Abstract
BACKGROUND AND PURPOSE: Physiologic and scanner variability of proton MR spectroscopy (MRS) measurements can limit the detection of subtle metabolite fluctuations. We assessed the variability of such measurements at 3T and compared two methods to obtain absolute concentrations.
METHODS: Variability over 14 days was assessed with short-echo, single-voxel proton MRS in 14 control subjects and in a phantom containing 50 mmol/L N-acetylaspartate (NAA). Spectra were analyzed by using LCModel, scaling factors determined with both the calibration phantom (CP), and water peak intensity (WP) methods. Relative (reflecting the systematic drift) and absolute variability (reflecting the magnitude of scanner variability) was determined.
RESULTS: For the phantom, initial (49 ± 1.7 mmol/L) and second measurements (50 ± 1.6 mmol/L) showed similar results, with small variability (relative, −0.6 ± 1.5 mmol/L; absolute, 1.1 ± 1.1 mmol/L). Control subjects had no systematic difference between the two scans for any measurement. Absolute variabilities in the temporal lobe for total NAA (NAA+NAAG) were 13% (CP) and 11% (WP). The largest variability (29%) was found for glutamate-glutamine (29%) with the CP method, and for myo-inositol with the WP method (28%). Absolute variability was smaller for the frontal lobe measurements (total NAA 7% and overall 6–18% for CP; total NAA 6% and overall 5–19% for WP). No significant difference was observed between the two methods.
CONCLUSION: Physiologic variability is the major source of measurement variability and accounts for 12% of the variability in temporal lobe total NAA. Therefore, total NAA variations must clearly exceed this before they can reliably be attributed to an effect of disease.
Proton (1H) MR spectroscopy (MRS) has been used to assess brain metabolites in patients with chronic neurologic diseases such as temporal lobe epilepsy (1–5). The metabolites typically assessed are the following: 1) NAA (N-acetylaspartate), a neuron specific metabolite (6) plus N-acetylaspartylglutamate (NAAG); 2) creatine plus phosphocreatine (Cr) and total trimethylamines (Cho), which may be concentrated in glial cells (6); and 3) myo-inositol (mI), a putative marker of gliosis and an organic osmolyte (7) involved in cellular volume control. In some neurologic diseases, such as epilepsy, metabolite changes can be observed both as chronic and permanent abnormalities (8) and as acute and transient changes (9). In this situation, knowledge of the degree of physiologic and machine variability of proton MRS measurements is of paramount interest.
To our knowledge, the current literature provides no information on metabolite variability for temporal or frontal lobes at 3T. Several studies have assessed MRS variability at 1.5T (10–19). Most studies assess only a single brain region, and therefore do not allow investigation of regional differences in variability. The predominant method for assessing variability has been the comparison of correlation coefficients and coefficients of variation. The degree of variability in MRS metabolites varies between studies, and is difficult to compare due to the use of different MRS sequences, voxel positions and analysis methods. The higher signal-to-noise ratio of measurements at 3T and above could result in reduced variability relative to studies by using lower magnetic field strengths, as suggested previously (14). On the other hand, increased variability in the homogeneity of the magnetic field may affect the accuracy of the methods used for determination of metabolite concentrations. At 3T, the preferred method for establishing absolute concentrations has not yet been evaluated.
We aimed to assess the physiologic and machine variability of metabolite concentrations in two brain regions over 2 weeks and to investigate the influence of the choice of scaling method on metabolite concentrations estimated by using the LCModel by comparing results obtained with either the internal water peak intensity or with a calibration phantom.
Methods
Single-voxel proton MRS was performed in 14 healthy control subjects (nine female, five male; mean age, 34 years) on two occasions 2 weeks apart. All studies were acquired within 3 months. None of the subjects had a history of disease that could affect metabolite concentrations.
MR Methods
All imaging was performed by using a 3T machine (Horizon LX echo speed; GE Medical Systems, Milwaukee, WI). Proton spectra were acquired by using pulse sequence of point-resolved spectroscopy with two chemical shift selective pulses for water suppression. Bilateral single-voxel spectra were acquired from each temporal lobe and both frontal lobes. Acquisition parameters were as follows: tip angle, 90°; TR/TE, 3000/30; 2048 data points, spectral width, 5000 Hz, and voxel dimensions, 2 × 2 × 2 cm. Voxels were shimmed to maximum line widths of 12 and 8 Hz for the temporal and frontal lobe voxels, respectively.
