Abstract
BACKGROUND AND PURPOSE: Perivascular (PV) spaces are known to distend and cause edema along the optic tract (OT) in pituitary-region tumors. Interstitial fluid may be retained in PV spaces when tumors block their drainage outlets to subarachnoid spaces. However, these spaces and their outlets have not been anatomically elucidated. Our purpose was to evaluate how often large PV spaces are present along the OT and demonstrate their superficial communication points to adjacent subarachnoid spaces.
METHODS: We examined serial histologic sections of 10 hemispheric blocks obtained from cadavers without cerebral abnormality.
RESULTS: Large PV spaces, 0.5–1.5 mm in maximum height, were always present along the middle portion of the OT. Perforation points of the largest spaces were noted at the medial sulcus of the OT in seven hemispheres and through the OT in three.
CONCLUSION: Large PV spaces are present along the middle portion of the OT. Their communication point to adjacent subarachnoid spaces was histologically demonstrated. The locations and variations of the outlet of large PV spaces explain the clinical features of edemas; these findings anatomically support the hypothesis that blockage of the outlets to subarachnoid spaces may play a role in distending the PV spaces and in causing edema in pituitary-region tumors. Only MR imaging has revealed this change; further pathologic investigations are awaited.
The advent of MR imaging has enabled detailed visualization of the perivascular (PV) spaces (called Virchow-Robin spaces) in the brain (1–6). Among the various MR images, heavily T2-weighted imaging can depict fine structures in and around the cerebrospinal fluid (CSF) space and are considered sufficiently sensitive for depicting the PV spaces, which are of CSF signal intensity (7).
Using the heavily T2-weighted pulse sequence, Saeki et al (8) highlighted the PV spaces along the OT in cases of pituitary region tumors from a new clinical viewpoint. Regarding tumors touching or compressing the optic pathway, the edema was visible in four of 25 pituitary adenomas, eight of 11 craniopharyngiomas (Fig 1), one germ cell tumor, and one malignant lymphoma. This observation showed that edema along the OT, which was previously thought to be specific for craniopharyngioma, is in fact seen in a range of pituitary region tumors. The change disappears or decreases after successful treatment. Comparison of the pretreatment and post-treatment MR images revealed that the change had been located at the normally present large PV spaces along the OT (Fig 1B and C) (8). This finding led the authors to propose that the edema is related to distension of normal PV spaces rather than regional inflammation from microscopic leakage of cyst contents.
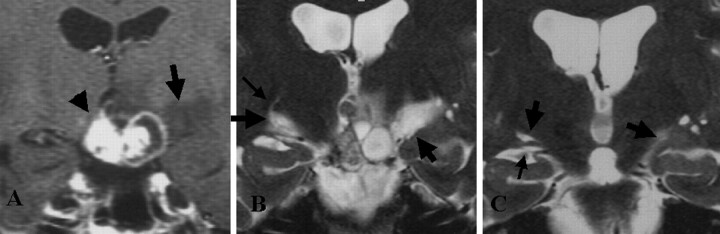
Fig 1.
Representative MR images show an edema along the OT in a 33-year-old woman with craniopharyngioma (reproduced from reference 8 with the permission of the ©American Society of Neuroradiology).
A, T1-weighted contrast-enhanced coronal MR image before treatment. High- and low-signal-intensity mass (arrowhead) is visible at the suprasellar cistern. Low-signal-intensity edema (arrow) is noted along the left OT.
B, Heavily T2-weighted coronal MR image before treatment shows bilateral edemas (thick arrows) along the OT. The left side is more prominent. The OTs are difficult to differentiate from the edemas. On the right side, a curvilinear area of high signal intensity (thin arrow) originates from the edema.
C, On this heavily T2-weighted coronal MR image obtained 3 months after surgery (similar level as in B), the edema disappears on the right side. A large PV space (left thick arrow), present in normal conditions, is visible along the right OT (thin arrow). Edema (right thick arrow) remains on the left side.
Previous animal experiments have revealed that extracellular or interstitial fluid leaves along the PV spaces, moving toward the subarachnoid space (9, 10). On the basis of such experiments, Saeki et al (8) hypothesized that the edema is due to the retention of interstitial fluid in PV spaces after tumors block their drainage outlets to the subarachnoid space. However, PV spaces along the OT and their outlets to the adjacent subarachnoid space have not been anatomically elucidated. This study was designed to provide background data about the formation mechanism of the edema along the OTs. Our purpose was to define the large PV spaces along the OT and to demonstrate the superficial perforation points of the vessels in these spaces (ie, communication routes between the subarachnoid space and the PV spaces along the OT).
