Abstract
BACKGROUND AND PURPOSE: Proton MR spectroscopy has demonstrated reduced levels of N-acetylaspartate (NAA) in normal-appearing occipital and frontal regions of patients with acute nonpenetrating traumatic brain injury (TBI). We studied the relationship of frontoparietal NAA, choline (Cho), and creatine (Cr) to test the hypothesis that reduction in NAA is predictive of cognitive outcome.
METHODS: Proton spectra were collected by using conventional 2D chemical shift imaging in five healthy children and seven children (6 weeks to 3 years) with severe (n = 4), moderate (n = 2), or mild (n = 1) TBI. Spectra in the anterior and posterior regions of the left and right frontoparietal areas were averaged for analysis by using LCModel, with a phantom-established basis function, for quantification of NAA, Cho, and Cr concentrations. Intellectual function, expressive language, and arithmetic capability were measured within 4 months of imaging.
RESULTS: NAA/Cho concentration was lower in TBI patients than in control subjects, but no group differences were present for Cho or Cr. Hemispheric levels for NAA, Cho, and Cr were higher on the left than on the right, but we found no effect of region and no interactions. Cognition was lower in the TBI group than the control group and correlated with NAA levels. Left frontal Cho was also correlated with arithmetic scores, whereas Cr was not significantly correlated.
CONCLUSION: NAA levels remain low after TBI and are related to cognitive function. Neurometabolite values are greater in the left frontoparietal region than in the right, and the left frontal Cho level is related to arithmetic ability.
Conventional MR imaging can be used to delineate the presence of blood, extra-axial fluid, and major disruption of tissue with any associated cerebral edema by using a combination of gradient echo, T1-weighted, and T2-weighted sequences, including fluid-attenuated inversion recovery (FLAIR). Newer sequences such as diffusion-weighted imaging and susceptibility-weighted imaging (1) provide additional information regarding disruption of white matter and the more sensitive detection of hemorrhage in relation to shear injury, respectively. However, the relation of MR imaging to cognitive outcome after traumatic brain injury (TBI) has been inconsistent across studies (2). Magnetization transfer imaging has been conducted in adults to evaluate axonal injury (3). However, there is growing interest in utilizing MR spectroscopy in TBI research to investigate changes in brain metabolites, such as reduced N-acetylaspartate (NAA) levels, which reflect a loss of neuronal and axonal integrity (4). The TBI literature suggests value in obtaining spectra from areas of the brain without MR imaging abnormalities (5–9). To date, much of the work has been performed in animal models (10) or adults (6, 8, 9, 11) by using single voxel techniques. Preliminary investigations of TBI in infants and children with single-voxel MR spectroscopy have revealed that reduced NAA and elevated lactate within a month after injury (5, 12) are predictive of poor long-term outcome, including effects on intellectual and neuropsychological functioning (12). However, the MR spectroscopy literature lacks information concerning the long-term effects of pediatric TBI on alterations in neurometabolites in relation to neurobehavioral outcome.
To measure late changes in neurometabolites after pediatric TBI, we applied the 2D chemical shift imaging (CSI) technique to acquire 1H MR spectra in school-aged children at least 6 weeks after injury. In addition, we measured their intellectual function and other cognitive abilities within 2 weeks of imaging and analyzed the findings in relation to the neurometabolites. We set out to test the hypothesis that reduction in NAA is predictive of cognitive outcome in children.
Methods
Subjects
Seven children with TBI associated with closed head trauma, (Table 1), and five uninjured control subjects were examined. Table 1 indicates that four of the patients had severe injuries, whereas two were moderately injured, and a single child had a mild injury, according to their lowest postresuscitation Glasgow Coma Scale score (13). Time postinjury ranged from 6 weeks to 3 years. Age at the time of imaging did not significantly differ between patients with TBI (mean ± standard deviation [SD], 10.66 ± 2.30 years) and healthy children (10.61 ± 1.92 years), with F(1,10) = 0.00 and P < .97. All of the children were right-hand dominant with the exception of one female control subject, who was ambidextrous. Three children with TBI and all uninjured control subjects were imaged within 1 month of their evaluation. All children were imaged within 4 months of their neuropsychological evaluation.