Proton data were processed by using the program LCModel http://s-provencher.com/pages/lcmodel.shtml (20), which allows deconvolution of spectra by using a basis set of reference spectra acquired from individual metabolites on our machine. Spectra with a signal-to-noise ratio of 8 or less were not included in the analysis. Results were expressed as absolute concentrations approximating millimoles per liter, which allowed us to make comparisons across centers using the same acquisition parameters. However, the results are presented as institutional units to emphasize on-site calibration.
Absolute metabolite concentrations were obtained by using two methods: one based on a scaling factor determined with a calibration phantom and another based on scaling relative to the unsaturated water peak. The standard procedure was followed for both scaling strategies (20). Results of both methods are presented.
Phantom Studies
Machine variability over the time of the study was assessed by using a phantom containing a model solution of 50 mmol/L NAA in 100 mmol/L phosphate, pH 7.2 containing 1 g/l NaN3. The phantom was imaged seven times with similar interscan intervals.
Statistical Analysis
Physiologic variability was assessed on the basis of human data. Variability between first and second measurements was first calculated by subtracting the second measurement from the first one (relative measurement variability). The relative measurement variability demonstrated whether the first measurement was systematically different (larger or smaller) from the second one. However, it did not reflect the whole measurement variability, as positive and negative differences cancelled each other out. Therefore, a rectified average was calculated by subtracting the larger measurement from the smaller measurement (absolute variability). For this calculation, all differences were positive and therefore additive. The absolute variability indicated the magnitude of the measurement error.
Scanner variability was assessed on the basis of phantom data. Analysis was performed using the same steps as for physiologic variability, by determining relative and absolute variability. The absolute measurement variability was based on a rectified average and therefore not affected by the random distribution of the data. All results were expressed as absolute values in institutional units and as percentage values.
Both relative and absolute variability were expressed as percentage values, to allow a comparison of the two methods used for determination of the concentrations (calibration phantom and water scaling). The difference in absolute variability obtained with the two methods was compared using Student t tests.
Results
Phantom Studies
Seven short-term variability measurements were taken during the study interval. The mean value obtained at the first measurement was 49.4 ± 1.7 mmol/L, and at the second measurement 50.0 ±1.6 mmol/L. The relative variability between the two phantom measurements was –0.6 ± 1.5, whereas the absolute variability was 1.11 ± 1.1. Expressed as a percentage value, the relative variability was –1.3% ± 3.0% (reflecting the systematic drift), and the absolute variability was 2.3% ± 2.3% (reflecting the magnitude of scanner variability).
Human Studies
Right-Left Differences.—
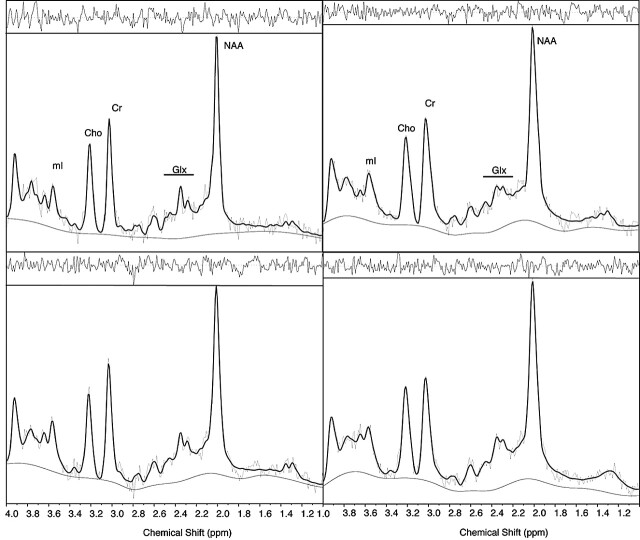
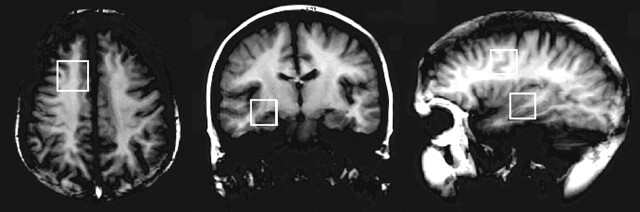
Each of the 14 subjects had two imaging sessions, yielding measurements of metabolites in each of the four voxels of interest (right and left temporal and right and left frontal lobe). Automatically selected receiver gain was either 29 or 30. The transmitter gain was 155.0 ± 7.6 (range, 144–174) for temporal lobe spectra and 153.6 (range 141–175) for frontal lobe spectra. Figure 1 shows examples of frontal and temporal lobe spectra recorded from the same volunteer at the two occasions. Figure 2 shows coronal and axial T1 images with the temporal and frontal lobe voxel positions marked, respectively.
