Abstract
Summary: Neuro-Behçet disease is one of the clinical forms of Behçet disease. We report a case of neuro-Behçet disease mimicked a brain tumor. This case was initially considered as a brain tumor from mass lesion with edema at left basal ganglia on radiologic images. The lesion, however, was not neoplasia by pathologic diagnosis. By using steroid therapy, the size had been markedly shrunken on the follow-up images. The clinical symptoms were also improved.
Behçet disease is an idiopathic inflammatory disease that is characterized mainly by skin lesions such as genital ulcers, aphthous stomatitis, and ophthalmologic lesions such as uveritis. Approximately 10–20% of patients have a CNS complication called neuro-Behçet disease (1), and the main lesion of neuro-Behçet disease is often located at the ventral brain stem. Neuro-Behçet disease causes various neurologic symptoms; therefore, it is often difficult to distinguish from multiple sclerosis or brain tumor.
We report a case of neuro-Behçet disease whose onset included symptoms of increased intracranial pressure, which made it difficult to differentiate this case from a brain tumor.
Case Report
A 33-year-old right-handed man visited our hospital at the emergency room in the morning with nausea, vomiting, and headache. Family history was unremarkable. He had a history of Behçet disease and genital ulcers seven years prior, after which oral aphthos ulcers and erythema nodosum repeatedly appeared. At that time, he was diagnosed at another hospital as having Behçet disease but never received steroid therapy. The patient had no significant symptoms and, thus, had not sought medical attention until recently.
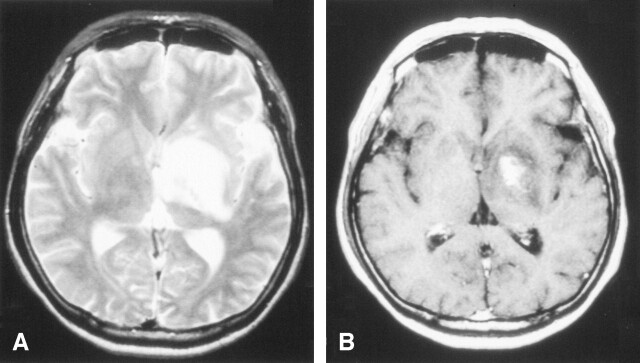
After admission, his consciousness gradually decreased. A head CT scan revealed a mass lesion at the left basal ganglia extending to the ventral site of the midbrain, causing compression to the surrounding tissue with cerebral edema. This lesion showed high intensity on the T2-weighted MR image, which exhibited a contrast effect of gadolinium (Fig 1A,-B).
Fig 1.
The diagnostic brain imaging on admission (obtained on January 3, 2002).
A, T2-weighted MR images (TR/TE, 4500/105) show a high-intensity area with a mild mass effect from the left basal ganglia to ventral site of midbrain.
B, Postcontrast T1-weighted images (TR/TE, 500/15). One part of the left basal ganglia shows a enhancement lesion.
General physical examination, including the skin and mucosa, was normal at this time, but needle prick reaction was positive. On hospital day 1, his consciousness was somnolent, but he responded to verbal stimuli. Papilloedema was negative, but visual acuity of the right eye was disturbed by a cataract complication. In the motor system, mild right hemiparesis was noted.
The laboratory data—which showed white blood cell count at 9100/mm3, C-reactive protein (CRP) at 2.95 mg/dL, and erythrocyte sedimentation rate of 85 mm/h, 118 mm/2 hours—indicated an increased inflammatory reaction. The serologic tests for syphilis, antinuclear antibody, anti-DNA antibody, and human leukocyte antigen-B51 (HLA-B51) were negative. The immune and complement systems were normal. The angiotensin-converting enzyme (ACE) level was within normal limits. CSF showed a mild pleocytosis of 26 mm3 (all mononuclear cells) and normal levels of protein, sugar, chlorine, and myelin basic protein, and oligoclonal bands were negative. Neither atypical cells nor bacteria were found cytologically in the CSF.