Methods
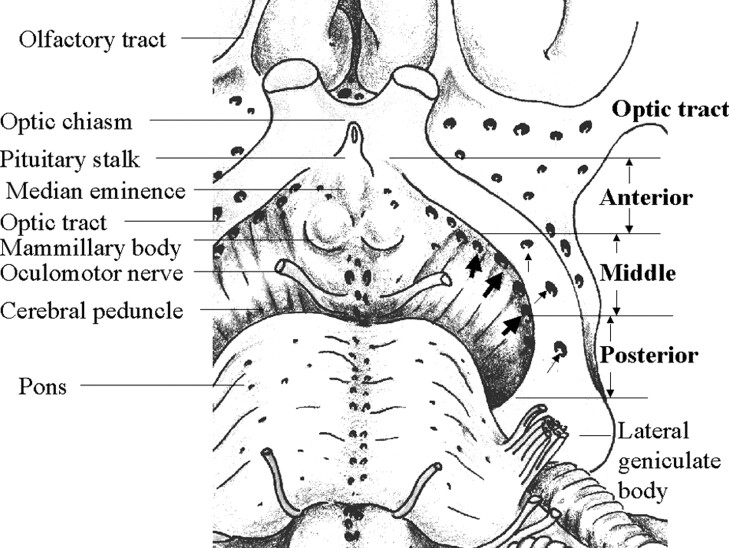
Ten hemispheres were obtained from five cadaveric brains from 72–86-year-old men and women who died from non-neurologic causes. The specimens were fixed in 60% ethanol solution for 4–6 months. In this study, the OT was anatomically defined as the optic pathway located between coronal sections, including the pituitary stalk and the anterior border of the lateral geniculate body (11, 12) After the whole length of the OT was exposed, the OT was divided and cut into three equal portions: anterior, middle, and posterior (Fig 2). Each portion was coronally cut into a 3–3.5-mm-long tissue block, which was embedded in paraffin. Sections 4 μm thick were stained by using hematoxylin-eosin and myelin staining. The sections were viewed by using low-power light microscopy at up to ×50 magnification.
Fig 2.
Schematic representation of the anatomy in an 85-year-old man shows the basal view of the OT and surrounding brain tissue in the left hemisphere. The whole length of the OT is exposed by removing the left temporal lobe. The tract is divided into three equal-sized portions: anterior, middle, and posterior. Numerous perforation points are present medial to (thick arrows) and through (thin arrows) the OT. These are mainly located in the middle and posterior portions of the OT.
On the basis of previous reports, the PV spaces were pathologically defined as spaces lined by a pial layer, including vessels, and surrounded by the brain parenchyma without ischemic or necrotic components (1–5). In addition, histologic similarities were compared between large PV spaces along the OT and around the anterior commissure where they had pathologically proved PV spaces.
Results
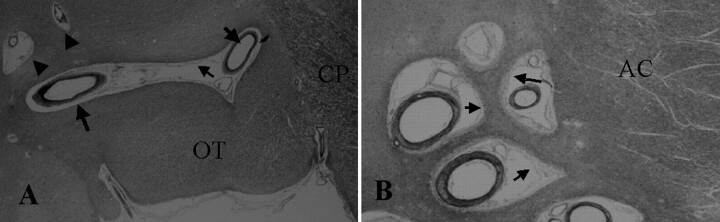
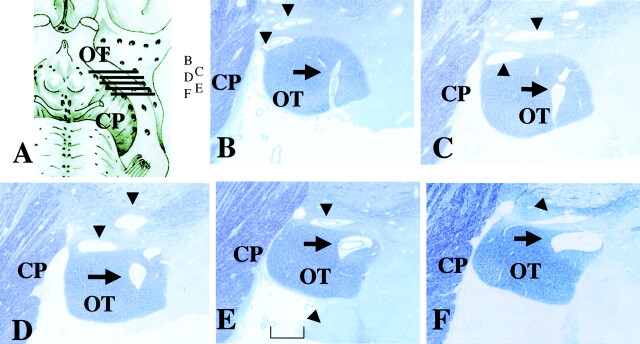
In all 10 hemispheres, several large and small PV spaces were always visible along the middle and posterior portions of the OT (Figs 3A, 4B–D, and 5B–F). Only a few were seen along the anterior portion of the OT. The largest space in each specimen was always present along the middle portion of the OT on the section including the mammillary body and the anterior portion of the cerebral peduncle (Figs 2, 4D, and 5F). It was horizontally long and narrow (Fig 4C) or oval (Fig 5F). The space was 0.5–1.5 mm in maximum height (Figs 3A, 4A and D, and 5A and F) and adjacent to or within the upper part of the OT (Figs 4C and 5F).
Fig 3.
Histologic samples obtained in a 79-year-old woman.