Fig 1.
Frontal (left) and temporal (right) lobe spectra from a volunteer in the first (top) and second (bottom) sessions. Superimposed solid line corresponded to the LCModel fit, with the baseline shown below. Residual of the LCModel fit is inset above each spectrum. Peaks are labeled for NAA (NAA), creatine plus phosphocreatine (Cr), total trimethylamines (Cho), mI (mI), and glutamine and glutamate (Glx).
Fig 2.
Axial, coronal, and sagittal T1-weighted images show the location of voxels used for MRS acquisition.
Table 1 shows the results obtained by using the phantom calibration method. Only one measurement demonstrated a side-to-side difference. In the first session, but not in the second session, NAA+NAAG was higher in the left temporal lobe (8.3 ± 0.9 vs. 7.8 ± 1.0, P = .04). Table 1 also shows the results by using the water scaling method. Several measurements show a side-to-side difference: Frontal lobe NAA and NAA+NAAG were increased on the left in both the first and second sessions (P ≤ .03), whereas in the temporal lobe mI (P = .02), and glutamine and glutamate (Glx) (P = .007) showed a difference in the first session, and NAA (P = .04) did in the second session. The comparison between the two methods indicated that the values for all metabolites were slightly higher by using the water peak method. A relatively smaller variance was found for NAA and NAA+NAAG by using the water peak method; in contrast mI had a smaller SD by using the calibration method.
TABLE 1:
Control measurements
| Method, Session, and Metabolite | Temporal Lobe | Frontal Lobe | ||
|---|---|---|---|---|
| Right | Left | Right | Left | |
| Calibration phantom | ||||
| First session (n = 14) | ||||
| NAA | 5.5 ± 1.0 | 6.0 ± 0.9 | 5.6 ± 0.4 | 5.7 ± 0.6 |
| NAA+NAAG | 7.8 ± 1.0 | 8.3 ± 0.9 | 6.9 ± 0.6 | 6.9 ± 0.8 |
| Cr | 4.8 ± 0.9 | 4.5 ± 0.6 | 4.0 ± 0.4 | 4.0 ± 0.3 |
| mI | 3.3 ± 0.7 | 3.2 ± 0.6 | 2.7 ± 0.3 | 2.8 ± 0.5 |
| Cho | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.1 |
| Glx | 8.1 ± 1.6 | 7.6 ± 1.7 | 7.8 ± 0.8 | 7.6 ± 0.8 |
| Second session (n = 14) | ||||
| NAA | 6.0 ± 1.0 | 5.8 ± 0.8 | 5.5 ± 0.5 | 5.7 ± 0.4 |
| NAA+NAAG | 7.5 ± 0.9 | 7.7 ± 1.0 | 6.9 ± 0.5 | 7.2 ± 0.7 |
| Cr | 4.5 ± 0.6 | 4.6 ± 0.5 | 3.9 ± 0.2 | 4.1 ± 0.3 |
| mI | 3.4 ± 0.6 | 3.1 ± 0.4 | 2.6 ± 0.4 | 2.7 ± 0.5 |
| Cho | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| Glx | 7.6 ± 1.6 | 7.9 ± 1.9 | 7.0 ± 0.8 | 7.5 ± 1.0 |
| Water peak intensity | ||||
| First session (n = 14) | ||||
| NAA | 6.5 ± 0.8 | 6.7 ± 0.7 | 6.8 ± 0.5 | 7.3 ± 0.5 |
| NAA+NAAG | 8.9 ± 0.9 | 9.2 ± 0.6 | 8.2 ± 0.4 | 8.8 ± 0.7 |
| Cr | 6.0 ± 0.8 | 5.7 ± 0.7 | 5.1 ± 0.5 | 5.3 ± 0.4 |
| mI | 4.9 ± 1.3 | 4.4 ± 1.1 | 3.6 ± 0.3 | 3.8 ± 0.8 |
| Cho | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 |