TABLE 1:
Clinical and demographic information for children with TBI
| Patient/Sex | GCS Score | Mechanism of Injury | Age at Injury (y) | Age at MRS (y) | Age at Testing (y)* | GOS Score | MR Imaging Finding |
|---|---|---|---|---|---|---|---|
| 1/M | 15 | Fall | 7.4 | 7.8 | 7.8 | 1 | Normal |
| 2/F | 8 | Hit by auto | 8.0 | 8.3 | 8.3 | 2 | Bilateral superior frontal gyrus gray matter hemosiderin deposit |
| 3/F | 3 | Hit by auto | 12.1 | 12.4 | 12.3 | 2 | R temporal lobe white matter atrophy |
| 4/F | 11 | Hit by auto | 8.2 | 9.9 | 10.2 | 1 | L frontal lobe gray matter and white matter encephalomalacia and shearing injury; bilateral middle frontal, inferior frontal, and gyrus rectus encephalomalacia; L orbital gyrus encephalomalacia |
| 5/F | 4 | Hit by auto | 10.1 | 10.2 | 10.4 | 2 | Bilateral frontal lobe white matter gliosis and L frontal lobe gray matter hemosiderin; L gyrus rectus gliosis; posterior corpus callosum gliosis |
| 6/M | 3 | Hit by auto | 10.5 | 11.8 | 11.5 | 1 | R frontal lobe white matter gliosis and hemosiderin |
| 7/F | 10 | Hit by auto | 11.1 | 14.2 | 14.5 | 1 | Bilateral gyrus rectus atrophy |
Note.—GCS indicates Glasgow Coma Scale; GOS, Glasgow Outcome Scale score (1 = good, 2 = moderate, 3 = severe); and MRS, MR spectroscopy.
Age at testing is reported for the evaluation closest in time to imaging.
MR Imaging Method
Data were acquired by using a 1.5T machine (Gyroscan Intera; Philips, Best, the Netherlands) with a receive-only circularly polarized head coil. In each subject, sagittal T1-weighted, axial T2-weighted, coronal FLAIR and axial gradient-echo imaging was performed before MR spectroscopy.
Phantoms
Three metabolite solution phantoms—one each for NAA, Cho, and Cr—were produced to create a reference “basis” spectrum file for the LCModel (Provencher; Ontario, Canada) MR spectroscopic analysis software package, which allowed quantification of the metabolites from the in vivo data (14). This is a well-established, commercially available product that uses a least-squares-fit algorithm. The LCModel documentation (15) was used as a guideline for the phantom constituents and concentrations. The measurements for the basis function were conducted by using the same pulse sequence, TR, and TE as that used for the in vivo studies. A series of spectra with various TRs and TEs were acquired to obtain T1 and T2 correction factors for water and each metabolite. A conversion factor between the signal intensity amplitude from one voxel in the CSI and that from the single voxel water spectrum was obtained from phantom measurements as well.
A phantom containing all three metabolites in concentrations similar to those found in the normal brain was made to analyze both the spatial homogeneity and temporal stability of the received signal intensity. The solution phantoms were made to be uniform and stable. Four sets of identical measurements were obtained in the transverse plane. Although only NAA, Cr, and Cho were quantified in this phantom, myo-inositol, glutamine, and glutamate were also added to the metabolite phantom at physiologic levels, as this allowed us to include the effects of possible interactions among all of the metabolites on the quantitative analysis. Finally, all phantoms contained sodium azide as a preservative and potassium phosphates as pH buffers.
