Abstract
BACKGROUND AND PURPOSE: Analogous to the CT hyperattenuated vessel sign (HMCAS), MR imaging may show hypo- or hyperintense vessels in acute ischemic stroke (AIS) patients. We assessed the diagnostic and prognostic strength of early MR imaging vessel signs in AIS patients treated with intravenous thrombolysis (IVT) within 3 hours of the onset of symptoms.
METHODS: We studied AIS patients both treated with IVT and stroke MR imaged within 3 hours of the onset of symptoms and at 2 hours and 24 hours after treatment. We assessed the presence or absence of early vessel signs (hyperintense fluid-attenuated inversion recovery sign [FLAIR HVS]; gradient-echo susceptibility vessel sign [GRE SVS]) compared with a combined MR angiography/perfusion-weighted imaging reference and their strength for predicting clinical outcome (favorable vs. poor, independent vs. dependent, or dead, death), recanalization (by clot composition and flow), and hemorrhage in uni- and multivariate analysis.
RESULTS: Fifty-six patients (age range, 76 years ± 13 years; median National Institutes of Health stroke scale score [NIHSSS], 11) met the inclusion criteria. Forty-four patients (78.6%) had a vessel occlusion at baseline; 22 of them (50%) recanalized. Nineteen patients (33.9%) suffered some form of intracranial hemorrhage (ICH), 24 patients (42.9%) had an independent outcome, 18 patients (32.1%) a favorable outcome, and 14 patients died. Compared with our combined reference for vessel status PWI/MRA, the sensitivities of CT HMCAS, FLAIR HVS, and GRE SVS were 40%, 66%, and 34%, respectively, and improved during the hours that followed. Localization was accurately reflected by FLAIR HVS but not by GRE SVS. Only NIHSSS and age were independent predictors for recanalization and all clinical outcomes in multiple logistic regression analysis.
CONCLUSION: Although early vessel signs can be helpful in the diagnosis of intravascular disease, they do not independently predict recanalization, ICH, or any of the three clinical outcomes in a multivariate logistic regression model. Thrombus composition as reflected by signal intensity characteristics on GRE and FLAIR does not predict the therapeutic effect of IVT.
At present, intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rt-Pa; Alteplase) is the only approved therapy for acute ischemic stroke (AIS) within 3 hours of symptom onset (1). Multiparametric stroke MR imaging protocols have taken an increasingly important role in the initial diagnosis and treatment of AIS (2, 3). Analogous to the CT hyperattenuated middle cerebral artery sign (HMCAS) that indicates intraluminal thrombus (4), early vessel signs on MR imaging have been described on fluid-attenuated inversion recovery (FLAIR) images (hyperintense vessel sign [HVS]) (5–7) and less frequently on gradient-echo (GRE; susceptibility vessel sign [SVS]) images (8, 9). In AIS, the presence of cerebral artery occlusion according to MRA or HMCAS is associated with higher baseline National Institutes of Health stroke scale score (NIHSSS) and worse outcome (10–12). Only two smaller and less homogenously sampled studies assessed the diagnostic value of these early MR imaging vessel signs in AIS <6 hours (9, 13). The present study had three objectives. First, we sought to determine the diagnostic accuracy of early MR imaging vessel signs in a large cohort of homogeneous AIS patients stroke MR imaged before and after standard treatment with IVT within 3 hours after stroke onset and at two early follow-up measurements. Second, we aimed to assess the prognostic value of early MR imaging vessel signs for clinical outcome, recanalization, and intracranial hemorrhage (ICH). Finally, we hypothesized that the MR imaging signal intensity characteristics of early MR imaging vessel signs may reflect the structure of the intraluminal thrombus in patients with a vessel occlusion and can predict response to recombinant tissue plasminogen activator (rt-PA; Alteplase).
Methods
Patients
All patients (n = 127) treated at our institution with IVT for AIS (September 1999–February 2004) were considered for inclusion. Alteplase was administered within 3 hours of onset, according to National Institute of Neurological Disorders and Stroke (NINDS) criteria (1), with the addition of specific MR imaging criteria. On the basis of pretreatment MR imaging findings, we treated patients with uncertain diagnoses after clinical assessment but confirmed ischemic stroke by MR imaging; we did not treat patients with evidence of acute or chronic cerebral hemorrhage on GRE imaging or patients with subacute diffusion-weighted (DW) imaging lesions (associated with FLAIR or T2 changes). No upper limit on age, NIHSSS, or lesion size was used for IVT eligibility. For inclusion, patients needed to have an interpretable baseline stroke MR imaging before IVT follow-up MR imaging study at 2 hours and 24 hours and MR imaging evidence of a supratentorial stroke. Clinical assessment was done by NIHSSS at presentation, modified Rankin score (mRS), and Barthel index (BI) at approximately day 90 (follow-up visit or phone call) or, if this was not available, forwarded from the latest assessment. Informed consent was obtained for all patients to participate in a natural history study of stroke.
