the brain, and is particularly abundant in the hippocampus, cortex and thalamus (Kleinridders, Ferris, Cai, & Kahn, 2014). Injection of insulin into the vena cava activates brain insulin signaling, and this is lost with knockout of the insulin receptor (Bruning et al., 2000). While insulin is synthesized outside of the central nervous system in the pancreatic β cells, it is readily measured in the CSF (Geijselaers et al., 2018). There is some evidence that small amounts of insulin may be synthesized locally in the brain, however the physiologic consequence of this remains unclear (Devaskar et al., 1994; Devaskar, Singh, Carnaghi, Rajakumar, & Giddings, 1993). The kinetics of peripheral insulin activating brain insulin signaling appear to be dependent on endothelial insulin receptors, which serve to traffic insulin into the local brain tissue (Gray, Aylor, & Barrett, 2017; Konishi et al., 2017). In addition to the IR, the brain also contains abundant levels of the closely related IGF-1 receptor (IGF1R), as well as its primary ligand, insulin-like growth factor 1 (IGF-1)(Bach, Shen-Orr, Lowe, Roberts, & LeRoith, 1991; Fernandez & Torres-Aleman, 2012). IGF1R is also capable of acting as a lower-affinity receptor for insulin and thus may be responsible for mediating some of the effects of insulin in the brain. IGF1R is capable of forming heterodimers with IR, although the functional and physiologic significance of these hybrid receptors in the brain remains uncertain (Belfiore, Frasca, Pandini, Sciacca, & Vigneri, 2009).
While it is clear the brain contains abundant insulin receptor and is responsive to insulin signaling, the functional significance of insulin signaling in the brain is still being elucidated. One of the first attempts to address this issue came from the development of the brain insulin receptor knockout mouse (Bruning et al., 2000). While these mice are viable, deletion of brain IR causes peripheral metabolic dysfunction by inducing hyperphagia, mild insulin resistance, and obesity. Consistent with these findings, inducible deletion of brain IR in mice exacerbates hyperglycemia (Koch et al., 2008). Thus, insulin signaling in the brain plays an essential role in regulating metabolic homeostasis.
Evidence suggests that IR/IGF1R signaling mediates a variety of functions across different brain regions. Viral-mediated deletion of the IR/IGF1R in the hippocampus impairs learning and memory, which may be due to downregulation of glutamate receptor signaling. In contrast, deletion of IR/IGF1R in the amygdala alters temperature homeostasis (Soto, Cai, Konishi, & Kahn, 2019). Furthermore, IR/IGF1R signaling regulates circadian rhythms by driving synthesis of the PERIOD protein in cultured neurons and the suprachiasmatic nucleus, serving as an important molecular link connecting feeding to circadian regulation (Crosby et al., 2019). Insulin exerts its effects in the brain by acting on multiple cell types, including neurons and glial cells. Insulin modulates the activity of hippocampal neurons and likely plays a role in regulating synaptic activity (van der Heide, Kamal, Artola, Gispen, & Ramakers, 2005). In Neuropeptide Y neurons insulin signaling also regulates feeding behavior (Loh et al., 2017). In astrocytes, insulin modulates glucose uptake and regulates metabolism in cooperation with IGF-1 (Fernandez et al., 2017). Furthermore, insulin regulation of astrocyte glucose uptake is critical for brain regulation of peripheral metabolism (Garcia-Caceres et al., 2016). Insulin signaling also plays an important role in astrocyte gliotransmission. Deletion of IR from astrocytes impairs secretion of ATP, leading to reduced dopamine levels and a depression-like behavioral phenotype (Cai et al., 2018). Taken together, these studies suggest brain insulin signaling plays an essential role in promoting cognitive function and healthy metabolism.
Given the wide array of evidence supporting a role for insulin in brain physiology, it is not surprising then that insulin resistance in the brain may also contribute to neurodegeneration. Evidence suggesting that disrupted insulin/IGF1 signaling play a role in AD pathogenesis has been accumulating for more than a decade (Craft et al., 2000; de la Monte & Wands, 2005; Hoyer, 2002; Rivera et al., 2005; Steen et al., 2005). This concept was further validated in 2012, when it was reported that the human AD brain, including the hippocampus, is insulin and IGF1 resistant when stimulated post-mortem (Talbot et al., 2012). Moreover, tau expression and phosphorylation are regulated by insulin/IGF1 signaling (Schubert et al., 2003, 2004). Animal studies have also demonstrated an essential role for insulin in neurodegeneration. Monkeys treated with streptozotocin (STZ), a toxin which makes the animals insulin deficient by killing pancreatic β cells, show evidence of AD, including increased levels of Aβ in the temporal cortex and changes in tau phosphorylation (Morales-Corraliza et al., 2016). Numerous studies in rodent models of AD demonstrate that disrupting insulin levels or signaling, through genetic, chemical or dietary methods, worsens AD phenotype (Hascup et al., 2019; Petrov et al., 2015; Ramos-Rodriguez et al., 2017; X. Wang et al., 2010).
The mechanism by which insulin resistance occurs in the brains of AD patients is uncertain, but studies suggest that both Aβ and tau proteins may play a role in this phenomenon. Aβ can compete with insulin in binding to the insulin receptor (Xie et al., 2002). Increasing concentrations of Aβ inhibit phosphorylation of the IR and block its normal signaling functions. It is, however, uncertain if Aβ directly inhibits IR in vivo as the estimated Ki of this interaction is in the micromolar range. This far exceeds normal physiologic concentrations of Aβ in the cerebrospinal fluid (Schirinzi et al., 2017). Micromolar concentrations of Aβ have also been shown to antagonize the effect of insulin when applied directly to synaptosomes (Heras-Sandoval, Ferrera, & Arias, 2012). Insulin signaling may also be disrupted by intracellular Aβ, which may have more relevance in vivo (H.-K. Lee, Kumar, Fu, Rosen, & Querfurth, 2009). Injection of Aβ oligomers into either the monkey or mouse brain disrupts insulin signaling in a manner that seems to be dependent on inducing neuroinflammation (Lourenco et al., 2013). Aβ oligomers may also promote metabolic dysfunction. Injection of Aβ oligomers into the brains of mice promotes glucose intolerance, suggesting that Aβ may play a role in promoting diabetes by damaging the hypothalamus (Clarke et al., 2015). In addition, tau also appears to play a role in promoting insulin resistance. Bot deletion of tau and hyperphosphorylation of tau impair insulin signaling (Marciniak et al., 2017)(Rodriguez-Rodriguez et al., 2017). Tau can also induce inflammasome activation in the brain, which may further contribute to increased ROS and impaired insulin signaling (Ising et al., 2019; Stancu et al., 2019). Thus, it appears that the Aβ and tau proteins likely have a shared role in causing dysregulated insulin signaling in the brain.
It is reasonable that Type 2 diabetes, a state of systemic insulin resistance, might promote Alzheimer’s disease progression by worsening brain insulin resistance. Interestingly, several groups have shown that a high fat diet is sufficient to promote brain insulin resistance and cognitive impairment in mice (Kothari et al., 2017; Spinelli et al., 2017; Wakabayashi et al., 2019). This cognitive dysfunction may occur in part due to increased levels of palmitic acid driving glutamate signaling dysfunction by altering glutamate receptor palmitoylation (Spinelli et al., 2017). It has long been known that inflammation plays an important role in promoting insulin resistance in peripheral tissues, and inflammation likely plays an important role in promoting insulin resistance in brain disease (Hotamisligil, 2006). Inflammation is also an established component of AD, and several studies in mice show that high fat diets increase biomarkers of inflammation in brain tissue, including increased cytokine levels and microglial reactivity (C. H. Lee et al., 2018; Nakandakari et al., 2019; Vinuesa et al., 2018). As stated above, Aβ oligomers promote insulin resistance in an inflammation-dependent manner (Lourenco et al., 2013). Interestingly, epidemiological evidence suggests that reducing inflammation may protect against AD (McGeer, Rogers, & McGeer, 2016). Therapeutic strategies seeking to modulate inflammation in AD may be a viable strategy to improve insulin sensitivity in the AD brain.
While the role of brain insulin resistance in AD is contentious, mouse studies and human clinical trials are seeking to understand how increasing insulin levels in the brain may impact the disease. Intranasal insulin administration rapidly increases brain insulin levels without significantly impacting peripheral tissues (Born et al., 2002). Increasing insulin in the brains of mice has cognitive and pathological benefits in neurodegenerative models (Chen et al., 2017; Kim et al., 2019; Sanguinetti et al., 2019). Clinical trials of intranasal insulin administration to combat dementia and AD are currently ongoing with mixed results to this point (Avgerinos et al., 2018).