A, Coronal section of the middle portion of the OT shows the cerebral peduncle (CP) (hematoxylin-eosin stain, original magnification ×20) Along the OT, a large 1.5-mm-high space has a pial layer (thin arrow) that lines its inner surface. Vessels (thick arrows) are present in the space, and no necrotic or ischemic changes are visible in the surrounding brain tissue. The histologic features are compatible with those of PV spaces around the anterior commissure shown in B. Small spaces (arrowheads) are present adjacent to the OT.
B, Coronal section at the anterior commissure (AC) and the lower basal ganglia shows multiple PV spaces (hematoxylin-eosin stain, original magnification × 40). Adjacent to the anterior commissure are multiple PV spaces with vessels lined by a pial layer (arrows). No necrotic or ischemic changes are visible in the surrounding brain tissue.
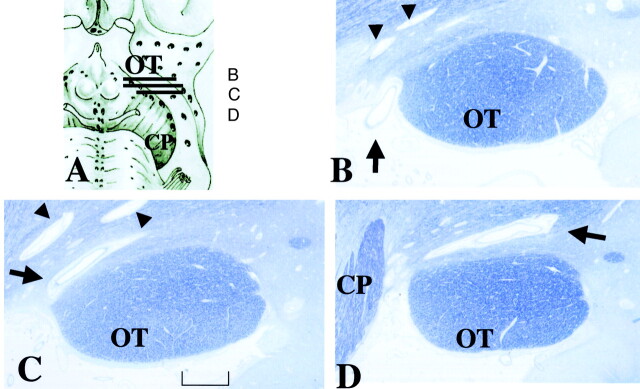
Fig 4.
Histologic samples obtained in an 86-year-old woman.
A, Section shows lines corresponding to sections in B–D.
B–D, Continuous thin sections show a penetrating vessel (arrow) from the perforation point at the subarachnoid space (B) to the largest PV space adjacent to the OT (C and D). Its maximum height is 0.5 mm. Sections show the perforation point medial to the OT, which corresponds to the large arrows in Figure 2. Small PV spaces (arrowheads) are present (myelin staining, original magnification ×25). CP indicates cerebral peduncle.
Fig 5.
Histologic samples obtained in an 81-year-old man. CP indicates cerebral peduncle.
A, Section shows lines corresponding to sections in B–F.
B–F, Continuous thin sections show a penetrating vessel from the perforation point in the subarachnoid space (B) to the largest PV space within the upper part of the OT (F). Its maximum height is 0.7 mm. Sections show the perforation point through the OT, which corresponds to the small arrows in Figure 2. A few small PV spaces (arrowheads) are visible along the OT (myelin staining, original magnification ×25). Bracket in E = 1 mm.
Large spaces along the OT included vessels and were lined with a pial layer (Fig 3A). The spaces along the OT and those around the anterior commissure had the same histologic features, as described in the previous reports (1–5) (Fig 3B). Therefore, the spaces along the OT were considered PV spaces (Figs 4 and 5).
The vessel passed the perforation point medial to the OT (the sulcus between the OT and tuber cinereum) in seven of 10 hemispheric blocks (Figs 2 and 4) and through the OT in the remaining three (Figs 2 and 5) before reaching the large PV space on the OT. Therefore, there were two variations in the communication point between the PV space along the OT and the adjacent subarachnoid space.
Discussion
Although our sample size was small, our histologic studies of 10 specimens showed the consistent presence of large PV spaces along the middle and posterior portions of the OT. The largest space in each specimen was present along the middle portion on the coronal section including mammillary body and anterior midbrain. The spaces possess histologic features compatible with those of spaces previously reported. Photographs from human anatomic atlases demonstrate similarly thin and small spaces in the same location on coronal brain sections but without correlation to their anatomic or clinical importance (13, 14). We believe that these large PV spaces along the OT are always present.
The present histologic studies also revealed that the outlets of the PV spaces along the OT to the adjacent subarachnoid space are present at two sites: medial to and through the OT.