Imaging Technique
Imaging was performed by using a 1.5T (General Electric, Twinspeed XL, Minneapolis, MN) clinical MR imaging scanner. Pretreatment scans were obtained in all patients, and follow-up scans were scheduled at 2 hours and 24 hours thereafter. The scanning protocol was standardized and included DW imaging, PWI, MRA, GRE and fast spin-echo FLAIR series having the following relevant parameters: 24-cm FOV; 7-mm-thick axial-oblique sections aligned with the AC-PC line; 20 sections contiguous, interleaved, and colocalized. The DW imaging parameters were as follows: TR/TE, 6000/72 ms; acquisition matrix, 128 × 128, with both b = 0 and b = 1,000 s/mm2; isotropic trace images of the DW imaging. FLAIR parameters were as follows: TR/TE, 9000/85 ms; TI, 1750 ms; matrix, 256 × 128. The following were the GRE T2* parameters: TR/TE, 800/20 ms; flip angle, 30°; matrix, 256 × 192. The follow-up scanning protocol included a higher resolution FLAIR series with 66 sections, 2-mm-thick contiguous axial-oblique sections, with TR/TE of 9000/92 ms, TI of 2200 ms, and a matrix of 256 × 128. Dynamic susceptibility contrast PWI images (0.1 mmol/kg gadolinium, power injector) were obtained with gradient-echo EPI; TE, 45 ms; 25 phases, at 2 seconds per phase; and a matrix of 64 × 64. Maps of normalized mean transit time (MTT; first moment method) were calculated by using concentration-time curves obtained from the PWI time-series. The 3D time-of-flight (TOF) MRA consisted of a single slab, approximately 7 cm thick, positioned over the circle of Willis; (TR/TE, 39/6.9 ms; flip-angle, 25°; FOV, 24 × 18 cm; matrix, 224 × 160) for an in-plane resolution of approximately 1 mm, reconstructed to 92 axial images, 1.6 mm thick with a 0.8-mm overlap. The MRA source images were postprocessed into maximum intensity projection images by using standard software tools. A subgroup of patients also had standard noncontrast CT performed before IVT (section thickness, 4 mm/8 mm) and were included in the analysis.
Data Analysis and Statistics
Complete history, physical and neurologic examinations, and NIHSSS were performed before imaging in all patients. Two experienced readers (P.D.S., J.A.C.) blinded to all clinical information independently rated FLAIR (HVS), GRE (SVS), CT (HMCAS) MRA/PWI (vessel occlusion on either one or both methods) at time points baseline, 2 hours, and 24 hours. Interpretations for each sequence for a single patient were performed on different days to avoid reader recognition or recall of findings from other sequences. The order of the presentation of the films was randomized and differed for each sequence and day.
Presence and localization of vessel occlusion were assessed on PWI and MRA, which when used in combination give concordant and more comprehensive information than either single sequence on the presence or absence and the site of vessel occlusion. In those patients in whom either PWI or MRA was of nondiagnostic quality, we based the assessment on the other sequence. Patients who had neither MRA or PWI of sufficient diagnostic quality were excluded from the study. The vessel status on PWI/MRA was chosen as reference and compared with early vessel signs by categorization into 2*2 tables with occlusion on MRA/PWI (yes/no) versus vessel sign (yes/no) to assess the measures of diagnostic accuracy (sensitivity, specificity, positive [PPV] and negative [NPV] predictive values, accuracy) and measures of significance (dependence, association, correlation). Recanalization/reperfusion was sufficiently proved by follow-up MRA and PWI in the same manner as was performed for the baseline MR imaging. If an occlusion was still present on MRA at the same site as before and the PWI lesion was more or less the same as at baseline, there was no recanalization. Substantial restoration of flow on MRA in parallel with a complete or nearly complete reperfusion according to PWI was graded as recanalization/reperfusion.
Localization for all assessments was categorized into ICA, M1, P1, A1, branch, and no occlusion. Recanalization according to MRA/PWI was assessed on the 24-hour scan. Presence or absence of hemorrhage was assessed on follow-up images on days 3–10, or, if not available, the 24-hour scan. The following variables were entered into the analysis: age, sex, Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification, antithrombotic therapy (acetylic salicylic acid [ASA], ASA/dipyridamol, clopidogrel) at time of stroke, times to imaging and treatment, FLAIR HVS and GRE SVS at different time points, recanalization, ICH, 90-day mRS and BI, and clinical outcomes: favorable versus poor (mRS 0–1 vs. 2–6), independent versus dependent or dead (mRS 0–2 vs. 3–6), death (mRS 6).