Cholesterol in Alzheimer’s Disease and Diabetes
The brain is a cholesterol rich organ containing approximately 25% of total cholesterol in the body. Unlike other organs, the brain must make its own cholesterol as cholesterol carrying lipoproteins cannot cross the blood-brain barrier (Bjorkhem & Meaney, 2004). In brain tissue, cholesterol is synthesized in a variety of cell types including both neurons and glia (Genaro-Mattos, Anderson, Allen, Korade, & Mirnics, 2019; van Deijk et al., 2017). Cellular production of cholesterol is primarily regulated by SREBP2, a transcription factor that facilitates the transcription of several cholesterol synthesis enzymes, including the rate-limiting enzyme HMG-CoA reductase (Brown & Goldstein, 1997). In addition to de-novo synthesis, cells in the brain can obtain cholesterol from neighboring cells by absorbing cholesterol-laden lipoprotein molecules, such as Apolipoprotein E (APOE), in a receptor mediated process. Cholesterol fulfills several important roles in the brain. It is abundant in myelin and lipid membranes and plays an important role in the organization of lipid rafts (Hussain et al., 2019). Oxidized cholesterol species may also function as signaling molecules (reviewed below). Glial derived cholesterol plays an essential role in neuronal synapse formation and maintenance (Mauch et al., 2001). Depleting cholesterol in cultured neural cells impairs intracellular signaling responses to a variety of important hormones (Fukui, Ferris, & Kahn, 2015). This finding is in general agreement with research demonstrating that cholesterol rich lipid membrane domains are required for cultured cells to respond normally to insulin (Ratcliffe et al., 2018). Moreover, it was recently shown that the insulin receptor requires membrane sterol content for its kinase activity (Delle Bovi, Kim, Suresh, London, & Miller, 2019). Thus various indispensable functions in the brain are dependent on cholesterol and cholesterol levels must be tightly regulated to maintain healthy brain function.
Evidence suggests that cholesterol is an important factor in AD. The strongest known genetic risk factor for late-onset AD is the E4 allele of the cholesterol transport lipoprotein ApoE (Corder et al., 1993; Lambert et al., 2009). Carrying two copies of the APOE4 allele greatly increases a person’s risk of developing late-onset AD compared to the more common APOE3 variant. In contrast, the APOE2 allele is protective against AD (Corder et al., 1993; C.-C. Liu, Liu, Kanekiyo, Xu, & Bu, 2013). Additionally, mutations in other proteins involved in maintaining cholesterol homeostasis, including CLU/APOJ and the cholesterol transporters ABCA1 and ABCA7, are also associated with increased AD risk (Hollingworth et al., 2011; Lambert et al., 2009; Nordestgaard, Tybjaerg-Hansen, Nordestgaard, & Frikke-Schmidt, 2015). Evidence also suggests that cholesterol synthesis is altered in the AD brain, as levels of the cholesterol synthesis enzyme DHCR24 are significantly reduced in AD brain samples (Greeve et al., 2000; Peri & Serio, 2008). Changes in the levels of brain cholesterol species may also occur in AD. Several studies indicate that cholesterol or its oxidized metabolites are increased in the CSF of AD patients compared to healthy controls (Leoni & Caccia, 2011; Mateos et al., 2011; Papassotiropoulos et al., 2002; Popp et al., 2012; Schonknecht et al., 2002; F. Wang & Jia, 2007). Other studies however, have shown reductions in oxidized cholesterol products in AD (Benussi et al., 2017; Kolsch et al., 2010). High serum cholesterol has also been correlated with a risk for AD (Anstey, Ashby-Mitchell, & Peters, 2017; Notkola et al., 1998), but this association would seem to be indirect given that cholesterol does not cross the blood-brain barrier.
How cholesterol and cholesterol associated lipoproteins influence Alzheimer’s disease remains uncertain, but research suggests that APOE4 promotes amyloid aggregation and impairs clearance from the brain by directly binding to amyloid beta (Huynh, Davis, Ulrich, & Holtzman, 2017; C.-C. Liu et al., 2013; Strittmatter et al., 1993). Furthermore, cholesterol itself has a role in regulating the production of Aβ. The amyloid precursor protein (APP), from which Aβ is derived, resides in cellular membranes and binds directly to cholesterol (Barrett et al., 2012). As stated above, cholesterol is a major structural component in lipid membranes and its depletion can potently disrupt organized cholesterol rich components of the membrane referred to as lipid rafts (Kabouridis, Janzen, Magee, & Ley, 2000; Pike, 2003). Changes in membrane cholesterol content and pharmacologic inhibition of cholesterol synthesis influence amyloid production, which may occur in part due to disruption of APP processing in lipid rafts, in addition to reduction of APP levels in the cellular membrane (Bhattacharyya, Barren, & Kovacs, 2013; Guardia-Laguarta et al., 2009; Vetrivel & Thinakaran, 2010). The enzymes which cleave APP to generate Aβ reside in the cellular membrane and their proteolytic capabilities are also influenced by membrane cholesterol content (Marquer et al., 2011; Wahrle et al., 2002). Thus factors which alter cholesterol levels or synthesis are likely to impact the rate of amyloid deposition in the brain by effecting the dynamics of amyloid production.
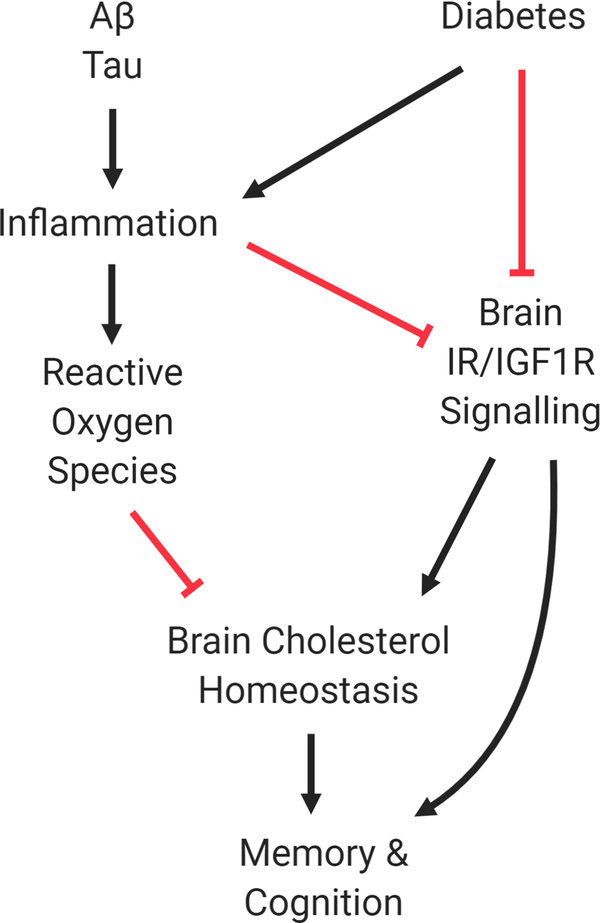
Interestingly, rodent studies suggest that diabetes potently disrupts brain cholesterol synthesis. In a study performed by Suzuki et al, it was first reported that in multiple mouse models of diabetes, brain SREBP2 levels are significantly reduced, resulting in impaired cholesterol synthesis (Suzuki et al., 2010). Brain cholesterol synthesis was rescued by intracerebroventricular administration of insulin, but not normalization of glucose. Furthermore, deletion of brain insulin receptor was sufficient to disrupt cholesterol synthesis. These data demonstrate the importance of insulin in maintaining cholesterol homeostasis in the central nervous system. In addition to disruption of SREBP2 and its downstream targets, insulin depletion also disrupts total levels of the upstream regulatory protein sterol regulatory element-binding protein cleavage-activating protein (SCAP), which is responsible for regulating both cholesterol and fatty acid synthesis (Suzuki, Ferris, Chee, Maratos-Flier, & Kahn, 2013). The effect of insulin depletion on brain cholesterol was further demonstrated in rats. Romano et al. found that insulin deficiency created with STZ treatment impairs mitochondrial function, cholesterol synthesis and cholesterol levels in rat cortex (Romano et al., 2018). These studies suggest that impaired brain cholesterol synthesis may be an underappreciated consequence stemming from insulin-insufficiency. This also raises the possibility that the cognitive dysfunction observed in diabetes patients stems in part from brain cholesterol dyshomeostasis. Cholesterol disruption may be further exacerbated with age, as evidence suggests that aging significantly impairs astrocyte cholesterol synthesis, possibly contributing to synapse loss (Boisvert, Erikson, Shokhirev, & Allen, 2018). Research has suggested that impaired brain cholesterol synthesis can potentiate insulin resistance in brain tissue by disrupting the conformation of IR in the cellular membrane, facilitating aberrant activation of the receptor (Martin-Segura et al., 2019). Cholesterol perturbation in the brain that occurs in diabetes may therefore promote neurodegeneration by disrupting a variety of important biological processes in the brain (Fig. 1).
