Abstract
Summary: Percutaneous injection of bone cement is a promising treatment for metastatic tumors. We present a case of hemorrhagic sacral metastases from hepatocellular carcinoma treated successfully by percutaneous sacroplasty with combined injections of bone cement and n-butyl cyanoacrylate resulting in pain relief.
Palliative treatment for symptomatic bone metastases is as important as the treatment of primary lesion to maintain patients’ quality of life. The effectiveness of percutaneous injection of polymethlymethacrylate (PMMA) for metastatic tumors in weight-bearing region such as vertebral bodies and pelvis has been reported (1–11); however, the treatment of extremely hypervascular lesions such as metastases from hepatocellular carcinoma (HCC) is challenging because of risk of hemorrhage. We report a case of percutaneous sacroplasty with a combination of bone cement and n-butyl cyanoacrylate (n-BCA) injection for a hemorrhagic metastasis of the sacrum resulting in persistent pain relief and reduction of tumor volume.
Case Report
A 76-year-old man with HCC presented with unbearable left low back pain. Preoperative MR imaging revealed a large osteolytic tumor in the left sacrum (Fig 1A). Percutaneous sacroplasty was requested because of failure of narcotics, radiation therapy, and transcatheter arterial embolization (TAE). The procedure was performed in the CT suite. Needle placement in the sacrum was performed using CT guidance, and the tip of an 11-gauge biopsy needle was positioned in the center of the tumor. Immediately after removal of an inner needle, gushing bleeding was noted through the needle. Two milliliters of a mixture of 50% n-BCA and 50% lipiodol was injected through the needle to control the bleeding, and the procedure was discontinued.
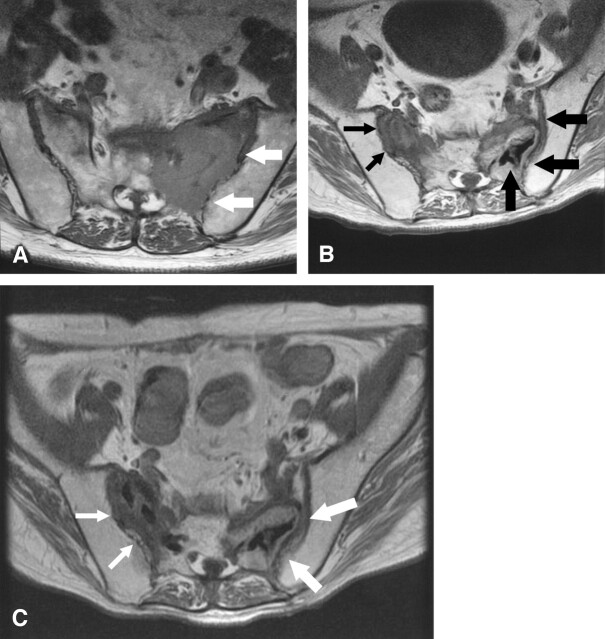
Fig 1.
Sequential MR imaging findings.
A, Axial T1-weighted MR image before the first pecutaneous sacroplasty shows a large osteolytic mass in left sacrum (arrows).
B, Follow-up axial T1-weighted MR image 3 months after the first percutaneous sacroplasty shows a slight reduction of left sacral metastasis (large arrows) and growth of right sacral metastasis (small arrows). Hypointensity in the left sacral tumor represents bone cement and n-BCA (curved arrow).
C, Follow-up axial T1-weighted MR image 35 days after the second percutaneous sacroplasty showed reduction of left sacral tumor (large arrows) and slight reduction of right sacral metastasis (small arrows).
A second attempt to alleviate the patient’s severe, persisting pain was made in the same manner 7 days after the first procedure. After placement of the needle into the tumor, a gushing hemorrhage was again recognized, and 2 mL of a mixture of 50% n-BCA and 50% lipiodol was injected. After injection of n-BCA, the residual material in the needle was pushed in coaxially by using an inner needle. Subsequently, 7 mL of the bone cement (70% PMMA and 30% barium powder mixture) was injected through the needle. Postprocedural CT confirmed cement distribution within the left sacral tumor. No complication occurred. Pain relief was apparent by the forth day after the procedure, and the patient became pain free by day 10. His general activities of daily living greatly improved, and pain relief was sustained.
Three months after the procedure, the patient began to complain of severe right lower back pain that was different from the previous left-sided pain. Follow-up MR imaging showed a reduction in size of the left sacral metastasis, but growth of the right sacral metastasis was noted (Fig 1B). CT-guided sacroplasty to right sacral metastasis was again requested. The second sacroplasty by using 3 mL of bone cement was performed without n-BCA injection because of slight bleeding and acceptable cement placement into the tumor was obtained (Fig 2). Transient numbness of the right lower extremity was noted, but it diminished the following day. Again, the pain greatly diminished by the third day after the second procedure, and he became ambulatory with a walker. Follow-up MR imaging obtained 35 days after the second procedure showed slight interval decrease in tumor size bilaterally (Fig 1C). Sepsis and intestinal bleeding led to the patient’s death 72 days after the second procedure. Pain recurrence was not recognized until death. The family donated his body for autopsy. Histologic analysis revealed complete necrosis of the tumor adjacent to the bone cement in metastases, and no viable metastatic lesion was noted (Fig 3).
