Abstract
BACKGROUND AND PURPOSE: This study was undertaken to assess the utility of contrast-enhanced MR angiography at 6 months after endovascular treatment of intracranial aneurysms with Guglielmi detachable coils.
METHODS: Contrast-enhanced MR angiography was performed in 47 patients at 6 and 12 months after endovascular treatment of intracranial aneurysms (48 aneurysms). Digital subtraction angiography (DSA) was used as reference and was performed at 12 months after the treatment in all patients. MR angiographs were analyzed independently by two senior radiologists. DSA and MR angiography findings were assigned into one of three categories: complete obliteration, residual neck, or residual aneurysm.
RESULTS: All examinations were assessable. Interobserver agreement was judged as very good for contrast-enhanced MR angiography (κ = 0.96), with one discrepancy between examiners. Comparison between MR angiography at 6 months and DSA at 12 months showed an excellent agreement between techniques (κ = 0.93). Two cases of complete occlusion at DSA were misclassified as a residual neck at 6-month MR angiography. All aneurysm recanalizations at DSA already were detected on MR angiography at 6 months. The size of aneurysm recanalization did not increase between both MR angiographs performed at 6 and 12 months.
CONCLUSION: Contrast-enhanced MR angiography after selective embolization of intracranial aneurysm seems to predict properly early aneurysm recanalizations.
Endovascular treatment of intracranial aneurysms has been routinely used for about 10 years and constitutes an excellent alternative to surgery. The long-term outcome of patients treated by Guglielmi detachable coils (GDCs) remains unknown and is currently being evaluated. Recurrences may occur due to coil compaction or aneurysm regrowing. Therefore, imaging follow-up has been recommended to identify potential aneurysmal regrowth and to assess the need for further treatment. Digital subtraction angiography (DSA) is seen as the criterion standard, because of its inherent high spatial resolution, but its high cost and risk of complications limit its use. Recently, MR angiography using time-of-flight (TOF) sequences has been suggested as a noninvasive technique for long-term aneurysm follow-up (1–5). A few studies have demonstrated the ability of contrast-enhanced MR angiography for the detection of aneurysm recanalization (5), but no consensus exists about the delay between the endovascular treatment and the first imaging control. DSA or MR angiography is usually performed at 3 months, 1 year, and 3 years after the treatment (6), but delay between initial treatment and imaging control changes according to the series with a range between 6 and 31 months (1, 2, 4, 6–18).
The aim of this study was to perform follow-up MR angiography at 6 and 12 months after treatment of intracranial aneurysms with GDC coils to assess the usefulness of early MR angiography for the detection of aneurysm recanalization.
Methods
Study Design
To evaluate the interest of contrast-enhanced MR angiography in the detection of aneurysm recanalization the first year after embolization, MR angiography was carried out at 6 and 12 months and DSA at 12 months after treatment. DSA was considered as the reference.
Patients
This study was conducted in 47 patients (24 women, 23 men; median age, 45.7 years; age range, 22–70 years) from April 1999 to December 2001, including 45 admitted for a subarachnoid hemorrhage (SAH) confirmed on brain CT scan (Tables 1 and 2). DSA demonstrated a total of 48 saccular aneurysms. Forty aneurysms were located in the anterior circulation and eight in the posterior circulation. The size of aneurysms ranged from 3 mm to 20 mm, and the ratio between the aneurysmal sac diameter and the neck was classified as smaller or larger.
TABLE 1:
Location of aneurysms (n = 48)
| Location | N |
|---|---|
| Internal carotid artery | 1 |
| Anterior communicating artery | 34 |
| Anterior cerebral artery | 4 |
| Middle cerebral artery | 1 |
| Basilar artery | 3 |
| Posterior cerebral artery | 2 |
| Superior cerebellar artery | 1 |
| Posterior inferior cerebellar artery | 2 |
TABLE 2:
Size of aneurysms in millimeters (n = 48)
| Size | N |
|---|---|
| <5 | 32 |
| 5–10 | 10 |
| 10–20 | 6 |
Patients were referred to the neuroradiology department for endovascular treatment according to our protocol. Therapeutic alternatives were discussed between neurosurgical and neurointerventional teams. All ruptured aneurysms were treated by using GDCs within a few days of SAH.