Figure 1 -.
Schematic of Diabetes in Brain Health. Under healthy conditions the brain has well-regulated insulin signaling and cholesterol synthesis. Diabetes leads to both impaired insulin signaling and cholesterol homeostasis in the brain to promote cognitive decline. Factors which promote inflammation, such as increased Tau and Amyloid abundance, may promote increased ROS and insulin resistance. Inhibition is represented with red lines while black arrows represent activation.
Lipid oxidation
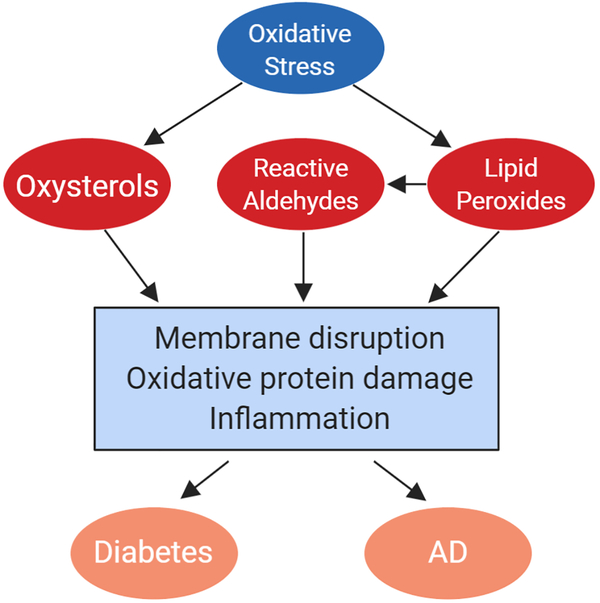
The central nervous system (CNS) is particularly susceptible to lipid oxidation as a result of its high oxygen consumption and lipid concentration. High metabolic activity and oxidative phosphorylation in the brain result in a propensity for ROS generation, and ROS can react with cholesterol to produce oxysterols or with fatty acids to produce lipid peroxides. These oxidized lipids have diverse signaling functions and likely contribute to cellular stress and dysfunction in a variety of diseases including Alzheimer’s disease and diabetes (Fig. 2).
Figure 2 -.
Lipid oxidation in diabetes and AD. Increased oxidative stress in the brain results in the oxidation of lipids. Cholesterol is oxidized to produce oxysterols such as 7KC, while fatty acids form lipid peroxides which can additionally generate reactive aldehydes such as 4-HNE. These products of lipid oxidation can harm cells in the brain through mechanisms including membrane disruption, oxidative damage to proteins, and inflammation. This oxidative stress is a factor which can promote both AD and diabetes induced cognitive decline.
Cholesterol can be oxidized either enzymatically or non-enzymatically. Non-enzymatically produced oxysterols include 7-ketocholesterol (7KC) and 7β-hydroxycholesterol (7βOHC). Some oxysterols can be produced both enzymatically and non-enzymatically, such as 7α-hydroxycholesterol (7αOHC) and 25-hydroxycholesterol (25OHC), while others are produced primarily by enzymatic reactions, such as 24(S)-hydroxycholesterol (24(S)OHC) and 27-hydroxycholesterol (27OHC). Oxysterols like 24(S)OHC and 25OHC have varied signaling and metabolic functions, and enzymatic control over their production contributes to cholesterol regulation and immune activation. ROS-generated oxysterols, like 7KC, can have other cellular effects such as promoting inflammation, oxidative stress, and apoptosis. Unlike cholesterol, oxysterols can cross the BBB. This allows for the clearance of excess cholesterol from the brain, primarily through the oxidation of cholesterol to 24(S)OHC by the enzyme cholesterol 24-hydroxylase, but also allows for other oxysterols to travel between the blood and the CNS. Cholesterol and oxysterols cannot be degraded in the brain, so oxysterols must cross the BBB and travel to the liver to be cleared.
Like cholesterol, fatty acids can be oxidized to produce both important signaling molecules and potentially cytotoxic ones. Polyunsaturated fats in the brain such as arachidonic acid and docosahexaenoic acid are the most commonly oxidized, as the methylene groups adjacent to double bonds are particularly susceptible to attack by enzyme catalytic sites or ROS (Gaschler & Stockwell, 2017). Lipid oxidation can be performed by several families of enzymes: cyclooxygenases (COXs), cytochrome p450s (CYPs), and lipoxygenases (LOXs). These enzymes oxidize lipids to create signaling molecules such as 5-HETE and prostaglandins. Non-enzymatic lipid oxidation occurs through less controlled mechanisms and can result in more toxic byproducts. Fatty acid oxidation by ROS generates lipid radicals which, if the radicals are not rapidly scavenged, can propagate oxidative damage to other membrane lipids. This chain reaction generates lipid peroxides, which can be further degraded to produce reactive aldehydes such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA). These reactive aldehydes are frequently used as biomarkers of lipid peroxidation, and they are biologically important in redox signaling and as effectors of oxidative damage to other macromolecules.
Oxidized lipids in Alzheimer’s disease and diabetes
Altered oxysterol levels are observed in Alzheimer’s disease and diabetes. Elevated 7-ketocholesterol (7KC) is found in AD patients in serum (Liang et al., 2016) and post-mortem brain tissue (Testa et al., 2016). 7βOHC, which is produced non-enzymatically, and 7αOHC, which is produced non-enzymatically and enzymatically, are both also elevated in AD brain tissue (Testa et al., 2016). 7KC is elevated in the serum of type 2 diabetes patients (Endo et al., 2008), where it is negatively correlated with glycemic control (Samadi et al., 2019). Other non-enzymatically produced oxysterols, including 7βOHC, are also elevated in serum in type 1 and type 2 diabetes patients (Ferderbar et al., 2007). Currently, research on oxysterols in the CNS in diabetes are extremely limited. 7KC is elevated in the cortex of rats after STZ treatment (Romano et al., 2018), but CNS oxysterols in other rodent models or in human diabetes have not been studied. Further research will be important to determine whether the oxysterol alterations observed in AD are also present in diabetes and whether this concordance is a mechanism by which diabetes contributes to AD risk.
7KC has been extensively studied as a pro-inflammatory and pro-apoptotic molecule, particularly in the contexts of atherosclerosis and age-related macular degeneration (AMD). Atherosclerosis research has shown that high concentrations of 7KC induce apoptosis in cultured macrophages in a ROS-dependent mechanism (Leonarduzzi et al., 2006) and 7KC can induce expression of the pro-inflammatory cytokines IL-6 and IL-1β in vitro (Larrayoz, Huang, Lee, Pascual, & Rodríguez, 2010; Watanabe et al., 2018). In AMD, a more relevant disease for learning about oxysterols in the brain, 7KC and other oxysterols are elevated in the retina (Moreira, Larrayoz, Lee, & Rodríguez, 2009; Rodriguez & Fliesler, 2009; Rodríguez & Larrayoz, 2010). 7KC in the retina increases IL-1β (Amaral, Lee, Chou, Campos, & Rodríguez, 2013) largely through TLR4 activation (Huang, Amaral, Lee, & Rodriguez, 2014). Microglia in the retina are particularly responsive to 7KC. 7KC acts as a potent chemoattractant for microglia in vitro and when injected into the retina (Indaram et al., 2015). Retinal microglia that uptake 7KC become more activated, increasing expression of pro-inflammatory cytokines IL-6 and IL-1β and decreasing expression of neurotrophic factors including BDNF and NGF (Indaram et al., 2015). Though the effects of 7KC in the brain parenchyma are less well studied, these data from the retina support a possible mechanism by which elevated 7KC in AD and diabetes promotes activation of the innate immune cells of the CNS.