MR Spectroscopic Method
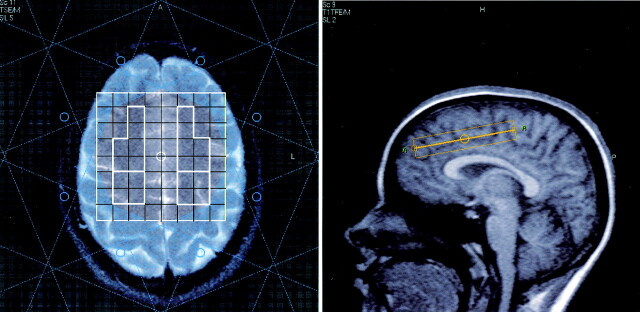
Proton spectra were collected using 2D CSI technique with the region of interest preselected by means of point-resolved spectroscopy (PRESS). This technique has the advantage that data from multiple voxels are acquired simultaneously, allowing us to compare metabolite levels in several regions in a relatively short data acquisition time. For each subject, an 11-section MR imaging localizer sequence was first performed so that the CSI volume of interest (VOI) of 80 × 80 × 15 mm could be precisely located. This enabled us to duplicate the VOI position on follow-up MR spectroscopy in the same subject and to ensure similar positioning among our sample of subjects. The 2D CSI plane was positioned obliquely, as depicted in Figure 1.
Fig 1.
Position of the spectroscopic imaging VOI, as viewed in the axial and sagittal planes. On the axial image, the outlined sections inside the VOI depict voxels typically selected for the four regions of interest, and the pattern surrounding the VOI is the area covered by the eight multiple regional saturation technique (MREST) pulses for saturating lipid signals from the scalp.
The anterior aspect of the plane was located >2 cm above the floor of the anterior cranial fossa, parallel to the anterior aspect of the body of the corpus callosum, and immediately above the cingulate gyrus at an angle of 25–30° to the anterior commissure-posterior commissure line. This position allowed simultaneous coverage of the frontal lobe, as well as the posterior frontoparietal region, in one 15-mm-thick CSI section to include predominantly white matter. The field of view for the 2D CSI was 200 × 200 mm, with 20 × 20 phase encoding steps, yielding 10-mm in-plane resolution.
Scalp lipid suppression was achieved by using eight MREST pulses that were incorporated into the spectroscopic software and saturated the voxels outside the VOI. The MREST pulses did not affect the signal intensity inside the quantified voxels. Each acquisition had 1024 time-domain sampling points,, and no subsequent apodization was performed. TR/TE was 1500/60 ms. This TE was chosen to avoid strong macromolecule baselines and coupled spins found at shorter TEs (e.g., 30 ms), while we minimized T2-weighting and signal intensity loss at longer TEs (e.g., 136 ms). NEX for 2D CSI was set to 1. In each subject, a single-voxel water reference spectrum was also obtained in the gray matter at the center of the 2D CSI plane for signal intensity calibration (TR/TE/NEX, 5000/31/4). This was based on the assumption that the water content of the gray matter is relatively constant (approximately 78%).
The first step in the data reduction was to import the localizer and 2D CSI data for each subject into a software package (Spectro Tool; Philips Medical Systems). This software allowed us to average a group of typically four to six voxels that included predominantly white matter in each of the four areas of interest (left and right frontal lobes and left and right posterior frontoparietal lobes). A separate program written Interactive Data Language (IDL, Research Systems, Denver, CO) was used to convert the data in each region into a format for use in LCModel. Other corrections entered into the LCModel analysis included the relaxation effects of metabolites in the phantom and in normal brain tissue (16). Water scaling was done in the LCModel analysis by using the data from the water reference spectrum.
Cognitive Testing
Patients with TBI completed a neuropsychological evaluation at baseline and at approximately 3, 6, 12, and 24 months after their injury as part of an ongoing study. One patient was also reevaluated at 36 months after injury. Healthy control subjects were matched to the patients by age and given a more limited battery of neuropsychological tests on one occasion. Cognitive testing of the patients and control subjects included the Wechsler Abbreviated Scale of Intelligence (17), a brief measure of intellectual function; the Formulated Sentences subtest of the Clinical Examination of Language Function (18), which measures expressive language; and the Calculations subtest of the Woodcock-Johnson Test (19), a measure of written arithmetic. Children with TBI were administered the Wechsler Abbreviated Scale of Intelligence at the 6-month evaluation (patients 1, 2, 3, 5, 6), the 24-month evaluation (patient 4), or 36 months after injury, (patient 7) (Table 1). Standard scores adjusted for age were analyzed for the cognitive tests.
Statistical Analysis
The metabolites were analyzed using a general linear mixed model with Statistical Analysis System software (SAS Institute; Cary, NC). Group (TBI, control) was the between-subject factor, and hemisphere (left, right) and region (frontal, posterior) were the within-subject factors.