For statistical analysis, we used a standard software package (SPSS 11.5.1). Demographic data, time intervals of examinations, and clinical scale scores are given as means with SDs or medians with interquartile range. For abnormally distributed data and ordinal scales, we used nonparametric tests in univariate analysis (Mann Whitney U test, MWU; Spearman signed rank correlation, SSR). For categorical variables such as the 2*two tables of diagnostic accuracy, we tested dependence (χ2) or Fisher’s exact test (FET) and strength of association (Cramer’s V) and correlation (SSR).
We performed a forward stepwise multiple logistic regression including the following variables: age, sex, NIHSSS, TOAST, DW imaging and PWI volumes at baseline, antithrombotic therapy, time(s), HVS and SVS at different time points, occlusion localization, and HMCAS to determine any independent predictors of recanalization, as well as ICH and the three clinical outcomes.
Results
Of the 127 AIS patients treated with IVT, 56 patients (27 female, 29 male), mean age 75.8 ± 13.1 years, median baseline NIHSSS 11 (0–37), met the inclusion criteria. Twenty-seven were on treatment with a platelet inhibitor immediately before stroke (aspirin [23], clopidogrel [2], aspirin plus clopidogrel [2]). Stroke etiology according to the TOAST classification was large artery atherosclerosis (4), cardioembolism (24), small vessel occlusion (3), other determined (6), and undetermined (19). The mean time to baseline MR imaging and IVT was 90.8 ± 29.5 minutes and 137.3 ± 27.7 minutes, respectively; 31 patients (55.4%) also had a baseline CT before IVT. Forty-four patients (78.6%) had a vessel occlusion at baseline as determined by MRA/PWI (ICA [9], M1 [13], A1 [1], P1 [5], Branch [16]); 22 of them (50%) recanalized. Nineteen patients (33.9%) suffered some form of ICH (hemorrhagic infarction and parenchymal hemorrhage, not differentiated into symptomatic versus asymptomatic ICH), 24 patients (42.9%) had an independent outcome, 18 patients (32.1%) had a favorable outcome, and 14 patients (25%) died.
Imaging
MRA and PWI were concordant in all cases where both sequences were interpretable (52). In the other four patients, MRA was diagnostic in three (2, ICA; 1, M1) and PWI in one patient (Branch). At baseline the diagnostic sensitivity, specificity, PPV, NPV, and accuracy was best for the FLAIR HVS, followed by CT HMCAS and GRE SVS (Table 1). At 2 hours, diagnostic strength improved for both FLAIR HVS and GRE SVS, although the latter was still substantially less accurate (Table 2). At 24 hours, both vessel signs yielded comparable diagnostic accuracy (Table 3). At 24 hours the diagnostic accuracy dropped for the FLAIR HVS but increased for the GRE SVS. The FLAIR HVS was more frequently missed in the subacute stage because of an increasing, equally hyperintense surrounding edema in the infarct with local swelling, which in addition effaced the dark CSF signal intensity contrasting with the HVS in hyperacute stages. In contrast, specificity and NPV improved on GRE SVS because of thrombus aging with increasing deoxyhemoglobin levels.