DSA
Endovascular treatment was performed on a digital subtraction system (Integris V 3000, Philips Medical Systems, Best, the Netherlands). Follow-up angiograms at 1 year included a selective injection of common carotid or vertebral arteries with intracranial views (frontal, sagittal) completed by additional views when necessary. Each angiogram was acquired at two images per second with a 512 × 512 matrix size and a 20-cm field of view. For each projection, a 8–16-mL bolus of iodinated contrast material (Iohexol, Omnipaque, Amersham Health, Cork, Ireland) was injected at a rate between 3 and 6 mL/s by using a power injector.
Imaging Technique
MR angiographs were performed on a Siemens 1.5T system (Magnetom Vision Siemens, Germany) with 25-mT/m maximum gradient strength. All the examinations were performed with a standard head coil. Patients were positioned with a 20-gauge intravenous catheter inserted into the antecubital vein. After scout images in three planes were obtained, the following sequences were programmed. A contrast-enhanced MR angiography FISP (fast imaging steady-state precession) sequence was acquired in the coronal plane. The acquisition volume was placed on the sagittal scout image in an oblique direction so that the volume included the cervical carotid arteries, the carotid siphons, the A1 and A2 segments of the anterior cerebral arteries, the M1 and M2 segments of the middle cerebral arteries, the basilar artery, and the initial segment of the posterior cerebral arteries. The parameters were as follows: repetition time/echo time, 6.8/2.3; flip angle, 35°; FOV, 25; and matrix, 150 × 512. The anteroposterior diameter was 60 mm, and the acquisition time 40 seconds. A bolus of 0.2 mmol/kg of gadolinium chelate (Gadodiamide Omniscan; Amersham Health) was injected at a rate of 2 mL/s by using an MR-compatible power injector (Spectris, Medrad, Pittsburgh, PA). The circulation time of contrast media from the antecubital vein to the carotid or vertebral arteries was estimated by using a test bolus before 3D MR angiography. Two-dimensional images (2D turboflash sequence; 3.3/1.4; flip angle, 8 °; FOV, 25; section thickness, 8 mm; and matrix, 88 × 128) were acquired every second for 60 seconds at the level of the carotid siphons. The start of the test bolus injection coincided with the start of the sequence. The circulation time was then calculated by means of signal intensity measurements within a ROI placed either over the carotid siphon or the basilar artery. Source images were then reconstructed by using a maximum intensity projection (MIP) algorithm. Multiple projections were obtained every 15° over 180° in lateral and anteroposterior views.
The first MR angiography was performed at 6 months (average, 6.06 months; range, 4–8 months) and the second MR angiography and DSA at 12 months (average, 12.3 months; range, 9–15 months). The time range between the first MR angiography and DSA at 1 year did not exceed 24 hours.
Image Analysis
Image quality of MR angiographs was judged in a consensual manner by two senior neuroradiologists (J.Y.G., M.P) according to the following criteria: image contrast, artifact (metal [GDC], motion), vessel overlap, and patency of intracranial arteries. Image contrast was graded as low when the signal intensity in the enhanced arterial lumen was only slightly higher than the signal intensity in the background, as moderate when the signal intensity was clearly higher and high when the signal intensity was optimal. Artifacts and vessel overlaps were judged as minor when they did not prevent the interpretation of images and major when they degraded the image quality.
For the detection of aneurysm recanalization, DSAs were reviewed by one trained radiologist (X.L.) and MR angiographs were interpreted in a blinded fashion by two trained radiologists (J.Y.G., M.P.) in a fully randomized order. Cases that led to a disagreement between observers were reviewed by both readers to reach a consensus.
Each aneurysm was assigned into three categories as suggested by Raymond et al (19–21): group 1, complete obliteration (absence of opacification of the aneurysmal sac including the neck); group 2, residual neck (persistence of any portion of the original defect of the arterial wall but without opacification of the aneurysmal sac); and group 3, residual aneurysm (any opacification of the aneurysmal sac). A recurrence was qualified as any increase in the size of the remnant or defined as a change of classification of the anatomic result.
Statistical Analysis
The first step of the analysis consisted of an evaluation of the level of interobserver agreement for each set of MR images contrast-enhanced MR angiography at 6 months and 12 months by the means of the κ statistic. The second step consisted of a comparison between MR angiographs and DSAs for the detection of a residual neck with the use of the same statistical tests. Kappa values higher than 0.6 suggested a substantial agreement, and values higher than 0.8 indicated an excellent agreement. P values less than .05 were regarded as significant.