| Glx | 9.8 ± 0.9 | 9.0 ± 1.3 | 9.5 ± 1.3 | 9.9 ± 1.0 |
| Second session (n = 14) | ||||
| NAA | 6.4 ± 0.8 | 6.8 ± 0.7 | 6.8 ± 0.5 | 7.2 ± 0.3 |
| NAA+NAAG | 8.5 ± 0.9 | 8.8 ± 0.9 | 8.4 ± 0.6 | 8.9 ± 0.6 |
| Cr | 6.0 ± 0.8 | 5.7 ± 0.7 | 5.1 ± 0.5 | 5.3 ± 0.4 |
| mI | 4.7 ± 1.2 | 4.0 ± 0.6 | 3.6 ± 0.6 | 3.7 ± 0.7 |
| Cho | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 |
| Glx | 9.9 ± 0.7 | 9.6 ± 1.0 | 9.4 ± 0.7 | 10 ± 0.8 |
Short-Term Variability
Imaging-to-imaging variability based on the scaling method is given in Table 2, and the corresponding results for the water peak scaling method is shown in Table 3. We observed no systematic difference between the initial and second imaging session for any metabolite in either the frontal or temporal lobes, as the relative variability was in some metabolites below and in others above zero. Overall, the variability appeared to be increased in the temporal lobe compared with the frontal lobe. With the phantom calibration method (Table 2), the absolute variability in the temporal lobe was between 10% and 20% for all metabolites except Glx, and it was larger than 20% for Glx. The absolute variability was slightly smaller for the frontal lobe measurements (6–18%). With the water scaling method (Table 3), the absolute variability in the metabolites measured in the temporal lobe was between 9% and 28%; here, the largest variability was found for mI and not for Glx. The absolute variability appeared to be slightly lower for the frontal lobe metabolites (5–19%), with NAA, NAA+NAAG and Cr demonstrating less than 10% variability between measurements. We noted no difference in the absolute variability between the two methods used.
TABLE 2:
Scan-to-scan variability for the calibration phantom method
| Lobe and Metabolite | Relative Variability |
Relative Variability (%) |
Absolute Variability |
Absolute Variability (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |
| Temporal lobe (n = 14) | ||||||||
| NAA | 0.2 ± 0.7 | −0.5 ± 1.0 | 7 ± 18 | −4 ± 13 | 0.9 ± 0.8 | 0.7 ± 0.3 | 15 ± 12 | 12 ± 6 |
| NAA+NAAG | 0.3 ± 1.2 | 0.5 ± 1.2 | −5 ± 17 | −9 ± 17 | 0.9 ± 0.8 | 1.1 ± 0.7 | 13 ± 12 | 16 ± 10 |
| Cr | 0.3 ± 1.0 | −0.0 ± 0.9 | −8 ± 27 | 0 ± 17 | 0.7 ± 0.8 | 0.6 ± 0.5 | 18 ± 22 | 13 ± 10 |
| mI | −0.1 ± 1.0 | 0.1 ± 0.5 | −0 ± 26 | −3 ± 17 | 0.7 ± 0.5 | 0.4 ± 0.2 | 22 ± 14 | 15 ± 9 |
| Cho | 0.0 ± 0.3 | 0.1 ± 0.2 | −5 ± 21 | −5 ± 12 | 0.3 ± 0.2 | 0.2 ± 0.1 | 18 ± 12 | 11 ± 6 |
| Glx | 0.5 ± 2.0 | −0.3 ± 3 | −10 ± 24 | −2.5 ± 39 | 1.4 ± 0.8 | 2 ± 2 | 20 ± 15 | 29 ± 25 |
| Frontal lobe (n = 14) | ||||||||
| NAA | 0.0 ± 0.6 | 0.0 ± 0.5 | 1 ± 10 | 0.5 ± 9 | 0.5 ± 0.3 | 0.4 ± 0.4 | 8 ± 4 | 6 ± 6 |
| NAA+NAAG | −0.3 ± 1.7 | −0.2 ± 0.7 | −4 ± 24 | 3 ± 10 | 0.8 ± 1.5 | 0.6 ± 0.4 | 12 ± 21 | 8 ± 6 |