Results
MR Imaging Findings
No abnormalities were found in the healthy control subjects. Table 1 summarizes the MR imaging results. Patient 1 had normal examination results, patient 3 had right temporal lobe atrophy, and patient 6 had gliotic change in the right frontal white matter, with hemosiderin. The remaining four children had bilateral frontal volume loss, two with superficial siderosis, and patient 4 had hemosiderin and encephalomalacia involving the left frontal gray and white matter, respectively. Figure 1 shows that the region of interest for spectroscopy was generally away from the site of most of these injuries.
Metabolite Levels in TBI and Control Groups
Table 2 shows the average of the LCModel least-squares analysis for each metabolite according to group, side, and region. Table 3 summarizes results of the statistical analysis of the metabolites.
TABLE 2:
Concentrations of NAA, Cho and Cr (mean ± SD in mmol/L)
| Group and Area | NAA |
Cho |
Cr |
NAA/Cr |
NAA/Cho |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | |
| Patients with TBI (n = 7) | ||||||||||
| Frontal | 9.64 ± 0.54 | 9.21 ± 0.74 | 2.26 ± 0.29 | 1.88 ± 0.34 | 9.94 ± 1.25 | 9.46 ± 1.47 | 0.98 ± 0.12 | 1.00 ± 0.19 | 4.33 ± 0.72 | 5.01 ± 0.80 |
| Parietal | 9.83 ± 1.04 | 9.35 ± 1.57 | 2.10 ± 0.31 | 1.93 ± 0.41 | 9.86 ± 1.70 | 8.92 ± 1.35 | 1.02 ± 0.16 | 1.05 ± 0.16 | 4.73 ± 0.42 | 4.98 ± 1.12 |
| Control subjects (n = 5) | ||||||||||
| Frontal | 10.46 ± 0.64 | 10.17 ± 0.54 | 2.05 ± 0.16 | 1.89 ± 0.36 | 10.26 ± 0.89 | 9.18 ± 0.96 | 1.02 ± 0.07 | 1.12 ± 0.13 | 5.13 ± 0.57 | 5.55 ± 1.22 |
| Parietal | 10.34 ± 0.42 | 9.63 ± 0.30 | 2.07 ± 0.52 | 1.76 ± 0.22 | 9.51 ± 1.33 | 8.65 ± 1.44 | 1.10 ± 0.13 | 1.14 ± 0.21 | 5.29 ± 1.47 | 5.52 ± 0.64 |
TABLE 3:
Summary of the analysis of metabolites for effects of group (G), side (S), and region (R) of brain
| Factor | NAA |
Ch |
Cr |
NAA/Cr |
NAA/Cho |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | P Value | F | df | P Value | F | df | P Value | F | df | P Value | F | df | P Value | |
| Group | 3.94 | 1,10 | 0.075 | 1.21 | 1,10 | .296 | 0.06 | 1,10 | .816 | 0.47 | 1,10 | .509 | 5.51 | 1,10 | .041 |
| Side | 7.11 | 1,10 | 0.0236 | 8.650 | 1,10 | .015 | 7.01 | 1,10 | .024 | 1.17 | 1,10 | .305 | 2.36 | 1,10 | .155 |
| Region | 0.21 | 1,10 | 0.660 | 0.45 | 1,10 | .520 | 2.23 | 1,10 | .901 | 0.13 | 1,10 | .729 | 0.23 | 1,10 | .644 |
| G × S | 0.02 | 1,10 | 0.894 | 0.06 | 1,10 | .809 | 0.17 | 1,10 | .69 | 0.81 | 1,10 | .389 | 0.07 | 1,10 | .798 |
| G × R | 1.92 | 1,9 | 0.196 | 0.00 | 1,9 | .956 | 0.28 | 1,9 | .611 | 0.06 | 1,10 | .808 | 0.060 | 1,10 | .808 |
The level of NAA tended to be lower in the TBI group than in the control group (Table 2), and this effect of group approached significance (Table 3). A significant effect of side was present, reflecting generally higher left frontoparietal NAA values relative to right hemisphere values (Table 3). When we averaged the results across groups and regions, the left frontoparietal NAA level was 10.01 ± 0.76 (mean ± SD in mmol/L) compared with 9.54 ± 0.99 for the right hemisphere. However, we observed no interactions of group with the side or region of the brain and no main effect of region. Although the group difference in Cho was not significant (Table 3), the laterality effect was significant, as levels were higher in the left hemisphere (overall 2.13 ± 0.33) than in the right hemisphere (1.88 ± 0.33). However, we found no significant interaction of group with either hemisphere or region of brain for Cho. Similar analysis of Cr also revealed a significant side effect with higher left (9.89 ± 1.29) than right (9.08 ± 1.29) frontoparietal values but no significant effect of group or region and no interaction.