The third step consisted in determining the sensitivity and specificity of MR angiography at six and 12 months; we considered category 1 as healthy patients and categories 2 and 3 as diseased patients.
Results
DSA
Initial Results.Immediately after embolization, 29 aneurysms were completely obliterated, 17 showed a residual neck, and two presented with a residual aneurysm related to technical difficulties (one 6-mm diameter aneurysm and one 4-mm diameter aneurysm).
One-Year Follow-Up. DSA at 1-year follow-up showed 27 complete obliterations (group 1), eight residual necks (group 2), and 13 residual aneurysms (group 3). Most of the aneurysms (8/13) in group 3 were considered as occluded immediately after embolization and evolved over time to the residual aneurysm category. Recurrences were found in a total of 15 of 48 treated aneurysms at the 1-year follow-up.
MR Angiography
Image Quality. The contrast of gadolinium-enhanced MR angiographic images was judged excellent in 88 cases and moderate in six cases. Visualization of vessels was not optimal in five cases, because of the low contrast of image. No motion artifact was seen on contrast-enhanced MR images. One minor GDC artifact was observed. Venous overlaps were present in one case and were considered as minor because they did not prevent the interpretation of vessel conspicuity.
Comparison Between 6-month and 12-month MR Angiography. Interobserver agreement was judged as very good and significant for contrast-enhanced MR angiographs at 6 and 12 months (κ = 0.96, P < .0001). One case was misclassified with a residual neck at 6 months and a complete occlusion at 12 months. In this case, an additional reading by both examiners together was performed to reach a consensus.
Among the 48 aneurysms, MR angiographs at both 6 and 12 months showed 16 aneurysm regrowths. The size of recanalization did not increase between both MR angiographs. The sensitivity and specificity of MR angiography for the detection of a residual neck or aneurysm were 100% and 92%, respectively, at the 6-month follow-up and 100% and 96%, respectively, at the 12–month follow-up.
Comparison of DSA and MR angiography
Comparison of early MR angiography at 6 months and DSA at 12 months (Table 3) (Figures 1–4) showed a good and significant agreement between techniques (κ = 0.93, P < .0001); however, two cases of complete occlusion of aneurysm at DSA were misclassified as a residual neck at the 6-month MR angiography. MR angiographs at 6 months showed 16 recurrences of 48 treated aneurysms. One case of complete occlusion of the aneurysm at DSA was misclassified because it was considered to be a recurrence with residual neck on contrast-enhanced MR angiography (Figure 5).
TABLE 3:
Results
| Class 1 | Class 2 | Class 3 | |
|---|---|---|---|
| DSA | 27 | 8 | 13 |
| MRA 6 months | 25 | 10 | 13 |
| MRA 12 months | 26 | 9 | 13 |
Fig 1.
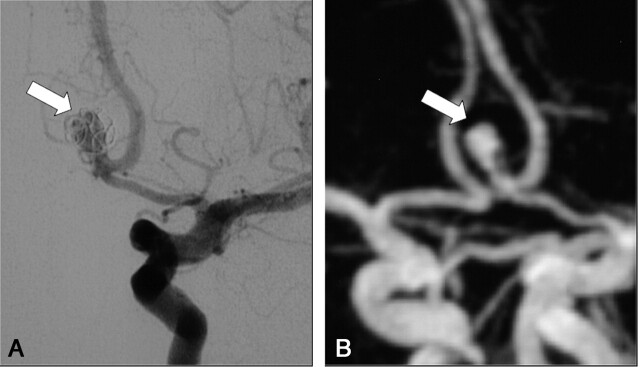
A, DSA of the left internal carotid artery at 9 months after treatment of a 7-mm aneurysm of the anterior communicating artery. The frontal head view shows a large recanalization (arrow) classified as residual aneurysm (class 3).
B, Contrast-enhanced MR angiograph with MIP reconstruction in the frontal plane (arrow) demonstrates a residual aneurysm (class 3) in accordance with DSA findings.
Fig 4.