Fatty acid peroxidation is also linked to AD and diabetes. 4-HNE and other reactive aldehydes, toxic products of fatty acid oxidation, are increased in the brains of patients with mild cognitive impairment (MCI) and AD (Barone et al., 2012; Scheff, Ansari, & Mufson, 2016), as well as in the plasma of AD patients (Selley, Close, & Stern, 2002). Lipid peroxidation markers are elevated in the plasma of type 2 diabetes patients and correlate positively with diabetes control, as measured by HbA1c (de Souza Bastos et al., 2016; Fatani, Babakr, NourEldin, & Almarzouki, 2016). Lipid peroxidation is also increased in the brain in a genetic rat model of diabetes (Raza, John, & Howarth, 2015) and in STZ-treated rats (Sözbir & Nazıroğlu, 2016). Fatty acid peroxidation could contribute both directly and indirectly to neuronal damage in disease. Oxidation of fatty acid tails changes the conformation of membrane phospholipids and dramatically alters lipid membrane properties, decreasing membrane fluidity and increasing permeability (Borst, Visser, Kouptsova, & Visser, 2000; Wong-ekkabut et al., 2007). These membrane disruptions are one direct mechanism by which fatty acid oxidation can be toxic to cells.
In addition to the direct damage of lipid peroxidation on membrane function, 4-HNE and other reactive aldehydes can covalently modify proteins and impair their function in the brain. Interestingly, 4-HNE modification of glutamate transporter 1 (GLT-1) is elevated in AD brains and in cultured rat synaptosomes treated with Aβ (Lauderback et al., 2001). GLT-1 is the glutamate transporter expressed by astrocytes that clears excess glutamate from synapses to protect neurons from excitotoxicity, and increased GLT-1 function has been proposed as protective in a mouse model of AD (Takahashi et al., 2015). Impaired astrocyte glutamate uptake as a result of HNE-mediated GLT-1 modification is one possible mechanism by which lipid peroxidation could contribute to neurodegeneration. 4-HNE modification of low density lipoprotein receptor-related protein 1 (LRP1) is also increased in AD (Owen et al., 2010), and could contribute to impaired export of Aβ out of the brain. Acrolein, another aldehyde byproduct of lipid peroxidation, increases tau phosphorylation in vitro (Gómez-Ramos, Díaz-Nido, Smith, Perry, & Avila, 2003).
Oxidative stress results in lipid oxidation, and cells in the brain have various strategies to prevent or clear lipid oxidative damage. Cells have many systems for maintaining redox balance and repairing oxidative damage, of which vitamin E and glutathione (GSH) are the most important for controlling lipid oxidation. Vitamin E is a lipophilic free radical scavenger, allowing it to embed in membranes and reduce ROS or terminate lipid radical chain reactions. Although vitamin E has shown promise in some mouse models of Alzheimer’s disease (Nakashima et al., 2004; Sung et al., 2004) and diabetes (Takemoto, Doi, & Masuoka, 2016), it has not shown benefits in human trials for MCI, AD (Lloret et al., 2009; Petersen et al., 2005), or diabetes (Ingram et al., 2012). GSH is an important antioxidant through several mechanisms. The enzyme glutathione peroxidase can use GSH to reduce peroxidized lipids. Glutathione S-transferases are a family of enzymes that conjugate GSH to many different xenobiotics, including 4-HNE (Singhal et al., 2015), to detoxify them. GSH is decreased in the brain in AD (Mandal, Saharan, Tripathi, & Murari, 2015) and diabetes (Mastrocola et al., 2005), which may contribute to the elevated levels of lipid peroxides and 4-HNE in these diseases.
Different cell types in the brain may have different roles in the clearance of peroxidized lipids. Rather than detoxify peroxidized fatty acids on their own, neurons preferentially export them in APOE+ lipoprotein particles which are endocytosed by astrocytes in vitro (Ioannou et al., 2019). These astrocytes incorporate neuron-derived peroxidized fatty acids into lipid droplets, from which they can be detoxified and exported or metabolized in the mitochondria. Glial lipid droplet production is also induced by neuronal oxidative stress in Drosophila (Bailey et al., 2015; L. Liu et al., 2015) and mice (L. Liu, MacKenzie, Putluri, Maletić-Savatić, & Bellen, 2017), suggesting a conserved strategy for sequestering and detoxifying oxidized lipids outside of neurons. Thus, therapies targeting lipid peroxides in the brain may benefit from future research focused on differential effects in neurons versus astrocytes and other glial cell types.
Unlike oxidized fatty acids, oxysterols cannot be degraded or detoxified in the brain and must be exported to the blood in order to travel to the liver and be detoxified there. The ATP-binding cassette transporter ABCG1 can export 7KC from cells to protect them from its pro-apoptotic effects (Terasaka, Wang, Yvan-Charvet, & Tall, 2007), and ABCG1 expression is promoted by signaling from the enzymatically produced oxysterol 24(S)OHC (Okabe et al., 2014). Interestingly, ABCG1 expression is decreased in diabetes (Daffu et al., 2015; Mauldin et al., 2006), while AD may result in decreased ABCG1 activity (Marchi et al., 2019). The receptor for advanced glycation end products (RAGE), which is implicated in inflammatory signaling in both Alzheimer’s disease and diabetes, can decrease ABCG1 transcription (Daffu et al., 2015) and may be a shared mechanism responsible for increased 7KC levels in the two diseases.
Conclusion
Diabetes is a complex disease and likely promotes neurodegeneration through multiple mechanisms. While the relative importance of various complications induced by diabetes on the health of the brain remains to be determined, it is reasonable to predict that therapeutic strategies seeking to ameliorate these disruptions may provide benefits in AD. As noted above, insulin signaling plays a role in healthy brain function and efforts to enhance insulin signaling in the brains of AD patients are ongoing (Avgerinos et al., 2018). Increased oxidative stress in diabetes results in increased lipid oxidation, and therapeutic strategies seeking either to reduce the production of oxidized lipids or enhance oxidized lipid clearance from the brain may be beneficial in AD. Clinical trials seeking to treat AD patients with antioxidants have so far failed to provide robust evidence of improving cognitive performance (Galasko et al., 2012; Lloret, Esteve, Monllor, Cervera-Ferri, & Lloret, 2019). However, the duration of treatment, stage of disease and ability of antioxidants to effectively act in the brain should be considered. The recent discoveries of biological processes which allow the brain to clear waste products may reveal novel strategies to enhance the clearance of oxidized lipids (Iliff et al., 2012; Louveau et al., 2015). Future research is required to determine if ameliorating one or more of these diabetes-mediated insults on the brain can positively impact the progression of AD.