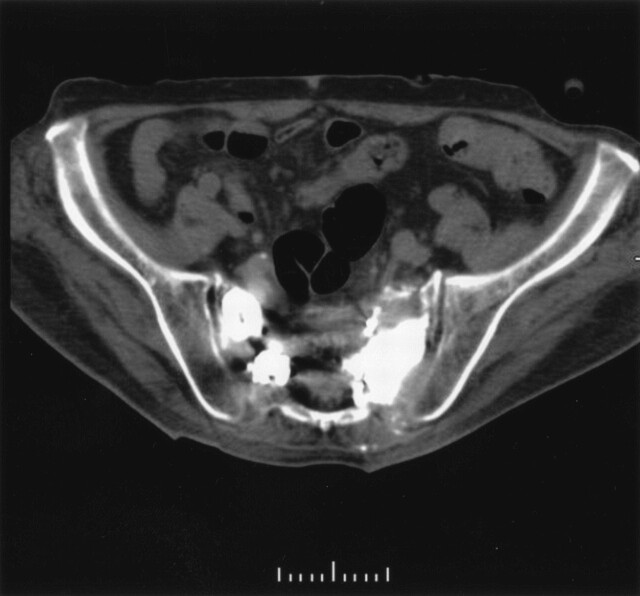
Fig 2.
CT after second percutaneous sacroplasty shows acceptable cement distribution in both metastases.
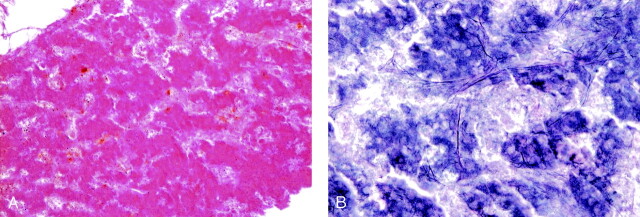
Fig 3.
Histologic analysis.
A, Specimen obtained from the tumor of the left sacrum adjacent to the injected cement. Hematoxylin and eosin staining demonstrates vast necrotic cells without apparent viable cancer cells (magnification×100).
B, The specimen from the same area as A. Silver stain shows presence of reticulin fibers (arrows) and cordlike structure of ghost cells (arrowheads) showing necrotic hepatocellular carcinoma tissue (magnification ×200).
Discussion
Bone metastases from HCC present as osteolytic, expansile, and hypervascular lesions (12–14). They usually cause severe pain and immobility, and high doses of opiate are required for pain relief (15). Radiation therapy and TAE are usually chosen for intolerable bone metastasis from HCC. Although radiation therapy can give partial or complete pain relief in 83.8% of patients (16), the effect is obtained only after a delay of several weeks. TAE also gives favorable outcome immediately if it is properly performed; however, the effect of radiation therapy and TAE tends to be transient because of the tumor’s intrinsic high neovascularity (15). To obtain permanent pain relief, the combination of radiation therapy and TAE is reported to be effective, but it is time consuming and cannot strengthen the weakened bone (15).
Percutaneous injection of bone cement is a promising technique as an alternative therapy for metastatic spinal tumors (1–11). In their series of 20 patients, Kaemmerlen et al (1) were the first to report the effectiveness of percutaneous injection of bone cement to metastatic tumors; they found that 80% of patients experienced pain relief. They were followed by Weill et al (2), who reported 73% of pain relief at 6 months after the procedures among 37 patients with spinal metastases. Cotten et al (3) found partial or complete pain relief in 36 of 37 patients, and pain relief occurred despite insufficient lesion filling. Cortet et al (4) also reported that thirty-six patients (97.3%) showed a decrease in their pain 48 hours after the procedure. This treatment has also been applied to pelvic metastases with favorable outcome (5–11). Weill et al (9) observed improvement in pain in 83% of 18 cases of acetabular malignancies. Marcy et al (10) described 82% of 18 pelvic metastatic patients experienced pain relief. Concerning metastasis from HCC, Hokotate et al (11) reported a case of acetabular metastasis from HCC in which there was sufficient pain relief and improved walking ability by cement injection. The clinical outcome of our case is consistent with those previous reports. In our case, although TAE and radiation therapy were performed before the first sacroplasty to the left-sided metastasis, immediate pain relief after sacroplasty is thought to be caused by the cement injection independent of the effect of TAE and radiation therapy. Moreover, the tumor reduction and immediate pain relief after second procedure to the right-sided metastasis without preprocedural TAE and radiation therapy suggested further the therapeutic effect of sacroplasty.