TABLE 1:
Early vessel signs at baseline
| PWI/MRA: Vessel Occlusion, Baseline | CT baseline HMCAS |
FLAIR baseline HVS |
GRE baseline SVS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | No | Yes | Total | No | Yes | Total | |
| No | 6 | 0 | 6 | 9 | 3 | 12 | 9 | 3 | 12 |
| Yes | 15 | 10 | 25 | 15 | 29 | 44 | 29 | 15 | 44 |
| Total | 21 | 10 | 31 | 24 | 32 | 56 | 38 | 18 | 56 |
| Sensitivity (%) | 40 | 65.9 | 34.1 |
| Specificity (%) | 100 | 75 | 75 |
| PPV (%) | 100 | 90.6 | 83.3 |
| NPV (%) | 28.6 | 37.5 | 23.7 |
| Accuracy (%) | 51.6 | 67.9 | 42.9 |
TABLE 2:
Early vessel signs at 2 hours
| PWI/MRA: Vessel Occlusion, 2 Hours | FLAIR 2-Hour HVS |
GRE 2-Hour SVS |
||||
|---|---|---|---|---|---|---|
| No | Yes | Total | No | Yes | Total | |
| No | 20 | 0 | 20 | 14 | 6 | 20 |
| Yes | 11 | 25 | 36 | 18 | 18 | 36 |
| Total | 31 | 25 | 56 | 32 | 24 | 56 |
| Sensitivity (%) | 69.4 | 50 | ||||
| Specificity (%) | 100 | 70 | ||||
| PPV (%) | 100 | 75 | ||||
| NPV (%) | 64.5 | 43.8 | ||||
| Accuracy (%) | 80.4 | 57.1 | ||||
TABLE 3:
Early vessel signs at 24 hours
| PWI/MRA: Vessel Occlusion, 24 Hours | FLAIR 24-Hour HVS |
GRE 24-Hour SVS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | No | Yes | Total | |||||
| No | 29 | 1 | 30 | 26 | 4 | 30 | ||||
| Yes | 13 | 13 | 26 | 12 | 14 | 26 | ||||
| Total | 42 | 14 | 56 | 38 | 18 | 56 | ||||
| Sensitivity (%) | 50 | 53.8 | ||||||||
| Specificity (%) | 96.7 | 86.7 | ||||||||
| PPV (%) | 92.9 | 77.8 | ||||||||
| NPV (%) | 69 | 68.4 | ||||||||
| Accuracy (%) | 75 | 71.4 | ||||||||
Only the FLAIR HVS was significantly associated and correlated with the findings (occlusion vs. patency) on PWI/MRA (two-tailed Pearson χ2, P = .011; SSR ρ and Cramer’s V = 0.34; both P = .011) at baseline. The accuracy (both P not significant) and correlation of CT HMCAS (SSR ρ = 0.3) and GRE SVS (SSR ρ = 0.08) compared with PWI/MRA was not significant at baseline. At 2 hours the association (P not significant) and correlation (SSR ρ = 0.19) of the GRE SVS again was not significant, while the FLAIR HVS was significantly associated and correlated with the findings on PWI/MRA (two-tailed Pearson χ2, P < .001; SSR ρ, and Cramer’s V = 0.67, both P < .001). At 24 hours, both FLAIR HVS (two-tailed Pearson χ2, P < .001; SSR ρ, and Cramer’s V = 0.54, both P < .001) and a slightly smaller GRE SVS (two-tailed Pearson χ2, P = .001; SSR ρ, and Cramer’s V = 0.43, both P = .001) were significantly associated and correlated with the findings on PWI/MRA.
Assessment and congruence of localization of an occlusion (or no occlusion) was significantly associated and correlated for FLAIR HVS (two-tailed Pearson χ2, P < .001; SSR ρ, 0.66; Cramer’s V, 0.49, both P < .001), less significantly associated and correlated for CT HMCAS (two-tailed Pearson χ2, P = .018; SSR ρ, 0.56; Cramer’s V, 0.51, both P < .018) and not at all for the GRE SVS (all P not significant; SSR ρ, 0.18). For the 2-hour time point accuracy of occlusion localization was significant for FLAIR HVS (two-tailed Pearson χ2, P < .001; SSR ρ, 0.73; Cramer’s V, 0.72, both P < .001) and less for the GRE SVS (two-tailed Pearson χ2, P < .001; SSR ρ, 0.29, Cramer’s V, 0.45, both P < .05). At 24 hours, the values were (two-tailed Pearson χ2, P < .001; SSR ρ, 0.61, Cramer’s V, 0.71, both P < .001) for FLAIR HVS and (two-tailed Pearson χ2, P < .001; SSR ρ, 0.43, Cramer’s V, 0.55, both P < .001) for GRE SVS. In all instances, where the localizations assessed on GRE and FLAIR did not match those on MRA/PWI, the occlusion was located more distally than on MRA/PWI. This was, however, more frequently the case on GRE (7/15) than on FLAIR (9/27) (Figure 1).
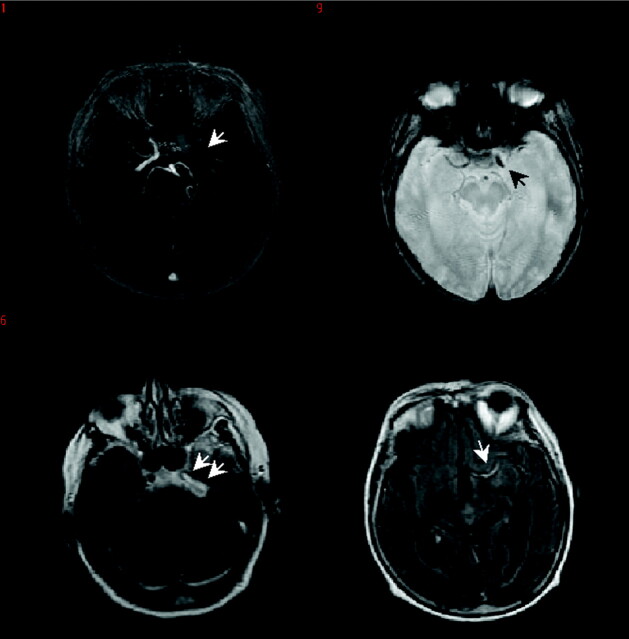
Fig 1.