| Cr | 0.1 ± 0.5 | −0.1 ± 0.3 | 7 ± 6 | −3 ± 13 | 0.4 ± 0.3 | 0.3 ± 0.2 | 11 ± 7 | 7 ± 6 |
| mI | 0.0 ± 0.5 | 0.0 ± 0.6 | −4 ± 23 | −5 ± 26 | 0.4 ± 0.4 | 0.4 ± 0.4 | 15 ± 18 | 18 ± 20 |
| Cho | 0.0 ± 0.2 | 0.0 ± 0.2 | −2.5 ± 15 | −3 ± 17 | 0.1 ± 0.1 | 0.1 ± 0.1 | 11 ± 11 | 12 ± 12 |
| Glx | 0.8 ± 1.0 | 0.0 ± 0.9 | −12 ± 17 | −1 ± 13 | 0.9 ± 0.9 | 0.6 ± 0.6 | 13 ± 16 | 9 ± 9 |
TABLE 3:
Scan-to-scan variability for the water peak intensity method
| Lobe and Metabolite | Relative Variability |
Relative Variability (%) |
Absolute Variability |
Absolute Variability (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | |
| Temporal lobe (n = 14) | ||||||||
| NAA | 0.1 ± 1.0 | −0.1 ± 0.7 | 2 ± 17 | −1 ± 11 | 0.8 ± 0.7 | 0.6 ± 0.3 | 12 ± 11 | 9 ± 5 |
| NAA+NAAG | 0.4 ± 1.0 | 0.5 ± 1.2 | 6 ± 12 | 7 ± 16 | 0.8 ± 0.7 | 0.8 ± 0.9 | 10 ± 9 | 10 ± 14 |
| Cr | 0.4 ± 0.9 | 0.2 ± 0.8 | 7 ± 18 | 4 ± 15 | 0.6 ± 0.8 | 0.7 ± 0.4 | 12 ± 15 | 12 ± 9 |
| mI | 0.0 ± 1.5 | 0.4 ± 0.9 | 7 ± 35 | 11 ± 25 | 1.2 ± 0.8 | 0.7 ± 0.7 | 28 ± 20 | 17 ± 21 |
| Cho | 0.1 ± 0.2 | 0.0 ± 0.2 | 9 ± 17 | 3 ± 12 | 0.5 ± 0.2 | 1.6 ± 1.5 | 15 ± 12 | 10 ± 7 |
| Glx | 0.1 ± 0.6 | −0.9 ± 2.0 | 2 ± 6 | −8 ± 19 | 0.5 ± 0.2 | 1.6 ± 1.5 | 5 ± 3 | 15 ± 13 |
| Frontal lobe (n = 14) | ||||||||
| NAA | 0.0 ± 0.5 | 0.1 ± 0.5 | 1 ± 7 | 2 ± 7 | 0.4 ± 0.3 | 0.4 ± 0.4 | 7 ± 5 | 5 ± 5 |
| NAA+NAAG | −0.3 ± 0.6 | −0.0 ± 0.9 | −3 ± 7 | 0 ± 10 | 0.5 ± 0.3 | 0.7 ± 0.6 | 6 ± 4 | 7 ± 7 |
| Cr | 0.0 ± 0.5 | −0.0 ± 0.5 | 1 ± 10 | −1 ± 9 | 0.4 ± 0.3 | 0.4 ± 0.3 | 8 ± 6 | 7 ± 6 |
| mI | 0.2 ± 1.2 | 0.2 ± 0.8 | −1 ± 17 | 6 ± 21 | 0.7 ± 1.0 | 0.7 ± 0.4 | 13 ± 10 | 19 ± 10 |
| Cho | 0.0 ± 0.5 | 0.0 ± 0.2 | 1 ± 15 | 4 ± 17 | 0.2 ± 0.1 | 0.2 ± 0.1 | 12 ± 9 | 12 ± 12 |
| Glx | 0.7 ± 1.0 | −1.0 ± 0.8 | 8 ± 12 | −9 ± 8 | 0.9 ± 0.8 | 1.0 ± 0.8 | 10 ± 9 | 9 ± 8 |
Discussion
In this study, we addressed the reliability of repeat measurements by examining short-term variability of temporal lobe and frontal lobe metabolite concentrations based on short-echo, single-voxel proton MRS at 3T. Two different methods for obtaining absolute concentrations with LCModel were compared. Biologic factors contributed to most of the variability between measurements, with machine factors being less important. Frontal lobe measurements were more precise compared with the temporal lobe, for all metabolites. We found no overall benefit in using either the phantom calibration or the water peak scaling method. However, the phantom calibration method gave slightly more precise measurements for mI, whereas the water peak method was slightly more precise for NAA and NAA+NAAG. These differences were not significant, but they may be of importance in the interpretation of individual results.