References
- Amaral J, Lee JW, Chou J, Campos MM, & Rodríguez IR (2013). 7-Ketocholesterol Induces Inflammation and Angiogenesis In Vivo: A Novel Rat Model. PLoS ONE, 8(2), e56099. 10.1371/journal.pone.0056099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Ashby-Mitchell K, & Peters R (2017). Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. Journal of Alzheimer’s Disease : JAD, 56(1), 215–228. 10.3233/JAD-160826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, & Bennett DA (2006). Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology, 67(11), 1960–1965. 10.1212/01.wnl.0000247053.45483.4e [DOI] [PubMed] [Google Scholar]
- Avgerinos KI, Kalaitzidis G, Malli A, Kalaitzoglou D, Myserlis PG, & Lioutas V-A (2018). Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: a systematic review. Journal of Neurology, 265(7), 1497–1510. 10.1007/s00415-018-8768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach MA, Shen-Orr Z, Lowe WLJ, Roberts CTJ, & LeRoith D (1991). Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Research. Molecular Brain Research, 10(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Koster G, Guillermier C, Hirst EMA, MacRae JI, Lechene CP, … Gould AP (2015). Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell, 163(2), 340–353. 10.1016/J.CELL.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, & Butterfield DA (2012). Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radical Biology and Medicine, 52(11–12), 2292–2301. 10.1016/J.FREERADBIOMED.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, Hadziselimovic A, … Sanders CR (2012). The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science (New York, N.Y.), 336(6085), 1168–1171. 10.1126/science.1219988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, & Vigneri R (2009). Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine Reviews, 30(6), 586–623. 10.1210/er.2008-0047 [DOI] [PubMed] [Google Scholar]
- Benussi L, Ghidoni R, Dal Piaz F, Binetti G, Di Iorio G, & Abrescia P (2017). The level of 24-Hydroxycholesteryl Esters is an Early Marker of Alzheimer’s Disease. Journal of Alzheimer’s Disease : JAD, 56(2), 825–833. 10.3233/JAD-160930 [DOI] [PubMed] [Google Scholar]
- Best JD, Taborsky GJJ, Halter JB, & Porte DJ (1981). Glucose disposal is not proportional to plasma glucose level in man. Diabetes, 30(10), 847–850. 10.2337/diab.30.10.847 [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, & Wang Y (2008). Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obesity Reviews : An Official Journal of the International Association for the Study of Obesity, 9(3), 204–218. 10.1111/j.1467-789X.2008.00473.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya R, Barren C, & Kovacs DM (2013). Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 33(27), 11169–11183. 10.1523/JNEUROSCI.4704-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Deary IJ, & Ryan CM (2008). Cognition and diabetes: a lifespan perspective. The Lancet. Neurology, 7(2), 184–190. 10.1016/S1474-4422(08)70021-8 [DOI] [PubMed] [Google Scholar]
- Biessels GJ, & Despa F (2018). Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nature Reviews. Endocrinology, 14(10), 591–604. 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkhem I, & Meaney S (2004). Brain cholesterol: long secret life behind a barrier. Arteriosclerosis, Thrombosis, and Vascular Biology, 24(5), 806–815. 10.1161/01.ATV.0000120374.59826.1b [DOI] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN, & Allen NJ (2018). The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Reports, 22(1), 269–285. 10.1016/j.celrep.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, & Fehm HL (2002). Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience, 5(6), 514–516. 10.1038/nn849 [DOI] [PubMed] [Google Scholar]
- Borst JW, Visser NV, Kouptsova O, & Visser AJW. (2000). Oxidation of unsaturated phospholipids in membrane bilayer mixtures is accompanied by membrane fluidity changes. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1487(1), 61–73. 10.1016/S1388-1981(00)00084-6 [DOI] [PubMed] [Google Scholar]
- Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, … Dening T (2009). Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. Journal of Alzheimer’s Disease : JAD, 18(3), 645–658. 10.3233/JAD-2009-1182 [DOI] [PubMed] [Google Scholar]
- Brown MS, & Goldstein JL (1997). The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell, 89(3), 331–340. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, … Kahn CR (2000). Role of brain insulin receptor in control of body weight and reproduction. Science (New York, N.Y.), 289(5487), 2122–2125. 10.1126/science.289.5487.2122 [DOI] [PubMed] [Google Scholar]
- Cai W, Xue C, Sakaguchi M, Konishi M, Shirazian A, Ferris HA, … Kahn CR (2018). Insulin regulates astrocyte gliotransmission and modulates behavior. The Journal of Clinical Investigation, 128(7), 2914–2926. 10.1172/JCI99366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dai C-L, Wu Z, Iqbal K, Liu F, Zhang B, & Gong C-X (2017). Intranasal Insulin Prevents Anesthesia-Induced Cognitive Impairment and Chronic Neurobehavioral Changes. Frontiers in Aging Neuroscience, 9, 136. 10.3389/fnagi.2017.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Lyra E Silva NM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, … De Felice FG (2015). Alzheimer-associated Abeta oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Molecular Medicine, 7(2), 190–210. 10.15252/emmm.201404183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, … Pericak-Vance MA (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science (New York, N.Y.), 261(5123), 921–923. 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, … Plymate S (2000). Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Annals of the New York Academy of Sciences, 903, 222–228. 10.1111/j.1749-6632.2000.tb06371.x [DOI] [PubMed] [Google Scholar]
- Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, … O’Neill JS (2019). Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell, 177(4), 896–909.e20. 10.1016/j.cell.2019.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Morstorf T, & Zhong K (2014). Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Research & Therapy, 6(4), 37. 10.1186/alzrt269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffu G, Shen X, Senatus L, Thiagarajan D, Abedini A, Pozo C. H. del, … Schmidt AM (2015). RAGE Suppresses ABCG1-Mediated Macrophage Cholesterol Efflux in Diabetes. Diabetes, 64(12), 4046–4060. 10.2337/DB15-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, & Wands JR (2005). Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. Journal of Alzheimer’s Disease : JAD, 7(1), 45–61. [DOI] [PubMed] [Google Scholar]
- de Souza Bastos A, Graves DT, de Melo Loureiro AP, Júnior CR, Corbi SCT, Frizzera F, … Orrico SRP (2016). Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. Journal of Diabetes and Its Complications, 30(8), 1593–1599. 10.1016/J.JDIACOMP.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Bovi RJ, Kim J, Suresh P, London E, & Miller WT (2019). Sterol structure dependence of insulin receptor and insulin-like growth factor 1 receptor activation. Biochimica et Biophysica Acta. Biomembranes, 1861(4), 819–826. 10.1016/j.bbamem.2019.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, & Zahm DS (1994). Insulin gene expression and insulin synthesis in mammalian neuronal cells. The Journal of Biological Chemistry, 269(11), 8445–8454. [PubMed] [Google Scholar]
- Devaskar SU, Singh BS, Carnaghi LR, Rajakumar PA, & Giddings SJ (1993). Insulin II gene expression in rat central nervous system. Regulatory Peptides, 48(1–2), 55–63. [DOI] [PubMed] [Google Scholar]
- Endo K, Oyama T, Saiki A, Ban N, Ohira M, Koide N, … Shirai K (2008). Determination of serum 7-ketocholesterol concentrations and their relationships with coronary multiple risks in diabetes mellitus. Diabetes Research and Clinical Practice, 80(1), 63–68. 10.1016/J.DIABRES.2007.10.023 [DOI] [PubMed] [Google Scholar]
- Fatani SH, Babakr AT, NourEldin EM, & Almarzouki AA (2016). Lipid peroxidation is associated with poor control of type-2 diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 10(2), S64–S67. 10.1016/J.DSX.2016.01.028 [DOI] [PubMed] [Google Scholar]
- Ferderbar S, Pereira EC, Apolinário E, Bertolami MC, Faludi A, Monte O, … Abdalla DSP (2007). Cholesterol oxides as biomarkers of oxidative stress in type 1 and type 2 diabetes mellitus. Diabetes/Metabolism Research and Reviews, 23(1), 35–42. 10.1002/dmrr.645 [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Hernandez-Garzon E, Perez-Domper P, Perez-Alvarez A, Mederos S, Matsui T, … Torres Aleman I (2017). Insulin Regulates Astrocytic Glucose Handling Through Cooperation With IGF-I. Diabetes, 66(1), 64–74. 10.2337/db16-0861 [DOI] [PubMed] [Google Scholar]
- Fernandez AM, & Torres-Aleman I (2012). The many faces of insulin-like peptide signalling in the brain. Nature Reviews. Neuroscience, 13(4), 225–239. 10.1038/nrn3209 [DOI] [PubMed] [Google Scholar]