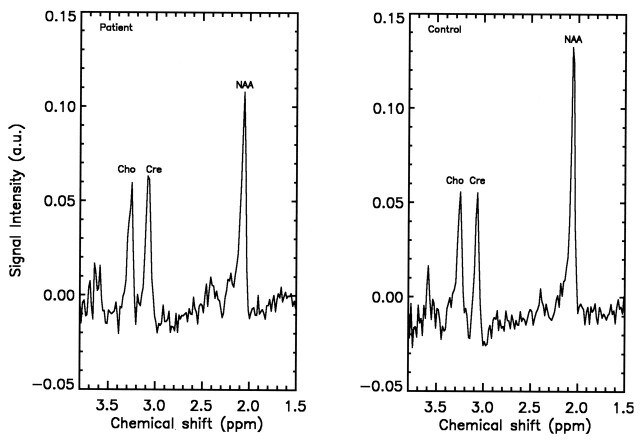
With evidence for greater reproducibility of brain metabolite peaks using ratios (20), we also analyzed the NAA/Cr and NAA/Cho ratios. Although analysis of the NAA/Cr ratio revealed no significant effects, NAA/Cho values were lower in the patients with TBI than in the control subjects (Table 3). However, we observed no significant interaction of group with either side or region for NAA/Cho. Figure 2 depicts sample spectra showing the metabolites of interest in the frontoparietal region in one patient and one control representative of the SNR. From the phantom metabolite homogeneity tests, mean values of the left-to-right metabolite ratios and 95% confidence intervals of the means were calculated to be 1.01 ± 0.05 for NAA, 0.99 ± 0.04 for Cho, and 0.99 ± 0.02 for Cr. Again, these tests were performed with the VOI oriented in the transverse plane. To exclude the possibility that the hemispheric asymmetry of neurometabolites could have been due to our acquiring the data with an oblique VOI, the homogeneity tests were repeated on the phantom with an oblique VOI producing essentially unchanged results showing no asymmetry.
Fig 2.
Average spectra obtained from the left frontal lobe of a patient versus that of a control demonstrates a decrease in NAA after TBI.
MR Spectroscopic Findings in Relation to Cognitive Test Data
As shown in Table 4, the control group performed above the level of the patients with TBI on all tests. Pearson correlation coefficients were computed for the average metabolite values and for each of the four regions. Table 5 summarizes these coefficients for the relation between the cognitive test variables and the neurometabolites, including the overall levels averaged across the four regions and the values for the left and right frontal regions. No correlation coefficient involving the parietal region was significant. Correlation coefficients were significant for arithmetic scores in relation to NAA values for each frontal lobe but not for the composite value averaged across all four regions. The correlation between left frontal NAA and intellectual level (on the Wechsler Abbreviated Scale of Intelligence) approached significance. Left frontal Cho was also related to arithmetic scores. Findings for Cr were limited to a correlation with expressive language that approached significance for the overall mean averaged across the four anatomic regions (Table 5). We found support for our hypothesis that reduction in NAA is demonstrated to predict some aspects of cognitive outcome, despite the small numbers.
TABLE 4:
Cognitive test findings in the TBI and control groups (mean ± SD)
| Test and Group | Result | t | P Value |
|---|---|---|---|
| WASI | 4.26 | .0017 | |
| Control | 113.80 ± 12.56 | ||
| TBI | 85.86 ± 10.19 | ||
| WJ Math | 5.54 | .0002 | |
| Control | 120.20 ± 14.99 | ||
| TBI | 84.86 ± 6.91 | ||
| CELF | 3.87 | .0031 | |
| Control | 117.00 ± 9.75 | ||
| TBI | 92.00 ± 11.82 |
Note.—CELF indicates Clinical Examination of Language Function; WASI, Wechsler Abbreviated Scale of Intelligence; and WJ Math, Woodcock Johnson Arithmetic Achievement subtest.