The mechanism of pain relief in patients with neoplastic lesions treated by cement injection is not well known. Stabilization of the destroyed bone in addition to the analgesic effect of PMMA due to the thermal effect in itself seemed to be main contributors for short-term pain relief. Long-term pain relief presumably was offered by reducing the tumor volume and long-term control of the tumor. Although the antitumoral effect of bone cement is not well known, exothermic effect of PMMA during polymerization possibly contribute for necrosis and volume reduction of the tumor (1, 17). PMMA particles may induce tumor necrosis factor mRNA and protein in a dose-dependent manner (18). Such necrotizing effect beyond the limit of the margin of PMMA may be the main factor in the persistent tumor control. Moreover, in our case, n-BCA injection might have played an additional role to control the growth of highly vascular metastatic tumors by embolization of feeding arteries.
Metastases from HCC are osteolytic, extremely hypervascular, and produce severe hemorrhage during the procedure, and insertion of the needle is challenging. Prevention of complication due to hemorrhage is a major indication for injecting n-BCA in all hemorrhagic lesions. The other complication during the procedure may be displacement of cement from the confines of the sacrum into the surrounding pelvic area, including sacral foramina (19). Therefore, it seems important to place the needle in the center of the tumor with sufficient margins to reduce the risks of cement migration in a hazardous area.
Conclusion
Percutaneous cement injection in preparation of n-BCA may be an alternative therapy for hypervascular metastases and superior to other treatments. In our case, the antitumoral and stabilization effects of bone cement and additional embolization effect of n-BCA were thought to be factors of persistent pain relief and tumor control.
References
- 1.Kaemmerlen P, Thiesse P, Bouvard H, et al. Percutaneous vertebroplasty in the treatment of metastases. Technic and results. J Radiol 1989;70:557–562 [PubMed] [Google Scholar]
- 2.Weill A, Chiras J, Simon JM, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology 1996;199:241–247 [DOI] [PubMed] [Google Scholar]
- 3.Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology 1996;200:525–530 [DOI] [PubMed] [Google Scholar]
- 4.Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma. Rev Rhum Engl Ed 1997;64:177–183 [PubMed] [Google Scholar]
- 5.Cotten A, Deprez X, Migaud H, et al. Malignant acetabular osteolyses: percutaneous injection of acrylic bone cement. Radiology 1995;197:307–310 [DOI] [PubMed] [Google Scholar]
- 6.Cotten A, Demondion X, Boutry N, et al. Therapeutic percutaneous injections in the treatment of malignant acetabularosteolyses. Radiographics 1999;19:647–653 [DOI] [PubMed] [Google Scholar]
- 7.Hierholzer J, Anselmetti G, Fuchs H, et al. Percutaneous osteoplasty as a treatment for painful malignant bone lesions of the pelvis and femur. J Vasc Interv Radiol 2003;14:773–777 [DOI] [PubMed] [Google Scholar]
- 8.Gangi A, Dietemann JL, Schultz A, et al. Interventional radiologic procedures with CT guidance in cancer pain management. Radiographics 1996;16:1289–1304 [DOI] [PubMed] [Google Scholar]
- 9.Weill A, Kobaiter H, Chiras J. Acetabulum malignancies: technique and impact on pain of percutaneous injection of acrylic surgical cement. Eur Radiol 1998;8:123–129 [DOI] [PubMed] [Google Scholar]
- 10.Marcy PY, Palussiere J, Descamps B, et al. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer 2000;8:500–503; erratum Support Care Cancer 2000;8: 510 [DOI] [PubMed] [Google Scholar]
- 11.Hokotate H, Baba Y, Churei H, et al. Pain palliation by percutaneous acetabular osteoplasty for metastatic hepatocellular carcinoma. Cardiovasc Intervent Radiol 2001;24:346–348 [DOI] [PubMed] [Google Scholar]
- 12.Liaw CC, Ng KT, Chen TJ, Liaw YF. Hepatocellular carcinoma presenting as bone metastasis. Cancer 1989;64:1753–1757 [DOI] [PubMed] [Google Scholar]
- 13.Golimbu C, Firooznia H, Rafii M. Hepatocellular carcinoma with skeletal metastasis. Radiology 1985;154:617–618 [DOI] [PubMed] [Google Scholar]
- 14.Robinson DL, Davaiah KA, Lawton RL. Hepatocellular carcinoma presenting as bone pain. J Surg Oncol 1986;31:100–103 [DOI] [PubMed] [Google Scholar]
- 15.Uemura A, Fujimoto H, Yasuda S, et al. Transcatheter arterial embolization for bone metastases from hepatocellular carcinoma. Eur Radiol 2001;11:1457–1462 [DOI] [PubMed] [Google Scholar]
- 16.Kaizu T, Karasawa K, Tanaka Y, et al. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol 1998;93:2167–2171 [DOI] [PubMed] [Google Scholar]
- 17.Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty [Suppl]. Bone 1999;25:17–21 [DOI] [PubMed] [Google Scholar]
- 18.Merkel KD, Erdmann JM, McHugh KP, et al. Tumor necrosis factor mediates orthopedic implant osteolysis. Am J Pathol 1999;154:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pommersheim W, Huang-Hellinger F, Baker M, Morris P. Sacroplasty: a treatment for sacral insufficiency fractures. AJNR Am J Neuroradiol 2003;24:1003–1007 [PMC free article] [PubMed] [Google Scholar]