- Fukui K, Ferris HA, & Kahn CR (2015). Effect of cholesterol reduction on receptor signaling in neurons. The Journal of Biological Chemistry, 290(44), 26383–26392. 10.1074/jbc.M115.664367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, … Aisen P (2012). Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Archives of Neurology, 69(7), 836–841. 10.1001/archneurol.2012.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Caceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, … Tschop MH (2016). Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell, 166(4), 867–880. 10.1016/j.cell.2016.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschler MM, & Stockwell BR (2017). Lipid peroxidation in cell death. Biochemical and Biophysical Research Communications, 482(3), 419–425. 10.1016/J.BBRC.2016.10.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijselaers SLC, Aalten P, Ramakers IHGB, De Deyn PP, Heijboer AC, Koek HL, … Biessels GJ (2018). Association of Cerebrospinal Fluid (CSF) Insulin with Cognitive Performance and CSF Biomarkers of Alzheimer’s Disease. Journal of Alzheimer’s Disease : JAD, 61(1), 309–320. 10.3233/JAD-170522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genaro-Mattos TC, Anderson A, Allen LB, Korade Z, & Mirnics K (2019). Cholesterol Biosynthesis and Uptake in Developing Neurons. ACS Chemical Neuroscience. 10.1021/acschemneuro.9b00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ramos A, Díaz-Nido J, Smith MA, Perry G, & Avila J (2003). Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. Journal of Neuroscience Research, 71(6), 863–870. 10.1002/jnr.10525 [DOI] [PubMed] [Google Scholar]
- Goodner CJ, Hom FG, & Berrie MA (1980). Investigation of the effect of insulin upon regional brain glucose metabolism in the rat in vivo. Endocrinology, 107(6), 1827–1832. 10.1210/endo-107-6-1827 [DOI] [PubMed] [Google Scholar]
- Gray SM, Aylor KW, & Barrett EJ (2017). Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia, 60(8), 1512–1521. 10.1007/s00125-017-4285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, Gomez-Isla T, Behl C, … Nitsch RM (2000). The human DIMINUTO/DWARF1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(19), 7345–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Coma M, Pera M, Clarimon J, Sereno L, Agullo JM, … Lleo A (2009). Mild cholesterol depletion reduces amyloid-beta production by impairing APP trafficking to the cell surface. Journal of Neurochemistry, 110(1), 220–230. 10.1111/j.1471-4159.2009.06126.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup ER, Broderick SO, Russell MK, Fang Y, Bartke A, Boger HA, & Hascup KN (2019). Diet-induced insulin resistance elevates hippocampal glutamate as well as VGLUT1 and GFAP expression in AbetaPP/PS1 mice. Journal of Neurochemistry, 148(2), 219–237. 10.1111/jnc.14634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heras-Sandoval D, Ferrera P, & Arias C (2012). Amyloid-beta protein modulates insulin signaling in presynaptic terminals. Neurochemical Research, 37(9), 1879–1885. 10.1007/s11064-012-0800-7 [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, … Williams J (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature Genetics, 43(5), 429–435. 10.1038/ng.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature, 444(7121), 860–867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Hoyer S (2002). The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. Journal of Neural Transmission (Vienna, Austria : 1996), 109(3), 341–360. 10.1007/s007020200028 [DOI] [PubMed] [Google Scholar]
- Huang J-D, Amaral J, Lee JW, & Rodriguez IR (2014). 7-Ketocholesterol-Induced Inflammation Signals Mostly through the TLR4 Receptor Both In Vitro and In Vivo. PLoS ONE, 9(7), e100985. 10.1371/journal.pone.0100985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain G, Wang J, Rasul A, Anwar H, Imran A, Qasim M, … Sun T (2019). Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids in Health and Disease, 18(1), 26. 10.1186/s12944-019-0965-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T-PV, Davis AA, Ulrich JD, & Holtzman DM (2017). Apolipoprotein E and Alzheimer’s disease: the influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins. Journal of Lipid Research, 58(5), 824–836. 10.1194/jlr.R075481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, … Nedergaard M (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science Translational Medicine, 4(147), 147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indaram M, Ma W, Zhao L, Fariss RN, Rodriguez IR, & Wong WT (2015). 7-Ketocholesterol Increases Retinal Microglial Migration, Activation and Angiogenicity: A Potential Pathogenic Mechanism Underlying Age-related Macular Degeneration. Scientific Reports, 5(1), 9144. 10.1038/srep09144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram KH, Hill H, Moellering DR, Hill BG, Lara-Castro C, Newcomer B, … Garvey WT (2012). Skeletal Muscle Lipid Peroxidation and Insulin Resistance in Humans. The Journal of Clinical Endocrinology & Metabolism, 97(7), E1182–E1186. 10.1210/jc.2011-2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou MS, Jackson J, Sheu S-H, Chang C-L, Weigel AV, Liu H, … Liu Z (2019). Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell, 177(6), 1522–1535.e14. 10.1016/J.CELL.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, … Heneka MT (2019). NLRP3 inflammasome activation drives tau pathology. Nature, 575(7784), 669–673. 10.1038/s41586-019-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS, Janzen J, Magee AL, & Ley SC (2000). Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. European Journal of Immunology, 30(3), 954–963. [DOI] [PubMed] [Google Scholar]
- Kim B-H, Kelschenbach J, Borjabad A, Hadas E, He H, Potash MJ, … Volsky DJ (2019). Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS (London, England), 33(6), 973–984. 10.1097/QAD.0000000000002150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Ferris HA, Cai W, & Kahn CR (2014). Insulin action in brain regulates systemic metabolism and brain function. Diabetes, 63(7), 2232–2243. 10.2337/db14-0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, … Bruning JC (2008). Central insulin action regulates peripheral glucose and fat metabolism in mice. The Journal of Clinical Investigation, 118(6), 2132–2147. 10.1172/JCI31073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch H, Heun R, Jessen F, Popp J, Hentschel F, Maier W, & Lutjohann D (2010). Alterations of cholesterol precursor levels in Alzheimer’s disease. Biochimica et Biophysica Acta, 1801(8), 945–950. 10.1016/j.bbalip.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, … Kahn CR (2017). Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proceedings of the National Academy of Sciences of the United States of America, 114(40), E8478–E8487. 10.1073/pnas.1710625114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari V, Luo Y, Tornabene T, O’Neill AM, Greene MW, Geetha T, & Babu JR (2017). High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochimica et Biophysica Acta. Molecular Basis of Disease, 1863(2), 499–508. 10.1016/j.bbadis.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, … Amouyel P (2009). Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature Genetics, 41(10), 1094–1099. 10.1038/ng.439 [DOI] [PubMed] [Google Scholar]
- Larrayoz IM, Huang J-D, Lee JW, Pascual I, & Rodríguez IR (2010). 7-Ketocholesterol–Induced Inflammation: Involvement of Multiple Kinase Signaling Pathways via NFκB but Independently of Reactive Oxygen Species Formation. Investigative Opthalmology & Visual Science, 51(10), 4942. 10.1167/iovs.09-4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, & Butterfield DA (2001). The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Aβ1–42. Journal of Neurochemistry, 78(2), 413–416. 10.1046/j.1471-4159.2001.00451.x [DOI] [PubMed] [Google Scholar]
- Lee CH, Kim HJ, Lee Y-S, Kang GM, Lim HS, Lee S-H, … Kim M-S (2018). Hypothalamic Macrophage Inducible Nitric Oxide Synthase Mediates Obesity-Associated Hypothalamic Inflammation. Cell Reports, 25(4), 934–946.e5. 10.1016/j.celrep.2018.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-K, Kumar P, Fu Q, Rosen KM, & Querfurth HW (2009). The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Molecular Biology of the Cell, 20(5), 1533–1544. 10.1091/mbc.e08-07-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonarduzzi G, Vizio B, Sottero B, Verde V, Gamba P, Mascia C, … Biasi F (2006). Early Involvement of ROS Overproduction in Apoptosis Induced by 7-Ketocholesterol. Antioxidants & Redox Signaling, 8(3–4), 375–380. 10.1089/ars.2006.8.375 [DOI] [PubMed] [Google Scholar]
- Leoni V, & Caccia C (2011). Oxysterols as biomarkers in neurodegenerative diseases. Chemistry and Physics of Lipids, 164(6), 515–524. 10.1016/j.chemphyslip.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Liang Q, Liu H, Zhang T, Jiang Y, Xing H, & Zhang A (2016). Discovery of serum metabolites for diagnosis of progression of mild cognitive impairment to Alzheimer’s disease using an optimized metabolomics method. RSC Advances, 6(5), 3586–3591. 10.1039/C5RA19349D [DOI] [Google Scholar]
- Liu C-C, Liu C-C, Kanekiyo T, Xu H, & Bu G (2013, February). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews. Neurology, Vol. 9, pp. 106–118. 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, MacKenzie KR, Putluri N, Maletić-Savatić M, & Bellen HJ (2017). The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metabolism, 26(5), 719–737.e6. 10.1016/J.CMET.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, … Bellen HJ (2015). Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell, 160(1–2), 177–190. 10.1016/J.CELL.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret A, Badía M-C, Mora NJ, Pallardó FV, Alonso M-D, & Viña J (2009). Vitamin E Paradox in Alzheimer’s Disease: It Does Not Prevent Loss of Cognition and May Even Be Detrimental. Journal of Alzheimer’s Disease, 17(1), 143–149. 10.3233/JAD-2009-1033 [DOI] [PubMed] [Google Scholar]