TABLE 5:
Pearson correlation coefficients between brain metabolite levels and cognitive scores
| Test | Composite |
L Frontal |
R Frontal |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cho | Cr | NAA | Cho | Cr | NAA | Cho | Cr | NAA | |
| WASI | |||||||||
| Correlation | −0.20 | −0.02 | 0.29 | −0.39 | 0.19 | 0.51 | 0.04 | −0.20 | 0.47 |
| P value | .55 | .94 | .36 | .21 | .54 | .09 | .91 | .54 | .12 |
| WJ MATH | |||||||||
| Correlation | −0.22 | 0.06 | 0.54 | −0.57 | 0.02 | 0.64 | 0.12 | −0.07 | 0.72 |
| P value | .49 | .84 | .07 | .06 | .95 | .03* | .71 | .84 | .01† |
| CELF | |||||||||
| Correlation | −0.03 | −0.50 | 0.21 | −0.01 | −0.11 | 0.32 | −0.09 | −0.47 | 0.38 |
| P value | .92 | .10 | .50 | .99 | .74 | .31 | .78 | .12 | .23 |
Note.—CELF indicates Clinical Examination of Language Function; WASI, Wechsler Abbreviated Scale of Intelligence; and WJ Math, Woodcock Johnson Arithmetic Achievement subtest. Correlations for L and R parietal regions were not significant and are not shown.
P < .05.
P < .01.
Discussion
The major finding on MR spectroscopy, which distinguished children with TBI at ages 7–12 years was lower NAA levels, especially when it was analyzed as a ratio to Cho. This finding of reduced NAA, which we obtained after postinjury intervals ranging from 6 weeks to 3 years, is consistent with previous reports of pediatric patients with TBI examined within 1 month postinjury by using the single-voxel technique (5, 12). Our findings extend previous pediatric TBI studies by demonstrating a residual loss of NAA at later postinjury intervals. We interpret the NAA data as evidence for frontoparietal neuronal and axonal injury. NAA also had the most robust relation to cognitive function, including intellectual function and arithmetic. In contrast, Cho and Cr had less consistent and generally lower correlations with the cognitive variables. Although expressive language, as measured by the CELF was also impaired in the patients with TBI relative to the uninjured control subjects, it was not related to the neurometabolite levels as demonstrated for intellectual function and arithmetic.
We also observed hemispheric asymmetry of NAA, Cho, and Cr, which were generally higher in the left hemisphere than in the right hemisphere and which did not interact with group or region. The Cho signal intensity that appears prominently on 1H MR spectroscopy during early brain development compared with the adult brain most likely represents the high levels of substrate needed for the formation of cell membranes and myelin (16, 21–23).
Whole-brain spectroscopy can be used to study global changes in total brain NAA (24). Use of the 2D CSI technique in the present study, however, facilitated analysis of hemispheric asymmetries while it limits acquisition time, a factor that is important for imaging brain injured children. Furthermore, the frequency-dependent volume shift for single-voxel techniques is not present for spectra of individual voxels defined by the 2D phase encoding in CSI, which is an advantage for analyzing brain symmetry. We have reviewed the literature on hemispheric asymmetries in brain metabolites to provide a developmental framework for interpreting the higher level of left than right frontoparietal NAA, Cho, and Cr. Findings for hemispheric asymmetry of neurometabolites have been inconsistent across spectroscopic studies of healthy adult comparison groups. Moore et al (25) found that the ratio of Cho to Cr and phosphocreatine was generally lower in the left temporal lobe than in the right temporal lobe in groups of adults with schizophrenia and healthy control subjects, whereas other investigators have noted individual variation in interhemispheric hippocampal asymmetry of NAA, Cho, and Cr (26). In contrast, Pouwels and Frahm (20) found a homogeneous distribution of NAA throughout the brain, with the highest regional concentrations of Cho and Cr in cerebellum; the intraindividual right-to-left concentration ratio for Cho in the frontal lobe was 0.90 ± 0.12 in seven adult subjects, a trend consistent with our findings. Interhemispheric asymmetries in brain metabolites should be interpreted with caution due to variation with repeated testing (26). Analysis of ratios enhances the reproducibility of interhemispheric asymmetries, at least in normal adults (26). In our cross-sectional study, the frontal Cho level greater on the left than on the right was confirmed when ratios were analyzed, but we did not evaluate its reproducibility. The possibility that the asymmetry in neurometabolites is an artifact is unsupported by data we acquired from a uniform phantom solution.