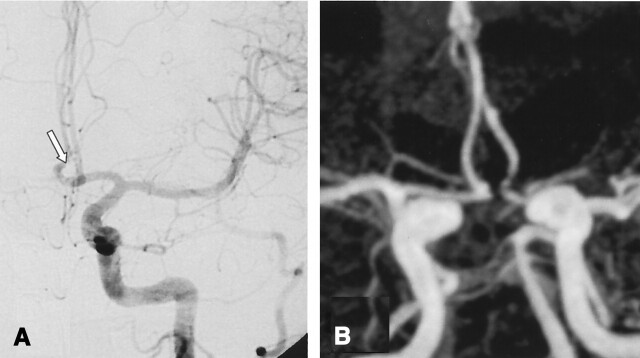
A, DSA of the left internal carotid artery performed at 12 months after treatment of a 3-mm aneurysm of the anterior communicating artery. The frontal head view shows a complete obliteration (class 1) at the site of the anterior communicating artery (arrow).
B, Contrast-enhanced MR angiograph with MIP reconstruction in the frontal view demonstrate a complete obliteration in agreement with DSA findings with minor coil artifact.
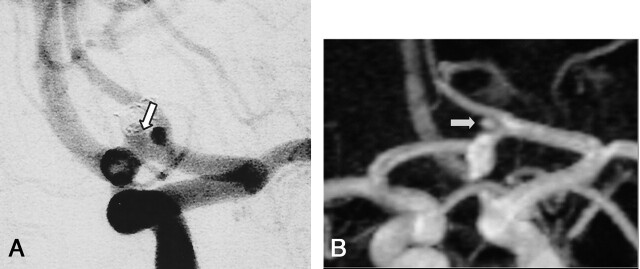
Fig 5.
A, DSA of the left internal carotid artery performed at 12 months after treatment of a 3-mm aneurysm of the anterior communicating (arrow). The frontal head view shows no residual neck or aneurysm (class 1).
B, Contrast-enhanced MR angiograph with MIP reconstruction in the frontal plane shows a hypersignal (arrow) at the site of the anterior communicating artery misinterpreted as a residual neck (class 2).
Comparison between 12-month MR angiography and DSA showed a good and significant agreement between techniques (κ = 0.96, P < .0001). Only one case was misclassified at MR angiography as a residual neck, whereas DSA showed a complete occlusion of aneurysm.
Discussion
Our study showed that contrast-enhanced MR angiography at 6 months after endovascular treatment of intracranial aneurysms with GDCs was highly sensitive to predict aneurysm recanalization on DSA at 1 year. Of 21 patients with an angiographically proved recanalization on DSA at 1 year, MR angiography at 6 months showed similar findings in all cases. Size of recanalization did not increase between both MR Angiographs performed at 6 and 12 months. Of 26 patients (27 aneurysms) with an angiographically complete aneurysm occlusion at 1 year, findings were similar in 24 patients on MR angiography at 6-month follow-up. Contrast-enhanced MR angiography has proved its effectiveness for the evaluation of supraaortic extracranial vessels (22, 23), but most previous studies used TOF sequences for imaging the circle of Willis (24–26). Only one study compared TOF and gadolinium-enhanced MR angiographs for the follow-up of patients treated with GDCs for an intracranial ruptured aneurysm, showing the higher sensitivity of contrast MR angiography for the detection of neck remnants (5).
For several reasons, contrast-enhanced MR angiographic sequences may have potential advantages over TOF for follow-up of intracranial aneurysms. First, this technique is much less sensitive to flow turbulences and saturation effects than the TOF technique because of the high signal intensity within the arterial lumen caused by the T1-shortening effect. This theoretically allows higher conspicuity of a residual aneurysmal neck than those observed with DSA. Second, these sequences demonstrated a relative insensitivity to artifacts in patients treated by means of detachable coils, which may degrade the image quality and hinder visualization of a residual neck. Finally, the intraarterial contrast enhancement caused by the gadolinium improves small-vessel conspicuity and allows better assessment of the relationship between an aneurysm and its parent vessel.