- Lloret A, Esteve D, Monllor P, Cervera-Ferri A, & Lloret A (2019). The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. International Journal of Molecular Sciences, 20(4). 10.3390/ijms20040879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loera-Valencia R, Cedazo-Minguez A, Kenigsberg PA, Page G, Duarte AI, Giusti P, … Winblad B (2019). Current and emerging avenues for Alzheimer’s disease drug targets. Journal of Internal Medicine, 286(4), 398–437. 10.1111/joim.12959 [DOI] [PubMed] [Google Scholar]
- Loh K, Zhang L, Brandon A, Wang Q, Begg D, Qi Y, … Herzog H (2017). Insulin controls food intake and energy balance via NPY neurons. Molecular Metabolism, 6(6), 574–584. 10.1016/j.molmet.2017.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, … De Felice FG (2013). TNF-alpha mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s beta-amyloid oligomers in mice and monkeys. Cell Metabolism, 18(6), 831–843. 10.1016/j.cmet.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, … Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Saharan S, Tripathi M, & Murari G (2015). Brain Glutathione Levels – A Novel Biomarker for Mild Cognitive Impairment and Alzheimer’s Disease. Biological Psychiatry, 78(10), 702–710. 10.1016/J.BIOPSYCH.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Marchi C, Adorni MP, Caffarra P, Ronda N, Spallazzi M, Barocco F, … Zimetti F (2019). ABCA1- and ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer’s disease. Journal of Lipid Research, jlr.P091033. 10.1194/jlr.P091033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak E, Leboucher A, Caron E, Ahmed T, Tailleux A, Dumont J, … Blum D (2017). Tau deletion promotes brain insulin resistance. The Journal of Experimental Medicine, 214(8), 2257–2269. 10.1084/jem.20161731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquer C, Devauges V, Cossec J-C, Liot G, Lecart S, Saudou F, … Potier M-C (2011). Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 25(4), 1295–1305. 10.1096/fj.10-168633 [DOI] [PubMed] [Google Scholar]
- Martin-Segura A, Ahmed T, Casadome-Perales A, Palomares-Perez I, Palomer E, Kerstens A, … Dotti CG (2019). Age-associated cholesterol reduction triggers brain insulin resistance by facilitating ligand-independent receptor activation and pathway desensitization. Aging Cell, 18(3), e12932. 10.1111/acel.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, & Boccuzzi G (2005). Oxidative and nitrosative stress in brain mitochondria of diabetic rats. Journal of Endocrinology, 187(1), 37–44. 10.1677/joe.1.06269 [DOI] [PubMed] [Google Scholar]
- Mateos L, Ismail M-A-M, Gil-Bea F-J, Leoni V, Winblad B, Bjorkhem I, & Cedazo-Minguez A (2011). Upregulation of brain renin angiotensin system by 27-hydroxycholesterol in Alzheimer’s disease. Journal of Alzheimer’s Disease : JAD, 24(4), 669–679. 10.3233/JAD-2011-101512 [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, & Pfrieger FW (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science (New York, N.Y.), 294(5545), 1354–1357. 10.1126/science.294.5545.1354 [DOI] [PubMed] [Google Scholar]
- Mauldin JP, Srinivasan S, Mulya A, Gebre A, Parks JS, Daugherty A, & Hedrick CC (2006). Reduction in ABCG1 in Type 2 Diabetic Mice Increases Macrophage Foam Cell Formation. Journal of Biological Chemistry, 281(30), 21216–21224. 10.1074/JBC.M510952200 [DOI] [PubMed] [Google Scholar]
- McGeer PL, Rogers J, & McGeer EG (2016). Inflammation, Antiinflammatory Agents, and Alzheimer’s Disease: The Last 22 Years. Journal of Alzheimer’s Disease : JAD, 54(3), 853–857. 10.3233/JAD-160488 [DOI] [PubMed] [Google Scholar]
- Mittal K, & Katare DP (2016). Shared links between type 2 diabetes mellitus and Alzheimer’s disease: A review. Diabetes & Metabolic Syndrome, 10(2 Suppl 1), S144–9. 10.1016/j.dsx.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Morales-Corraliza J, Wong H, Mazzella MJ, Che S, Lee SH, Petkova E, … Mathews PM (2016). Brain-Wide Insulin Resistance, Tau Phosphorylation Changes, and Hippocampal Neprilysin and Amyloid-beta Alterations in a Monkey Model of Type 1 Diabetes. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 36(15), 4248–4258. 10.1523/JNEUROSCI.4640-14.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira EF, Larrayoz IM, Lee JW, & Rodríguez IR (2009). 7-Ketocholesterol Is Present in Lipid Deposits in the Primate Retina: Potential Implication in the Induction of VEGF and CNV Formation. Investigative Opthalmology & Visual Science, 50(2), 523. 10.1167/iovs.08-2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakandakari SCBR, Munoz VR, Kuga GK, Gaspar RC, Sant’Ana MR, Pavan ICB, … Pauli JR (2019). Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain, Behavior, and Immunity, 79, 284–293. 10.1016/j.bbi.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Nakashima H, Ishihara T, Yokota O, Terada S, Trojanowski JQ, Lee VM-Y, & Kuroda S (2004). Effects of α-tocopherol on an animal model of tauopathies. Free Radical Biology and Medicine, 37(2), 176–186. 10.1016/j.freeradbiomed.2004.04.037 [DOI] [PubMed] [Google Scholar]
- Nordestgaard LT, Tybjaerg-Hansen A, Nordestgaard BG, & Frikke-Schmidt R (2015). Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 11(12), 1430–1438. 10.1016/j.jalz.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, … Nissinen A (1998). Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology, 17(1), 14–20. 10.1159/000026149 [DOI] [PubMed] [Google Scholar]
- Okabe A, Urano Y, Itoh S, Suda N, Kotani R, Nishimura Y, … Noguchi N (2014). Adaptive responses induced by 24S-hydroxycholesterol through liver X receptor pathway reduce 7-ketocholesterol-caused neuronal cell death. Redox Biology, 2, 28–35. 10.1016/J.REDOX.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, & Breteler MM (1999). Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology, 53(9), 1937–1942. 10.1212/wnl.53.9.1937 [DOI] [PubMed] [Google Scholar]
- Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, … Butterfield DA (2010). Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: Implications for Aβ accumulation in AD brain. Free Radical Biology and Medicine, 49(11), 1798–1803. 10.1016/J.FREERADBIOMED.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A, Lutjohann D, Bagli M, Locatelli S, Jessen F, Buschfort R, … Heun R (2002). 24S-hydroxycholesterol in cerebrospinal fluid is elevated in early stages of dementia. Journal of Psychiatric Research, 36(1), 27–32. [DOI] [PubMed] [Google Scholar]
- Peri A, & Serio M (2008). Neuroprotective effects of the Alzheimer’s disease-related gene seladin-1. Journal of Molecular Endocrinology, 41(5), 251–261. 10.1677/JME-08-0071 [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, … Alzheimer’s Disease Cooperative Study Group. (2005). Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. New England Journal of Medicine, 352(23), 2379–2388. 10.1056/NEJMoa050151 [DOI] [PubMed] [Google Scholar]
- Petrov D, Pedros I, Artiach G, Sureda FX, Barroso E, Pallas M, … Camins A (2015). High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochimica et Biophysica Acta, 1852(9), 1687–1699. 10.1016/j.bbadis.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Pike LJ (2003). Lipid rafts: bringing order to chaos. Journal of Lipid Research, 44(4), 655–667. 10.1194/jlr.R200021-JLR200 [DOI] [PubMed] [Google Scholar]
- Popp J, Lewczuk P, Kolsch H, Meichsner S, Maier W, Kornhuber J, … Lutjohann D (2012). Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. Journal of Neurochemistry, 123(2), 310–316. 10.1111/j.1471-4159.2012.07893.x [DOI] [PubMed] [Google Scholar]
- Ramos-Rodriguez JJ, Spires-Jones T, Pooler AM, Lechuga-Sancho AM, Bacskai BJ, & Garcia-Alloza M (2017). Progressive Neuronal Pathology and Synaptic Loss Induced by Prediabetes and Type 2 Diabetes in a Mouse Model of Alzheimer’s Disease. Molecular Neurobiology, 54(5), 3428–3438. 10.1007/s12035-016-9921-3 [DOI] [PubMed] [Google Scholar]
- Ratcliffe LE, Vazquez Villasenor I, Jennings L, Heath PR, Mortiboys H, Schwartzentruber A, … Wharton SB (2018). Loss of IGF1R in Human Astrocytes Alters Complex I Activity and Support for Neurons. Neuroscience, 390, 46–59. 10.1016/j.neuroscience.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza H, John A, & Howarth FC (2015). Increased Oxidative Stress and Mitochondrial Dysfunction in Zucker Diabetic Rat Liver and Brain. Cellular Physiology and Biochemistry, 35(3), 1241–1251. 10.1159/000373947 [DOI] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, & de la Monte SM (2005). Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. Journal of Alzheimer’s Disease : JAD, 8(3), 247–268. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Cha RH, Mielke MM, Pankratz VS, Boeve BF, … Lowe VJ (2014). Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. Journal of Nuclear Medicine : Official Publication, Society of Nuclear Medicine, 55(5), 759–764. 10.2967/jnumed.113.132647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rodriguez P, Sandebring-Matton A, Merino-Serrais P, Parrado-Fernandez C, Rabano A, Winblad B, … Cedazo-Minguez A (2017). Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain : A Journal of Neurology, 140(12), 3269–3285. 10.1093/brain/awx256 [DOI] [PubMed] [Google Scholar]