An 82-year-old female patient with cardioembolic stroke, baseline NIHSSS of 18 received rt-PA 88 minutes after symptom onset. Stroke MR imaging at 40 minutes after symptom onset. MRA (upper left) shows left-sided ICA occlusion extending into the MCA (arrows). This occlusion is seen on GRE (upper right, arrows) as a GRE SVS only at the level of the MCA, a FLAIR HVS can be seen at the level of the ICA and MCA (lower row, arrows).
Univariate Analysis
In the univariate analyses, baseline variables such as age, times to treatment or imaging, baseline lesion volumes did not differ for the dichotomous outcomes recanalization, and hemorrhage (trend toward larger DW imaging and PWI volumes in patients with any ICH). Patients who died had an unfavorable or dependent outcome, were significantly older, and had larger baseline lesion volumes than those who did not (two-tailed all P < .003, t test). Times to treatment and to baseline MR imaging were significantly shorter in patients who died (two-tailed both P < .05, t test). Baseline NIHSSS was not associated with recanalization but was significantly higher in patients with hemorrhage, unfavorable, dependent or dead outcomes, and death (two-tailed all P ≤ .001, MWT). Antithrombotic therapy and sex were not associated with recanalization. A cardioembolic as opposed to other etiologies (TOAST) was significantly associated with recanalization (two-tailed χ2, P = .032; Cramer’s V, 0.45; P = .032). Neither sex, antithrombotic therapy, nor TOAST classification was associated with hemorrhage or any of the three clinical outcomes (death, favorable vs. poor, independent vs. dependent or dead).
Although not predictive at baseline, presence of either vessel sign at 2 hours was significantly associated and correlated with nonrecanalization at 24 hours (two-tailed χ2, P = .006; Cramer’s V = 0.41, P = .005). ICH was significantly associated with high baseline NIHSSS (two-tailed P < .001, MWU), poor outcomes (two-tailed χ2, all P < .02), and trendwise death (P = .051, FET). Presence of a baseline or 2 hours MRA/PWI occlusion or FLAIR HVS was associated with ICH (all P < .05), GRE SVS was at neither time point.
A CT HMCAS at baseline was associated with an unfavorable outcome and death (two-tailed χ2, both P < .05). A baseline FLAIR HVS was associated with death, a 24-hour FLAIR HVS with an unfavorable outcome (two-tailed χ2, both P < .05). Independent (41% vs. 32%) and favorable (36% vs. 23%) outcomes were more frequently seen in recanalization, although this did not reach statistical significance.
Multivariate Analysis
Multiple logistic regression was performed for the following dichotomous outcomes: recanalization, hemorrhage, dependent or dead, poor, and death. For recanalization only cardioembolic stroke (TOAST) entered the model (P = .03 OR 8.0, 95% confidence interval [CI] 1.23–52.25). For ICH (P = .002, OR 1.12, 95% CI 1.04–1.2) only NIHSSS entered the model. In other words, having a cardioembolic stroke as opposed to the other etiologies coded in TOAST increases the odds of recanalization by 8.0, and a 1-point increase in NIHSSS increases the odds of ICH by 1.12. For all clinical outcomes, only NIHSSS and age entered the models, increasing the odds of a worse outcome with increasing NIHSSS and increasing age by 1.1–1.55 (all P < .01). For death, in addition to NIHSSS and age also a lower time to rt-PA improved the model (P = .02, OR 0.96, 95% CI 0.93–0.99)—that is, for each minute increase in time to rt-PA the odds for death are reduced by 0.96.
Discussion
Occlusion of a cerebral artery is the major cause of stroke and modern MR techniques such as MRA and PWI have been shown to be sensitive tools for detection of acute cerebrovascular occlusive disease (14). The CT HMCAS has been widely described and presented in the literature (15). It has a low overall incidence and a sensitivity of 47% in proved MCA occlusion, which corresponds to the 40% we found in the 31 patients also imaged with CT before IVT.