The choice of method may also be important for studies of specific metabolites. The strategy using a calibration phantom relies on consistent homogeneity of the magnetic field throughout the coil volume to provide a consistent flip angle in all regions of the sample. Homogeneity is likely to vary more with higher magnetic field strengths. The water scaling strategy compares the unsuppressed water signal intensity in the basis set and the sample with an assumed tissue water concentration. There is potential error with this method arising from the possibility that the water relaxation time changes due to changes in compartmentation with disease or with altered tissue composition.
Most of the previous studies investigating MRS variability were performed by using 1.5T machines, with about half by using short-TE methods. The sample size in most of the studies is similar to the number of subjects included in the current dataset. In contrast to all but one report, the current study assessed two different single voxels and compared the difference between them. Bartha et al (14) assessed a cortical and a subcortical voxel, whereas the present study focused on frontal and temporal lobes, two areas commonly investigated in epilepsy patients. In our study, variability was lower for the frontal lobe compared with the temporal lobe. One explanation for this observation is the effect of greater magnetic susceptibility in reducing spectral quality in spectra recorded from the temporal lobe relative to those recorded from the frontal lobe (9). Frontal lobe spectra also had a line width consistently narrower than those of the temporal lobe (9); this made the fit more robust.
The presentation of the metabolites and methods of variability assessment vary greatly between studies. It is therefore not easy to compare the various findings. The reported variability of repeated total NAA measurements obtained using the same scanner ranges from 5% to 20%, with several studies describing variability of around 15% (10, 11, 16, 17). Few studies examined the effects of the magnetic field strength on the MRS measurements. In a single subject who was imaged at 1.5 and 4T, the signal-to-noise ratio increased up to 80% with the higher field strength. The resulting increase in precision was approximately 40% (14), whereas the quantified metabolite concentrations were not different in relation to the field strengths (14). Another group comparing spectra recorded from phantoms and five subjects at field strengths of both 1.5 and 3T found that the spectra from certain phantoms displayed a significantly improved resolution at 3T compared with 1.5T (21). However, in human subjects, only short-TE (20 ms) spectra and not long-TE (272 ms) spectra showed a moderate improvement in sensitivity (20%) at 3T compared with 1.5T (21). These findings of less-than-expected improvements were explained by an increased line width at higher field strength, but they may also be related to the increased susceptibility and changes in relaxation times associated with higher magnetic field strength. The change in T2 with increasing magnetic field strength is small, with most investigators concluding that there is no significant change or a small reduction in T2 relaxation time with increasing magnetic field strength (22). However, the literature generally agrees that an increase in T1 relaxation time accompanies increasing magnetic field strength (23).
Footnotes
Supported by the Brain Imaging Research Foundation, the National Health and Medical Research Council of Australia, and Neuroscience Victoria.