- Rodriguez IR, & Fliesler SJ (2009). Photodamage Generates 7-keto- and 7-hydroxycholesterol in the Rat Retina via a Free Radical-mediated Mechanism. Photochemistry and Photobiology, 85(5), 1116–1125. 10.1111/j.1751-1097.2009.00568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez IR, & Larrayoz IM (2010). Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. Journal of Lipid Research, 51(10), 2847. 10.1194/JLR.R004820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S, Mitro N, Giatti S, Diviccaro S, Pesaresi M, Spezzano R, … Melcangi RC (2018). Diabetes induces mitochondrial dysfunction and alters cholesterol homeostasis and neurosteroidogenesis in the rat cerebral cortex. The Journal of Steroid Biochemistry and Molecular Biology, 178, 108–116. 10.1016/j.jsbmb.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Samadi A, Gurlek A, Sendur SN, Karahan S, Akbiyik F, & Lay I (2019). Oxysterol species: reliable markers of oxidative stress in diabetes mellitus. Journal of Endocrinological Investigation, 42(1), 7–17. 10.1007/s40618-018-0873-5 [DOI] [PubMed] [Google Scholar]
- Sanguinetti E, Guzzardi MA, Panetta D, Tripodi M, De Sena V, Quaglierini M, … Iozzo P (2019). Combined Effect of Fatty Diet and Cognitive Decline on Brain Metabolism, Food Intake, Body Weight, and Counteraction by Intranasal Insulin Therapy in 3xTg Mice. Frontiers in Cellular Neuroscience, 13, 188. 10.3389/fncel.2019.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Ansari MA, & Mufson EJ (2016). Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiology of Aging, 42, 1–12. 10.1016/J.NEUROBIOLAGING.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi T, Di Lazzaro G, Sancesario GM, Colona VL, Scaricamazza E, Mercuri NB, … Sancesario G (2017). Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer’s disease. Journal of Neural Transmission (Vienna, Austria : 1996), 124(12), 1621–1625. 10.1007/s00702-017-1786-8 [DOI] [PubMed] [Google Scholar]
- Schonknecht P, Lutjohann D, Pantel J, Bardenheuer H, Hartmann T, von Bergmann K, … Schroder J (2002). Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neuroscience Letters, 324(1), 83–85. 10.1016/s0304-3940(02)00164-7 [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, … White MF (2003). Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 23(18), 7084–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, … Bruning JC (2004). Role for neuronal insulin resistance in neurodegenerative diseases. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3100–3105. 10.1073/pnas.0308724101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, & Hardy J (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Molecular Medicine, 8(6), 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley ML, Close DR, & Stern SE (2002). The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease. Neurobiology of Aging, 23(3), 383–388. 10.1016/S0197-4580(01)00327-X [DOI] [PubMed] [Google Scholar]
- Singhal SS, Singh SP, Singhal P, Horne D, Singhal J, & Awasthi S (2015). Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicology and Applied Pharmacology, 289(3), 361–370. 10.1016/J.TAAP.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M, Cai W, Konishi M, & Kahn CR (2019). Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proceedings of the National Academy of Sciences of the United States of America, 116(13), 6379–6384. 10.1073/pnas.1817391116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözbir E, & Nazıroğlu M (2016). Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metabolic Brain Disease, 31(2), 385–393. 10.1007/s11011-015-9769-7 [DOI] [PubMed] [Google Scholar]
- Spinelli M, Fusco S, Mainardi M, Scala F, Natale F, Lapenta R, … Grassi C (2017). Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nature Communications, 8(1), 2009. 10.1038/s41467-017-02221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu I-C, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, … Dewachter I (2019). Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathologica, 137(4), 599–617. 10.1007/s00401-018-01957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, … de la Monte SM (2005). Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? Journal of Alzheimer’s Disease : JAD, 7(1), 63–80. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, & Roses AD (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 90(5), 1977–1981. 10.1073/pnas.90.5.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee VM-Y, Trojanowski JQ, & Praticò D (2004). Early vitamin E supplementation in young but not aged mice reduces Aβ levels and amyloid deposition in a transgenic model of Alzheimer’s disease. The FASEB Journal, 18(2), 323–325. 10.1096/fj.03-0961fje [DOI] [PubMed] [Google Scholar]
- Suzuki R, Ferris HA, Chee MJ, Maratos-Flier E, & Kahn CR (2013). Reduction of the cholesterol sensor SCAP in the brains of mice causes impaired synaptic transmission and altered cognitive function. PLoS Biology, 11(4), e1001532. 10.1371/journal.pbio.1001532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Lee K, Jing E, Biddinger SB, McDonald JG, Montine TJ, … Kahn CR (2010). Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metabolism, 12(6), 567–579. 10.1016/j.cmet.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Kong Q, Lin Y, Stouffer N, Schulte DA, Lai L, … Lin C-LG (2015). Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. The Journal of Experimental Medicine, 212(3), 319–332. 10.1084/jem.20140413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K, Doi W, & Masuoka N (2016). Protective effect of vitamin E against alloxan-induced mouse hyperglycemia. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1862(4), 647–650. 10.1016/J.BBADIS.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Talbot K, Wang H-Y, Kazi H, Han L-Y, Bakshi KP, Stucky A, … Arnold SE (2012). Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of Clinical Investigation, 122(4), 1316–1338. 10.1172/JCI59903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka N, Wang N, Yvan-Charvet L, & Tall AR (2007). High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proceedings of the National Academy of Sciences of the United States of America, 104(38), 15093–15098. 10.1073/pnas.0704602104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa G, Staurenghi E, Zerbinati C, Gargiulo S, Iuliano L, Giaccone G, … Gamba P (2016). Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biology, 10, 24–33. 10.1016/J.REDOX.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagelatos NT, & Eslick GD (2013). Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiologic Reviews, 35, 152–160. 10.1093/epirev/mxs012 [DOI] [PubMed] [Google Scholar]
- van Deijk A-LF, Camargo N, Timmerman J, Heistek T, Brouwers JF, Mogavero F, … Verheijen MHG (2017). Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia, 65(4), 670–682. 10.1002/glia.23120 [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, & Ramakers GMJ (2005). Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. Journal of Neurochemistry, 94(4), 1158–1166. 10.1111/j.1471-4159.2005.03269.x [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, & Thinakaran G (2010). Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochimica et Biophysica Acta, 1801(8), 860–867. 10.1016/j.bbalip.2010.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa A, Bentivegna M, Calfa G, Filipello F, Pomilio C, Bonaventura MM, … Saravia F (2018). Early Exposure to a High-Fat Diet Impacts on Hippocampal Plasticity: Implication of Microglia-Derived Exosome-like Extracellular Vesicles. Molecular Neurobiology. 10.1007/s12035-018-1435-8 [DOI] [PubMed] [Google Scholar]
- Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, … Golde TE (2002). Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiology of Disease, 9(1), 11–23. 10.1006/nbdi.2001.0470 [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Yamaguchi K, Matsui K, Sano T, Kubota T, Hashimoto T, … Iwatsubo T (2019). Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer’s disease. Molecular Neurodegeneration, 14(1), 15. 10.1186/s13024-019-0315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, & Jia J-P (2007). [Correlation of cholesterol 24-hydroxylase and ATP-binding cassette transporter A1 polymorphisms with Alzheimer’s disease]. Zhonghua yi xue za zhi, 87(9), 614–618. [PubMed] [Google Scholar]
- Wang X, Zheng W, Xie J-W, Wang T, Wang S-L, Teng W-P, & Wang Z-Y (2010). Insulin deficiency exacerbates cerebral amyloidosis and behavioral deficits in an Alzheimer transgenic mouse model. Molecular Neurodegeneration, 5, 46. 10.1186/1750-1326-5-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Yamaguchi T, Ishihara N, Nakamura S, Tanaka S, Oka R, … Tatsuno I (2018). 7-Ketocholesterol induces ROS-mediated mRNA expression of 12-lipoxygenase, cyclooxygenase-2 and pro-inflammatory cytokines in human mesangial cells: Potential role in diabetic nephropathy. Prostaglandins & Other Lipid Mediators, 134, 16–23. 10.1016/J.PROSTAGLANDINS.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Wong-ekkabut J, Xu Z, Triampo W, Tang I-M, Peter Tieleman D, & Monticelli L (2007). Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophysical Journal, 93(12), 4225–4236. 10.1529/BIOPHYSJ.107.112565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, & Martins R (2002). Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 22(10), RC221. https://doi.org/20026383 [DOI] [PMC free article] [PubMed] [Google Scholar]