Recent studies reported increased intensity in cerebral blood vessels on FLAIR and intravascular signal intensity loss on GRE in AIS patients with a sensitivity varying in between 40% and 100% (5, 7, 9, 13). The use of such signs could be additional or confirmatory information with regard to vessel patency, hemodynamic status, and thrombus composition (16, 17), thus improving diagnostic and prognostic strength of stroke MR imaging. The presence of hyperintense vessels on FLAIR images is thought to indicate the presence of slow flow or stasis in small arteries, veins, or collateral vessels. The mechanism of increased intravascular signal intensity on FLAIR images in AIS is most likely due to a combination of slow flow (intravoxel phase dispersion, TOF effects), flow-related enhancement (slow, but not static flow), and clot signal intensity (oxyhemoglobin) (18). T1 shortening due to methemoglobin formation in an aging thrombus (18) seems an unlikely cause for HVS within 3 hours. The term “FLAIR HVS” is used to describe an intraluminal disease in a major brain vessel or to refer to parenchymal vessel enhancement on postcontrast FLAIR images (6, 18, 19). The substrate for the GRE SVS most likely is paramagnetic deoxyhemoglobin causing signal intensity loss, a feature also used for the diagnosis of acute ICH (20). Therefore, older retracted clots and more heterogeneous clots can be seen on GRE (16, 17).
Cosnard et al (13) investigated 53 patients within 6 hours (28 patients <3 hours) and found a similar sensitivity and specificity for TOF-MRA (67%, 71%) and FLAIR HVS (65%, 85%). They suggested that time-consuming MRA techniques in the assessment of hyperacute AIS may be unnecessary in patients with negative HVS (13). It is interesting also that they had false-positive findings, probably because both slow flow and occluding clot may produce this sign. A flaw of this study, however, is that sensitivity was measured compared with presence of an infarct on FLAIR images at follow-up 1–5 days after symptom onset and not compared with another vascular imaging technique performed simultaneously. Toyoda et al (7) studied 60 patients with proved cerebral vessel occlusion (25 patients <3 hours; 14 patients 3–6 hours) and found a sensitivity of 100% within 3 hours after stroke onset. The FLAIR HVS corresponded to the localization on TOF-MRA and PWI in 80.0% and 87.5%. The authors concluded that FLAIR imaging may be used to assess the area of hypoperfusion in lieu of PWI (7). As Wolf stated in his editorial on Toyoda et al’s article, for potential measures of hemodynamic stress one needs to know what exactly is being measured (18), because slow flow can also represent an adequate compensatory response to proximal stenosis or occlusion (21). Another vessel sign less frequently reported is the GRE SVS (8, 9). Flacke et al (9) studied 23 patients within 6 hours and found a sensitivity of 82% in a subgroup of 11 patients who underwent angiography as opposed to 54% for the CT HMCAS; both had 100% specificity. Although there was a significant correlation of the GRE SVS with baseline NIHSSS, neither sign predicted clinical outcome in MCA stroke.
None of the studies discussed above investigated IVT patients, and most of these studies performed MR imaging only in part of their patients within 6 hours or even 3 hours after stroke onset, the currently accepted time window for IVT. Hyperacutely, we found the diagnostic accuracy of the FLAIR HVS to be substantially higher and better than that of the CT HMCAS and the GRE SVS, consistent with the findings of others albeit not nearly close to 100% when compared with MRA/PWI. The diagnostic accuracy of the baseline GRE SVS was substantially smaller than reported by Flacke et al, even lower than that of the CT HMCAS. The good sensitivity of FLAIR HVS may in part be explained by the fact that it also shows slow flow and not only thrombus (18). Nevertheless, in our series of 56 patients we only had three false-positive assessments and an excellent consistency of occlusion localization of FLAIR HVS with MRA/PWI. The diagnostic accuracy for both MR imaging vessel signs improved at 2 hours, although only for the GRE SVS at 24 hours. This is likely due to increased susceptibility by increasing deoxyhemoglobin content seen with thrombus aging (22). This may also be an explanation why GRE SVS showed the thrombus more distally than the FLAIR HVS, MRA, and PWI did, as deoxygenation is more likely to take part in the more distal areas (17). In addition to that, susceptibility artifacts and the vessel course at the skullbase may reduce the sensitivity of GRE for ICA occlusions. In fact, an ICA occlusion was seen on GRE only once. Therefore, with regard to diagnostic accuracy for vessel occlusion, if MRA and PWI are not available, the FLAIR HVS gives a reasonably accurate assessment of vessel status in hyperacute stroke patients, even in the first 3 hours.