Recent MR imaging investigation of regional and hemispheric variation in cortical and subcortical development (27, 28) is relevant to our finding of a hemispheric asymmetry in NAA, Cho, and Cr in children. These asymmetries may be related to the age-related specialization of the left frontoparietal region for language in typically developing children (28, 29), a possibility that is also consistent with the decline in frontoparietal cortical gray matter after age 6 years that is associated with prolonged myelination. Concurrent spectroscopic and morphometric studies linked with measures of language development could investigate this hypothesis. Whether the lateralized gradient for NAA, Cho, and Cr facilitates functional hemispheric specialization or is a product of this process is unknown.
Limitations of this initial study include the small sample size, heterogeneous population, and limited range of neuropsychological testing. A larger sample size, including the spectrum of postresuscitation Glasgow Coma Scale scores, would also facilitate analysis of TBI severity, which was not possible in the present study. Longitudinal investigation is also needed to determine the effect of TBI on cognitive development in relation to changes in neurometabolites, especially NAA. The use of an intermediate TE of 60 ms improved the signal-to-noise of the spectra but did not allow us to quantify glutamine and glutamate. We also acknowledge that myo-inositol is important to include in a future study.
Conclusion
Our research extends prior reports of acutely decreased NAA levels after TBI in children (5, 12) to later phases of recovery and shows that residual levels are moderately related to cognitive function on measures of arithmetic and intellectual ability. In contrast, levels of Cho and Cr did not differ between the groups of brain-injured children and healthy children. The relation of Cho and Cr to cognitive variables was weaker and less consistent relative to the correlations of arithmetic and intellectual function with NAA. We also found hemispheric lateralized asymmetry of NAA, Cho, and Cr in typically developing children and brain-injured children. These increased hemisphere neurometabolite levels, left greater than right, may be related to an age-related increase in left hemisphere specialization for language with associated loss of cortical gray matter and ongoing myelination in this region. Longitudinal spectroscopic investigation of children is necessary to evaluate this interpretation of our data, pending replication in another cross-sectional study.
Acknowledgments
We are indebted to Xiaoqi Li, MS, for data management and statistical analysis and to Stacey Martin for assistance with word processing and editing.
Footnotes
Supported by National Institute of Health grants R01 NS-21889 and R21 NS-45573.
Presented at the 41st Annual Meeting of the American Society of Neuroradiology, Washington, DC, April 31, 2003.
References
- 1.Tong K, Holshouser B, Shutter L, Chiou P, Herigauk G, Haacke E. High resolution susceptibility weighted imaging (SWI) improves detection of hemorrhagic lesions in adults with traumatic brain injury: correlation with severity of injury and outcome. Proceedings of ISMRM 10th Scientific Meeting. Berkeley: International Society for Magnetic Resonance in Medicine2002. :46 .