Clinical applications of this method for the assessment of intracranial vessels are limited. The short time window between the arterial and the venous phase of contrast enhancement may lead to a major venous enhancement that degrades the image quality, preventing an accurate delineation of the residual aneurysmal sac. The delay between arterial and venous maximal enhancement is much shorter in the brain compared than in the extracranial vessels (27), and this explains that individual timing is required to optimize the contrast of image. In the future, time resolution of MR angiographs will be probably improved by using the dynamic-DSA technique, making it possible to distinguish the different phase of contrast-enhancement (28, 29). By using the present imaging protocol, the false-positive neck remnant observed in our series may be explained by the peripheral contrast enhancement of the organized thrombus or by the vasa vasorum contrast enhancement within the adventitial layer of the aneurysm wall. Although platinum is relatively inert and does not elicit a strong inflammatory reaction, a progression of inflammatory changes definitely occurs with time after aneurysms coiling. Metens et al (27) evaluated this technique in 32 patients admitted for a suspected intracranial aneurysm. Findings showed high sensitivity and specificity (100% and 96%, respectively). A previous study assessed the feasibility and utility of contrast-enhanced MR angiography at 1 year after treatment of aneurysms of the anterior communicating artery with GDCs (5). One case of complete occlusion was misclassified as small residual neck on contrast-enhanced MR angiography. The sensitivity of MR angiography for the detection of a residual neck at 1-year follow-up was higher (100%), but its specificity was lower (93%). Gadolinium-enhanced MR angiography is sensitive in terms of screening and therapeutic choice with regard to aneurysmal recanalizations. Even if MR angiography sometimes mistakenly detects a neck remnant, this technique has the advantage of not missing aneurysm recanalizations that may require an additional treatment. Most previous series that assessed aneurysm follow-up after embolization demonstrated the potential of MR angiography techniques without contrast as three-dimensional TOF MR angiography. The sensitivity of this technique for the detection and the exclusion of a residual flow within the aneurysm ranged from 71% to 91% and from 89% to 100%, respectively (1, 4, 16, 18, 30, 31). To improve image quality and reduce the saturation effects, some authors have suggested the use of contrast material in addition to three-dimensional TOF MR angiography (16). These data, however, must be confirmed in larger patient groups.
Usually this follow-up is conducted by performing DSA or MR angiography at 1 year and 3 years after treatment (6). To the best of our knowledge, few reports have addressed the role of an early (3–6 months) angiography for the follow-up of intracranial coiled aneurysms. Thornton et al (15) performed DSA at 6 months, 1 year, and 2 years after endovascular treatment of 141 intracranial aneurysms. In this study (15), among the initially completely occluded aneurysms, only one recurrence (1.8%) was observed that was already demonstrated at 6-month. Among 24 aneurysms with a neck remnant, 15 were depicted at 6 months. Raymond et al (19) evaluated the long-term results of selective embolization of intracranial aneurysms in 277 aneurysms and showed that only 46.9% (24/51 aneurysms) of all recurrences found on delayed angiograms at 36 months had been depicted on angiograms at 6 months. According to Raymond et al, when a residual neck is visible at the end of the procedure, performing a 6-month angiographic control to detect early recurrence is recommended, but long-term angiographic control is always required as well to detect delayed recurrences. In the present study, only 32% (15/48 aneurysms) of aneurysm recurrence was observed at 6 months, and this rate did not increase at the 12-month follow-up. This lower rate of aneurysm recanalization compared with Raymond et al’s study might be explained by aneurysm features, because, of 48 aneurysms, 32 measured less than 5 mm in diameter and 34 were located at the anterior communicating artery in our series. The similar rate of recurrence between 6-month MR angiography and 12-month DSA may be explained by the short delay between examinations in our series, whereas Raymond et al (19) performed delayed angiograms at 36 months.
Several shortcomings may be pointed out in the present study. First, a selective catheterization of the internal carotid artery was not systematically performed in the present study, and this may potentially degrade the image quality. Second, the lack of rotational angiography with 3D reconstructions does not allow interpretation of intracranial angiograms with accuracy, especially for analysis of neck remnants. Finally, source images from MR angiograms were not included for image analysis, and this may lead to a misinterpretation of aneurysm recurrence.
Conclusion
Our study showed that early contrast-enhanced MR angiography after embolization of intracranial aneurysm may be useful to predict early recanalization of aneurysm. Imaging follow-up in the present study showed that 6-month contrast-enhanced MR angiography constituted a reliable technique for the detection of neck remnant. In our institution, contrast MR angiography is currently used as the first-year examination for patient follow-up. If the aneurysm is completely excluded from the circulation at 6-month MR angiography, patients are followed-up only by MR angiography for a 3-year period. If a small residual flow is detected within the aneurysm, MR angiography is performed every 12-months and patients undergo DSA if any change is observed. The issue of long-term efficacy, however, has not yet been resolved and requires further studies with larger groups of patients.