We hypothesized that early MR imaging vessel signs can predict response to rt-PA, risk of hemorrhage, and clinical outcome. The presence of cerebral artery occlusion in AIS is associated with higher baseline NIHSSS and worse outcome whether assessed by MRA (10) or presence of a HMCAS (11, 12). There are only limited data addressing the prognostic strength of early MR imaging vessel signs in AIS (18). Patients without HVS seem to have smaller infarctions (13) and, in cases of proximal occlusion, better collateral status (6). Although we think that the FLAIR HVS is congruent with assessment and localization of a vessel occlusion on PWI, in agreement with Wolf’s findings, we do not believe that FLAIR HVS can be a substitute for PWI with regard to assessment of microvascular perfusion (18). In contrast to Flacke et al’s findings (9), a FLAIR HVS at baseline was associated with a worse clinical outcome and hemorrhage, and if the HVS was still present 2 hours after the first MR imaging, a persistent occlusion with worse outcome and also hemorrhage was significantly more likely.
Another hypothesis was generated by in vitro studies showing that the MR imaging signal intensity characteristics of intraluminal thrombus especially on GRE SVS may reflect thrombus composition and therefore allow to predict whether an occlusive clot is susceptible or resistant to fibrinolytic therapy (16, 17). In specific, retracted older clots are more resistant to fibrinolysis and have fewer red blood cells and therefore a higher signal intensity on GRE, respectively, less signal intensity loss due to less deoxyhemoglobin. In our patients, however, neither recanalization nor any clinical outcome was predicted by the GRE SVS in uni- or multivariate analysis. Only well-established predictors for outcome such as age and baseline stroke severity (NIHSSS) entered the models. All patients had rt-PA and recanalization was therefore more evenly distributed over the given range of outcomes, which may explain the present but nonsignificant association of recanalization and outcome in this special patient sample. The case fatality rate was 25%, which is higher than the 17% of the rt-PA treatment arm in NINDS (1). This is likely due to the fact that the mean age was 7 years higher in our cohort than in the NINDS trial, and patients who died were on an average—and significantly—10 years older (mean 83 vs. 73 years) than survivors. Advanced age is known to be an independent predictor of poor outcome in stroke (23). Furthermore, the rates of independent and favorable outcomes are consistent with the NINDS trial. Another interesting finding is that antithrombotic therapy directly before thrombolysis was neither associated with ICH, nor with an increased recanalization rate nor with an independent or favorable outcome or death. Concomitant antithrombotic therapy seemed not to play any role at all. Our study was, of course, not powered to detect such an effect; however, the bleeding risk with antithrombotics after thrombolysis does not appear to be any higher than without. This is interesting when taking into account that in Europe the approval regulations prohibit fibrinolysis in such pretreated patients.
This study is new for several reasons. It is the first of its size in the early time window of 3 hours in a prospective, independent, and blinded fashion. It is the first to assess the role of MR imaging vessel signs in patients treated with rt-PA. It is also the first to assess the changes intraindividually over time at two additional imaging sessions within 24 hours. Therefore, it is suited to not only assess the diagnostic accuracy of early MR imaging vessel signs, but also render information about their prognostic value. To the best of our knowledge, no clinical study to date has taken thrombus composition as being reflected by GRE (and FLAIR) images and its role for rt-PA response into account. Obviously, there are also limitations to this analysis. The choice of combined MRA/PWI as a reference for vessel occlusion and recanalization rather than conventional angiograpy may be less accurate than for instance DSA. Although the size of our patient sample is larger and substantially more homogeneous with regard to time window, follow up assessments and patients than that of others, the sample size may still be too small to detect any prognostic effects other than those known from clinical trials and the presence of a proximal vessel occlusion itself. Another limitation is the differentiation of ICH into present and absent only, which limits the prognostic analysis for this variable. Further studies may show whether MR imaging vessel signs have a stronger predictive value for glycoprotein IIb/IIIa receptor antagonists such as abciximab, tirofiban, and eptifibatide rather than for fibrinolytics. Conclusion
The FLAIR HVS can be very helpful for the detection of intravascular disease and its duration, especially if MRA and/or PWI are of limited quality in an individual patient. The GRE SVS is of limited diagnostic value in the very early time window and has an increasing accuracy with time due to thrombus aging. Early MR imaging vessel signs, however, do not independently predict ICH, recanalization, and clinical outcome in a multivariable logistic regression model and, according to our study, do not predict therapeutic effect of IVT.