- 2.Donders J, Strom D. Neurobehavioral recovery after pediatric head trauma: injury, pre- injury, and post-injury issues. J Head Trauma Rehabil 2000;15:792–803 [DOI] [PubMed] [Google Scholar]
- 3.Sinson G, Bagley LJ, Cecil KM, et al. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury. AJNR Am J Neuroradiol 2001;22:143–151 [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil 2001;16:149–164 [DOI] [PubMed] [Google Scholar]
- 5.Ashwal S, Holshouser BA, Shu SK, et al. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr Neurol 2000;23:114–125 [DOI] [PubMed] [Google Scholar]
- 6.Brooks WM, Stidley CA, Petropoulos H, et al. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma 2000;17:629–640 [DOI] [PubMed] [Google Scholar]
- 7.Cecil KM, Hills EC, Sandel ME, et al. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg 1998;88:795–801 [DOI] [PubMed] [Google Scholar]
- 8.Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol 1998;19:1879–1885 [PMC free article] [PubMed] [Google Scholar]
- 9.Garnett MR, Cadoux-Hudson TA, Styles P. How useful is magnetic resonance imaging in predicting severity and outcome in traumatic brain injury? Curr Opin Neurol 2001;14:753–757 [DOI] [PubMed] [Google Scholar]
- 10.Rubin Y, Cecil K, Wehrli S, McIntosh TK, Lenkinski RE, Smith DH. High-resolution 1H NMR spectroscopy following experimental brain trauma. J Neurotrauma 1997;14:441–449 [DOI] [PubMed] [Google Scholar]
- 11.Condon B, Oluoch-Olunya D, Hadley D, Teasdale G, Wagstaff A. Early 1H magnetic resonance spectroscopy of acute head injury: four cases. J Neurotrauma 1998;15:563–571 [DOI] [PubMed] [Google Scholar]
- 12.Brenner T, Freier MC, Holshouser BA, Burley T, Ashwal S. Predicting neuropsychologic outcome after traumatic brain injury in children. Pediatr Neurol 2003;28:104–114 [DOI] [PubMed] [Google Scholar]
- 13.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;2:81–84 [DOI] [PubMed] [Google Scholar]
- 14.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679 [DOI] [PubMed] [Google Scholar]
- 15.Provencher S. LCModel(1) & LCMgui(2) User’s Manual. 2003. . Available at http://s-provencher.com/pages/lcm-manual.shtml. Accessed February 1, 2003
- 16.Kreis R, Ernst M, Ross BD. Absolute quantitation of water and metabolites in the human brain, II: Metabolite concentrations. J Magn Reson 1993;102:9–19 [Google Scholar]
- 17.Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual. San Antonio: Psychological Corporation;1999
- 18.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals. 3rd ed. San Antonio: Psychological Corporation;1995
- 19.Woodcock RW, Johnson MB. Woodcock-Johnson Tests of Achievement-Revised: Examiner’s Manual. Itasca, IL: Riverside;1990
- 20.Pouwels P, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized portion MRS. Magn Reson Med 1998;39:53–60 [DOI] [PubMed] [Google Scholar]
- 21.Cady E, Penrice J, Amess P, et al. Lactate, N-acetyl-aspartate, choline, and creatine concentrations and spin-spin relation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med 1996;36:878–886 [DOI] [PubMed] [Google Scholar]
- 22.Huppi PS, Fusch C, Boesch C, et al. Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high-performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res 1995;37:145–150 [DOI] [PubMed] [Google Scholar]
- 23.Huppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res 1991;30:574–578 [DOI] [PubMed] [Google Scholar]
- 24.Gonen O, Viswanathan AK, Catalaa I, Babb J, Udupa J, Grossman RI. Total brain N-acetylaspartate concentration in normal, age-grouped females: quantitation with non-echo proton NMR spectroscopy. Magn Reson Med 1998;40:684–689 [DOI] [PubMed] [Google Scholar]
- 25.Moore CM, Bonello CM, Sherwood AR, Cohen BM, Renshaw PF, Yurgulen-Todd DA. Mesial temporal lobe Cho to Cr(PCr) ratio asymmetry in chronic schizophrenics. Schizophr Res 2002;57:35–42 [DOI] [PubMed] [Google Scholar]
- 26.Maton B, Londono A, Sawrie S, Knowlton R, denHollander J, Kuznieck R. Reproducibility of proton magnetic resonance spectroscopy imaging measurements of normal human hippocampus at 1.5 T: clinical implications. J Neuroimaging 2001;11:194–201 [DOI] [PubMed] [Google Scholar]
- 27.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 2002;44:4–16 [DOI] [PubMed] [Google Scholar]
- 28.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci 2003;6:309–315 [DOI] [PubMed] [Google Scholar]
- 29.Sowell ER, Thompson PM, Rex D, et al. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex 2002;12:17–26 [DOI] [PubMed] [Google Scholar]