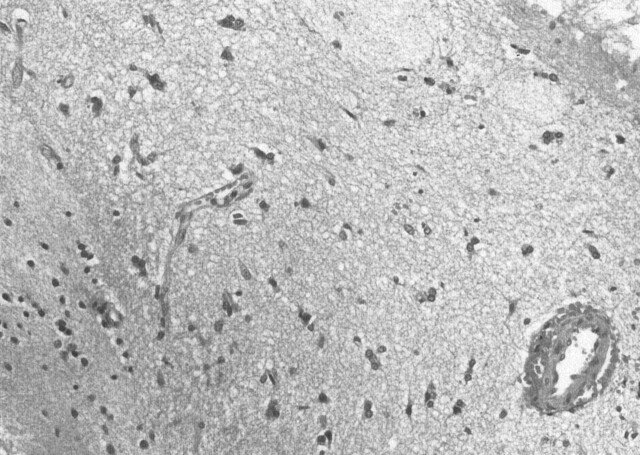
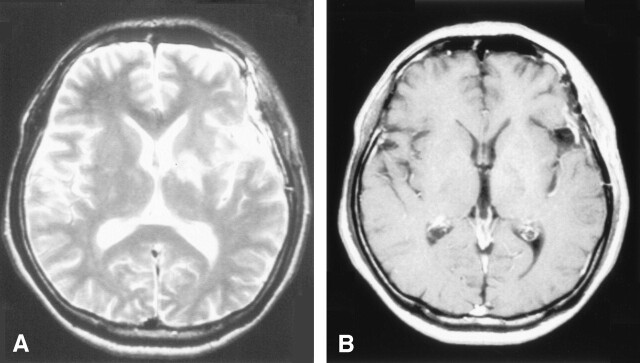
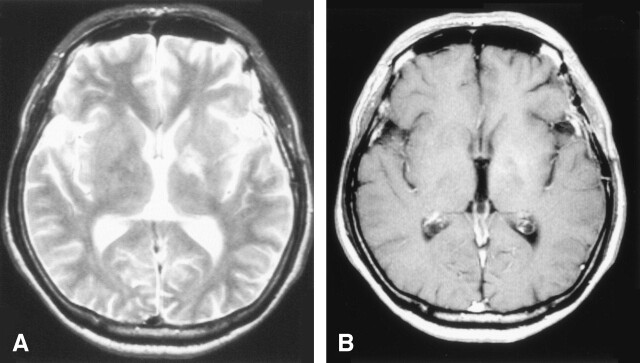
Under the diagnosis of brain tumor, glycerol was administered to prevent the progression of cerebral edema, after which the neurologic symptoms promptly improved. Cerebral angiography showed neither tumor stains nor other vascular abnormalities. To establish the final diagnosis, a craniotomic biopsy was performed on hospital day 14. The histopathologic findings excluded neoplastic lesions, showing only mild reactive gliosis without inflammatory cell infiltration (Fig 2). On hospital day 23, the patient was treated with high-dose methylprednisolone (m-PSL), a 3-day intravenous infusion of m-PSL with dose of 1000 mg/day, followed by an oral administration of prednisolone with dose of 30 mg/day. As the result, a remarkable improvement of the neurologic symptoms was achieved. Also the shrinkage of the cerebral lesion was noted on the MR images (Figs 3, 4).
Fig 2.
Histopathologic findings of biopsy specimen. It showed mild gliosis and neither neoplastic changes nor inflammatory cells infiltration. (H&E stain, original magnification × 20)
Fig 3.
The follow-up MR images (first time) at 7 days after the start of steroid therapy. The lesion had a reduction in size after the administration of steroids.
A, T2-weighted MR images
B, Postcontrast T1-weighted images
Fig 4.
The follow-up MR images (second time) at about a month after the start of steroid therapy. The lesion had a more reduction in size compared with previous images.
A, T2-weighted MR images
B, Postcontrast T1-weighted images
Discussion
In the past, this patient presented with the four main symptoms of Behçet disease, namely, mucocutaneous lesions (genital ulcers, oral mucosal recurrent aphthous ulcers, and erythema nodosum) and cataract complications (in the absence of uveritis and iridocyclitis). The patient was negative for HLA-B51, leukocytosis, increased serum CRP, an accelerated erythrocyte sedimentation rate, but mild pleocytosis of the CSF. He had a positive pinprick sensation test. Therefore, this patient presented the complete form of Behçet disease from his total clinical course, according to the criteria for clinical diagnosis of Behçet disease (2).
It has been reported that the appearance of the neurologic symptoms take several years and sometimes more than 10 years after the onset of the initial symptoms of Behçet disease (3). In this patient, nearly 7 years had passed after the initial onset of Behçet disease before the appearance of neurologic symptoms. This long clinical course made it difficult to judge whether these neurologic symptoms were related to Behçet disease, because he did not show any typical symptom of Behçet disease at the time.
Initially, a brain tumor was strongly suspected in this patient, because clinical onset with local neurologic symptoms was associated with increased intracranial pressure and the imaging demonstration of a space-occupying lesion involving mainly the left basal ganglia and reaching into the midbrain; however, cerebral angiography failed to demonstrate any vascular abnormalities.
The other possible differential diagnoses of this lesion included malignant lymphoma, multiple sclerosis, and sarcoidosis. Contrary to a report that the predominant site for lesions of multiple sclerosis is the dorsal brain stem (tegmentum) (2), the ventral site was involved in this patient. Myelin basic protein was within normal limits, and oligoclonal bands were negative in the CSF. Multiple sclerosis was excluded from these findings. The absence of typical skin lesions and failure to detect an increased level of ACE ruled out sarcoidosis. Thus, neuro-Behçet disease and brain tumors including malignant lymphoma remained in the differential diagnosis, ultimately requiring histopathologic diagnosis. Therefore, we performed a craniotomic biopsy and histologically excluded brain tumor.
It has been reported that steroid administration is effective for the treatment of neuro-Behçet disease (4). Also, in this patient, steroid therapy after biopsy improved the clinical symptoms and shrank the lesion on MR imaging, thus supporting the clinical diagnosis. If we had started steroid therapy before pathologic diagnosis, the differential diagnosis from malignant lymphoma would have been more difficult, because malignant lymphoma also has a response to steroid therapy.