References
- 1.Kuzniecky R. Magnetic resonance spectroscopy in focal epilepsy: 31P and 1H spectroscopy. Rev Neurol (Paris) 1999;155:495–498 [PubMed] [Google Scholar]
- 2.Kuzniecky R, Hugg JW, Hetherington H. Relative utility of 1H spectroscopic imaging and hippocampal volumetry in the lateralization of mesial temporal lobe epilepsy. Neurology 1998;51:66–71 [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi S, Kubota F, Akata T, et al. A study of the relationship between the seizure focus and 1H-MRS in temporal lobe epilepsy and frontal lobe epilepsy. Psychiatry Clin Neurosci 2000;54:455–459 [DOI] [PubMed] [Google Scholar]
- 4.Li LM, Cendes F, Antel SB, et al. Prognostic value of proton magnetic resonance spectroscopic imaging for surgical outcome in patients with intractable temporal lobe epilepsy and bilateral hippocampal atrophy. Ann Neurol 2000;47:195–200 [PubMed] [Google Scholar]
- 5.Capizzano AA, Vermathen P, Laxer KD, et al. Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. AJNR Am J Neuroradiol 2002;23:1359–1368 [PMC free article] [PubMed] [Google Scholar]
- 6.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993;13:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonaka M, Kohmura E, Yamashita T, et al. Kainic acid-induced seizure upregulates Na(+)/myo-inositol cotransporter mRNA in rat brain. Brain Res Mol Brain Res 1999;70:179–186 [DOI] [PubMed] [Google Scholar]
- 8.Danielsen ER, Ross B. Magnetic Resonance Spectroscopy of Neurological Diseases. New York: Marcel Dekker;1999
- 9.Wellard RM, Briellmann RS, Prichard JW, Syngeniotis A, Jackson GD. Myoinositol abnormalities in temporal lobe epilepsy. Epilepsia 2003;44:815–821 [DOI] [PubMed] [Google Scholar]
- 10.Marshall I, Wardlaw J, Cannon J, Slattery J, Sellar RJ. Reproducibility of metabolite peak areas in 1H MRS of brain. Magn Reson Imaging 1996;14:281–292 [DOI] [PubMed] [Google Scholar]
- 11.Bertolino A, Callicott JH, Nawroz S, et al. Reproducibility of proton magnetic resonance spectroscopic imaging in patients with schizophrenia. Neuropsychopharmacology 1998;18:1–9 [DOI] [PubMed] [Google Scholar]
- 12.Brooks WM, Friedman SD, Stidley CA. Reproducibility of 1H-MRS in vivo. Magn Reson Med 1999;41:193–197 [DOI] [PubMed] [Google Scholar]
- 13.Hoshino Y, Yoshikawa K, Inoue Y, et al. Reproducibility of short echo time proton magnetic resonance spectroscopy using point-resolved spatially localized spectroscopy sequence in normal human brains. Radiat Med 1999;17:115–120 [PubMed] [Google Scholar]
- 14.Bartha R, Drost DJ, Menon RS, Williamson PC. Comparison of the quantification precision of human short echo time (1)H spectroscopy at 1.5 and 4.0 Tesla. Magn Reson Med 2000;44:185–192 [DOI] [PubMed] [Google Scholar]
- 15.Maton B, Londono A, Sawrie S, Knowlton R, denHollander J, Kuzniecky R. Reproducibility of proton magnetic resonance spectroscopy imaging measurements of normal human hippocampus at 1.5 T: clinical implications. J Neuroimaging 2001;11:194–201 [DOI] [PubMed] [Google Scholar]
- 16.Hsu YY, Chen MC, Lim KE, Chang C. Reproducibility of hippocampal single-voxel proton MR spectroscopy and chemical shift imaging. AJR Am J Roentgenol 2001;176:529–536 [DOI] [PubMed] [Google Scholar]
- 17.Li BS, Babb JS, Soher BJ, Maudsley AA, Gonen O. Reproducibility of 3D proton spectroscopy in the human brain. Magn Reson Med 2002;47:439–446 [DOI] [PubMed] [Google Scholar]
- 18.Binesh N, Yue K, Fairbanks L, Thomas MA. Reproducibility of localized 2D correlated MR spectroscopy. Magn Reson Med 2002;48:942–948 [DOI] [PubMed] [Google Scholar]
- 19.Chard DT, McLean MA, Parker GJ, MacManus DG, Miller DH. Reproducibility of in vivo metabolite quantification with proton magnetic resonance spectroscopic imaging. J Magn Reson Imaging 2002;15:219–225 [DOI] [PubMed] [Google Scholar]
- 20.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679 [DOI] [PubMed] [Google Scholar]
- 21.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med 2001;45:765–769 [DOI] [PubMed] [Google Scholar]
- 22.de Graaf R, ed. In Vivo NMR Spectroscopy. New York: John Wiley & Sons,1998. :95
- 23.Wansapura JP, Holland SK, Dunn RS, Ball WS Jr. NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging 1999;9:531–538 [DOI] [PubMed] [Google Scholar]