Footnotes
Presented at the World Stroke Congress, Vancouver, BC, Canada, June 23–26, 2004.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2.Warach S. Measurement of the ischemic penumbra with MRI: it’s about time. Stroke 2003;34:2533–2534 [DOI] [PubMed] [Google Scholar]
- 3.Schellinger PD, Fiebach JB, Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: present status. Stroke 2003;34:575–583 [PubMed] [Google Scholar]
- 4.Pressman BD, Tourje EJ, Thompson JR. An early CT sign of ischemic infarction: increased density in a cerebral artery. AJR Am J Roentgenol 1987;149:583–586 [DOI] [PubMed] [Google Scholar]
- 5.Maeda M, Yamamoto T, Daimon S, et al. Arterial hyperintensity on fast fluid-attenuated inversion recovery images: a subtle finding for hyperacute stroke undetected by diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2001;22:632–636 [PMC free article] [PubMed] [Google Scholar]
- 6.Kamran S, Bates V, Bakshi R, et al. Significance of hyperintense vessels on FLAIR MRI in acute stroke. Neurology 2000;55:265–269 [DOI] [PubMed] [Google Scholar]
- 7.Toyoda K, Ida M, Fukuda K. Fluid-attenuated inversion recovery intraarterial signal: an early sign of hyperacute cerebral ischemia. AJNR Am J Neuroradiol 2001;22:1021–1029 [PMC free article] [PubMed] [Google Scholar]
- 8.Chalela JA, Haymore JB, Ezzeddine MA, et al. The hypointense MCA sign. Neurology 2002;58:1470. [DOI] [PubMed] [Google Scholar]
- 9.Flacke S, Urbach H, Keller E, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology 2000;215:476–482 [DOI] [PubMed] [Google Scholar]
- 10.Derex L, Nighoghossian N, Hermier M, et al. Early detection of cerebral arterial occlusion on magnetic resonance angiography: predictive value of the baseline NIHSS score and impact on neurological outcome. Cerebrovasc Dis 2002;13:225–229 [DOI] [PubMed] [Google Scholar]
- 11.Tomsick T, Brott T, Barsan W, et al. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale score before ultraearly thrombolytic therapy. AJNR Am J Neuroradiol 1996;17:1–7 [PMC free article] [PubMed] [Google Scholar]
- 12.Berge E, Nakstad PH, Sandset PM. Large middle cerebral artery infarctions and the hyperdense middle cerebral artery sign in patients with atrial fibrillation. Acta Radiol 2001;42:261–268 [PubMed] [Google Scholar]
- 13.Cosnard G, Duprez T, Grandin C, et al. Fast FLAIR sequence for detecting major vascular abnormalities during the hyperacute phase of stroke: a comparison with MR angiography. Neuroradiology 1999;41:342–346 [DOI] [PubMed] [Google Scholar]
- 14.Warach S, Li W, Ronthal M, Edelman RR. Acute cerebral ischemia: evaluation with dynamic contrast-enhanced MR imaging and MR angiography. Radiology 1992;182:41–47 [DOI] [PubMed] [Google Scholar]
- 15.von Kummer R, Meyding-Lamade U, Forsting M, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol 1994;15:9–15 [PMC free article] [PubMed] [Google Scholar]
- 16.Blinc A, Keber D, Lahajnar G, et al. Magnetic resonance imaging of retracted and nonretracted blood clots during fibrinolysis in vitro. Haemostasis 1992;22:195–201 [DOI] [PubMed] [Google Scholar]
- 17.Taber KH, Hayman LA, Herrick RC, Kirkpatrick JB. Importance of clot structure in gradient-echo magnetic resonance imaging of hematoma. J Magn Reson Imaging 1996;6:878–883 [DOI] [PubMed] [Google Scholar]
- 18.Wolf RL. Intraarterial signal on fluid-attenuated inversion recovery images: a measure of hemodynamic stress? AJNR Am J Neuroradiol 2001;22:1015–1016 [PMC free article] [PubMed] [Google Scholar]
- 19.Essig M, von Kummer R, Egelhof T, et al. Vascular MR contrast enhancement in cerebrovascular disease. AJNR Am J Neuroradiol 1996;17:887–894 [PMC free article] [PubMed] [Google Scholar]
- 20.Fiebach JB, Schellinger PD, Gass A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke 2004;35:502–507 [DOI] [PubMed] [Google Scholar]
- 21.Pantano P, Toni D, Caramia F, et al. Relationship between vascular enhancement, cerebral hemodynamics, and MR angiography in cases of acute stroke. AJNR Am J Neuroradiol 2001;22:255–260 [PMC free article] [PubMed] [Google Scholar]
- 22.Linfante I, Llinas RH, Caplan LR, Warach S. MRI features of intracerebral hemorrhage within 2 hours from symptom onset. Stroke 1999;30:2263–2267 [DOI] [PubMed] [Google Scholar]
- 23.Davalos A, Toni D, Iweins F, et al. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 1999;30:2631–2636 [DOI] [PubMed] [Google Scholar]