The brain stem has been the most common site for neuro-Behçet disease (5), followed by the cerebral basal ganglia. There is high risk in proceeding with a craniotomic biopsy in cases with a main lesion located in the brain stem, so this method is rarely used and reports of neuro-Behçet disease with a pathologic diagnosis are few. It is said that histopathologic findings in autopsy cases of neuro-Behçet disease are characterized by perivascular infiltration of leukocytes and microglia, degeneration of oligodendroglia, diapedetic bleeding, and perivascular softening or necrosis (6, 7).
Several similar cases of neuro-Behçet disease (7–11) diagnosed on the basis of the appearance of cutaneous lesions and other characteristic clinical symptoms have been reported, as well as the effectiveness of steroid therapy. Craniotomic biopsies were performed in only two cases, one reported by Yoshimura et al (7), and the other by Geny et al (9). In the case reported by Yoshimura et al, the histopathologic findings showed necrosis with macrophage infiltration and a small amount of glial and lymphocytic cells, but no perivascular inflammation. In the other case reported by Geny et al, the histopathologic findings showed mild perivascular inflammation without necrosis. The typical case of neuro-Behçet disease usually demonstrates multifocal necrotizing lesions with inflammatory cell reaction but without specific vascular changes (12). The difference in the pathologic findings of neuro-Behçet disease seems to depend on the stage or degree of the inflammation.
In the histopathologic findings of this case, only mild reactive gliosis was observed without an inflammatory cell infiltration and no neoplastic change. These might not be specific findings of neuro-Behçet disease, although, in general, it did not conflict with the previous reports of histologic findings. The specimen of brain tissue obtained by biopsy was too limited to cover the whole spectrum of typical histologic findings. Thus, it might be difficult to diagnose neuro-Behçet disease by pathologic findings alone. But at least we were able to rule out a brain tumor (including malignant lymphoma) and concluded that it was consistent with a neuro-Behçet disease because of typical symptoms in total clinical course and a marked response to steroid therapy.
Conclusion
The craniotomic biopsy was useful to differentiate the mass lesion associated with neuro-Behçet disease from a brain tumor. We should keep such a case of neuro-Behçet disease in our mind when we find intracranial mass lesion on radiologic images and carefully diagnose.
Acknowledgments
We thank Tadashi Shigematsu, Akihiko Hino, Masahito Fujimoto, Suzuko Moritani, Masamichi Bamba (Saiseikai Shiga Hospital), Kenji Yoshikawa, Kyoko Itoh (Kyoto Prefectural University of Medicine) and Satoru Mori (Matsushita Memorial Hospital), for their help with this manuscript.
References
- 1.Cavara V, D’Ermo F. A case of neuro-Behçet’s syndrome. Acta XVIII Concillum Ophthal 1954;3:1489–1505 [Google Scholar]
- 2.Motomura S, Tabira T, Kuroiwa Y. A clinical comparative study of multiple sclerosis and neuro-Behçet’s syndrome. J Neurol Neurosurg Psychiatry 1980;43:210–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simizu T, Ehrlich GE, Inaba G, et al. Behçet disease (Behçet syndrome). Semin Arthritis Rheum 1979;8:223–260 [DOI] [PubMed] [Google Scholar]
- 4.Inaba G. Behçet’s disease. In: Vinken PJ, et al eds. Handbook of Clinical Neurology. Amsterdam: Excerpta Medica;1989. :593–610
- 5.Kojima S, Fukutake T, Hirayama K, et al. Magnetic resonance imaging in neuro-Behçet’s disease. Clin Neurol 1987;27:458–464 [PubMed] [Google Scholar]
- 6.Hadfield MG, Aydin F, Lippman HR, et al. Neuro-Behçet’s Disease. Clin Neuropathol 1997;16:55–60 [PubMed] [Google Scholar]
- 7.Yoshimura J, Toyama M, Sekihara Y, et al. Neuro-Behçet’s disease mimicking a thalamic tumor. No Shinkei Geka 2001;29:527–531 [PubMed] [Google Scholar]
- 8.Kermode AG, Plant GT, MacManus DG, et al. Behçet’s disease with slowly enlarging midbrain mass on MRI: resolution following steriod therapy. Neurology 1989;39:1251–1252 [DOI] [PubMed] [Google Scholar]
- 9.Geny C, Cesaro P, Heran F, et al. Pseudotumoral neuro-Behçet’s disease. Surg Neurol 1993;39:374–376 [DOI] [PubMed] [Google Scholar]
- 10.Neudorfer M, Feiler OV, Geyer O, et al. Behçet’s disease presenting as a cerebral tumour. Neuroradiology 1993;35:145. [DOI] [PubMed] [Google Scholar]
- 11.Litvan I, Roig C, Rovira A., et al. Behçet’s syndrome masquerading as tumor. Neuroradiology 1987;29:103. [DOI] [PubMed] [Google Scholar]
- 12.Lindenberg R, Haymarker W. Tissue reactions in the grey matter of the central nervous system. In: Haymarker W, Adams RD, eds. Histology and Histopathlogy of the Nervous System. Springfield, IL: CC Thomas,1982. :1037–1228