Fig 2.
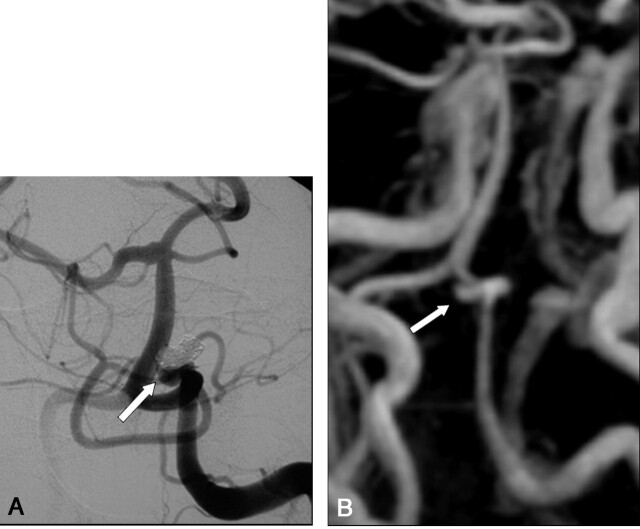
A, DSA of the left vertebral artery performed at 12 months after treatment of a 6-mm aneurysm of the left posterior inferior cerebellar artery. The frontal head view shows (arrow) an opacification of residual aneurysm (class 3).
B, Contrast-enhanced MR angiograph with MIP reconstruction in the frontal plane demonstrates (arrow) a residual aneurysm (class 3).
Fig 3.
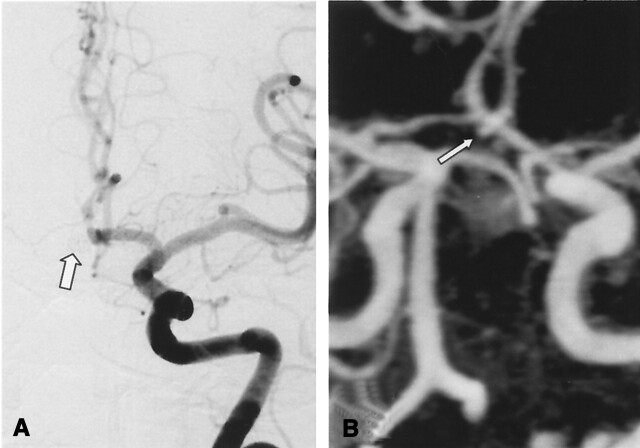
A, DSA of the left internal carotid artery at 11 months after treatment of a 3-mm aneurysm of the anterior communicating artery. The frontal head view shows a residual neck (arrow) of 2 mm-diameter (class 2).
B, Contrast-enhanced MR angiograph with MIP reconstruction in the frontal plane demonstrates (arrow) a residual neck (class 2) in accordance with DSA.
References
- 1.Boulin A, Pierot L. Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital subtraction angiography. Radiology 2001;219:108–113 [DOI] [PubMed] [Google Scholar]
- 2.Michardiere R, Bensalem D, Martin D, et al. Comparison of MRA and angiography in the follow-up of intracranial aneurysms treated with GDC. J Neuroradiol 2001;28:75–83 [PubMed] [Google Scholar]
- 3.Anzalone N, Triulzi F, Scotti G. Acute subarachnoid haemorrhage: 3D time-of-flight MR angiography versus intra-arterial digital angiography. Neuroradiology 1995;37:257–261 [DOI] [PubMed] [Google Scholar]
- 4.Kahara VJ, Seppanen SK, Ryymin PS, et al. MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow-up of intracranial aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:1470–1475 [PMC free article] [PubMed] [Google Scholar]
- 5.Leclerc X, Navez JF, Gauvrit JY, et al. Aneurysms of the anterior communicating artery treated with Guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol 2002;23:1121–1127 [PMC free article] [PubMed] [Google Scholar]
- 6.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–356 [DOI] [PubMed] [Google Scholar]
- 7.Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysma. Part II. Clinical results. Neurosurgery 1999;45:531–537; discussion 537–538 [DOI] [PubMed] [Google Scholar]
- 8.Murayama Y, Suzuki Y, Vinuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I. In vitro study. AJNR Am J Neuroradiol 1999;20:1986–1991 [PMC free article] [PubMed] [Google Scholar]
- 9.Solander S, Ulhoa A, Vinuela F, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg 1999;90:857–864 [DOI] [PubMed] [Google Scholar]
- 10.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–293 [DOI] [PubMed] [Google Scholar]
- 11.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery 1998;43:1016–1025 [DOI] [PubMed] [Google Scholar]
- 12.Casasco AE, Aymard A, Gobin YP, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg 1993;79:3–10 [DOI] [PubMed] [Google Scholar]
- 13.McDougall CG, Halbach VV, Dowd CF, et al. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393–399 [DOI] [PubMed] [Google Scholar]
- 14.Byrne JV. Long-term outcomes of Guglielmi detachable coil packing for acutely ruptured cerebral aneurysms. AJNR Am J Neuroradiol 1999;20:1184. [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton J, Debrun GM, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 2002;50:239–249; discussion 249–250 [DOI] [PubMed] [Google Scholar]
- 16.Anzalone N, Righi C, Simionato F, et al. Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2000;21:746–752 [PMC free article] [PubMed] [Google Scholar]
- 17.Weber W, Yousry TA, Felber SR, et al. Noninvasive follow-up of GDC-treated saccular aneurysms by MR angiography. Eur Radiol 2001;11:1792–1797 [DOI] [PubMed] [Google Scholar]
- 18.Gonner F, Heid O, Remonda L, et al. MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1324–1328 [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003. :1398–1403 [DOI] [PubMed]
- 20.Roy D, Milot G, Raymond J Endovascular treatment of unruptured aneurysms. Stroke,2001. :1998–2004 [DOI] [PubMed]
- 21.Roy D, Raymond J, Bouthillier A, et al. Endovascular treatment of ophthalmic segment aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:1207–1215 [PMC free article] [PubMed] [Google Scholar]
- 22.Remonda L, Heid O, Schroth G. Carotid artery stenosis, occlusion, and pseudo-occlusion: first-pass, gadolinium-enhanced, three-dimensional MR angiography: preliminary study. Radiology 1998;209:95–102 [DOI] [PubMed] [Google Scholar]
- 23.Remonda L, Senn P, Barth A, et al. Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. AJNR Am J Neuroradiol 2002;23:213–219 [PMC free article] [PubMed] [Google Scholar]
- 24.Talagala SL, Jungreis CA, Kanal E, et al. Fast three-dimensional time-of-flight MR angiography of the intra-cranial vasculature. J Magn Reson Imaging 1995;5:317–323 [DOI] [PubMed] [Google Scholar]
- 25.Parker DL, Tsuruda JS, Goodrich KC, et al.Contrast-enhanced magnetic resonance angiography of cerebral arteries. A review. Invest Radiol 1998;33:560–572 [DOI] [PubMed] [Google Scholar]
- 26.Isoda H, Takehara Y, Isogai S, et al. MRA of intracranial aneurysm models: a comparison of contrast-enhanced three-dimensional MRA with time-of-flight MRA. J Comput Assist Tomogr 2000;24:308–315 [DOI] [PubMed] [Google Scholar]
- 27.Metens T, Rio F, Baleriaux D, et al. Intracranial aneurysms: detection with gadolinium-enhanced dynamic three-dimensional MR angiography-initial results. Radiology 2000;216:39–46 [DOI] [PubMed] [Google Scholar]
- 28.Aoki S, Yoshikawa T, Hori M, et al. MR digital subtraction angiography for the assessment of cranial arteriovenous malformations and fistulas. AJR Am J Roentgenol 2000;175:451–453 [DOI] [PubMed] [Google Scholar]
- 29.Griffiths PD, Hoggard N, Warren DJ, et al. Brain arteriovenous malformations: assessment with dynamic MR digital subtraction angiography. AJNR Am J Neuroradiol 2000;21:1892–1899 [PMC free article] [PubMed] [Google Scholar]
- 30.Derdeyn CP, Graves VB, Turski PA, et al. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. AJNR Am J Neuroradiol 1997;18:279–286 [PMC free article] [PubMed] [Google Scholar]
- 31.Brunereau L, Cottier JP, Sonier CB, et al. Prospective evaluation of time-of-flight MR angiography in the follow-up of intracranial saccular aneurysms treated with Guglielmi detachable coils. J Comput Assist Tomogr 1999;23:216–223 [DOI] [PubMed] [Google Scholar]