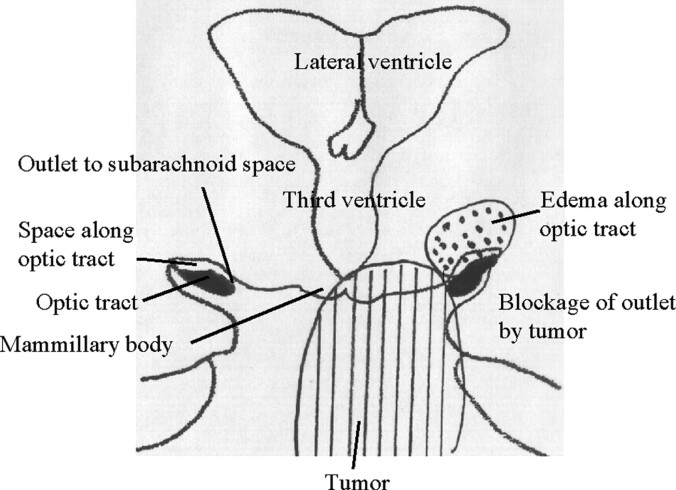
The present anatomic findings of possible drainage outlets of interstitial fluid from PV spaces along the OT may explain clinical features of the edema along the OT in pituitary region tumors (Fig 6). First, the location of the outlets to the subarachnoid space in the middle portion of the OT may explain why the edema is common in tumors in which the main mass is located behind the optic chiasm; examples of these include craniopharyngiomas, lymphomas, and germinomas (8). Tumors in the pituitary region commonly extend behind the optic chiasm and involve the posterior lobe system and the floor of the third ventricle (6, 8, 15). Second, the change is occasionally and remarkably unilateral and deviated to the side of tumoral extension (8) (Fig 1). This explanation is feasible because the outlets to the subarachnoid space are not on the midline; they are present bilaterally and off the midline. Third, the presence of at least two outlet points may explain why the change does not always occur in a larger tumor. To produce the edema, a tumor must be larger in a patient with an outlet point through the OT, rather than in a patient with the point medial to the OT. Likewise, even a small tumor may have accompanied edema when the points are located medially (8, 15). The distance between tumors and outlet points may play a role in how readily tumor causes PV spaces to be distended. Therefore, this present study has provided supportive anatomic evidence for dilated PV spaces along the OT as a formation mechanism of the edema along the OT in pituitary-region tumors (Fig 6).
Fig 6.
Schematic representation shows a possible new mechanism for edema along the OT in pituitary-region tumors. Right, In the normal anatomy, the PV space along the OT communicates with the adjacent subarachnoid space through a thin channel medial to the OT. Left, Tumor can block this channel with mechanical, inflammatory, or adhesive processes. The PV space retains interstitial fluid and distends along the OT (dotted area).
In addition to such anatomic considerations, other factors, such as the extent and degree of peritumoral edema, the growth speed, the invasiveness of malignant tumors, and tumor adhesion to the surrounding tissue, influence the edema formation (8, 15). Clinicoanatomic correlation between patients with tumors in the pituitary region and healthy subjects is important, because this pattern of edema has not been pathologically proved in humans. This change may be associated with a new pattern of brain edema in addition to vasogenic, cytotoxic, osmotic, hydrostatic, and interstitial edemas (16), or it may be an unrecognized variation of one of these mechanisms. Accordingly, the clinical application of this knowledge has great potential. Because this new type of brain edema has been revealed by using MR imaging, pathologic investigations are required. An MR imaging system of higher field strength may succeed in demonstrating the detailed pathologic anatomy of this edema.
Acknowledgments
We express sincere gratitude to Dr. Umeo Ito, one of the worldwide pioneering researchers on brain edema, for his indispensable advice in preparing this manuscript; to our colleagues in our department; and to Dr. Kenro Sunami and the other medical staff at Kawatetsu Chiba Hospital for their cooperation in collecting the clinical data.
References
- 1.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer W. Brain MR: Pathologic correlation with gross and histopathology, I: lacunar infarction and Virchow-Robin spaces. AJNR Am J Neuroradiol 1988;9:621–628 [DOI] [PubMed] [Google Scholar]
- 2.Elster AD, Richardson DN. Focal high signal on MR scans of the midbrain caused by enlarged perivascular spaces: MR-pathologic correlation. AJNR Am J Neuroradiol 1991;11:1119–1122 [DOI] [PubMed] [Google Scholar]
- 3.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol 1989;10:929–936 [PMC free article] [PubMed] [Google Scholar]
- 4.Jungreis CA, Kanal E, Hirsch WL, Martinez AJ, Moossy J. Normal perivascular spaces mimicking lacunar infarction: MR imaging. Radiology 1988;169:101–104 [DOI] [PubMed] [Google Scholar]
- 5.Song CJ, Kim JH, Kier EL, Bronen RA. MR imaging and histologic features of subinsular bright spots on T2-weighted MR images: Virchow-Robin spaces of the extreme capsule and insular cortex. Radiology 2000;214:671–677 [DOI] [PubMed] [Google Scholar]
- 6.Seeger JF. Normal variations of the skull and its contents. In: Zimmerman RA, Gibby WA, Carmody RF, eds. Neuroimaging. New York: Springer-Verlag;2000. :437
- 7.Mamata Y, Muro I, Matsumae M, et al. Magnetic resonance cisternography for visualization of intracisternal fine structures. J Neurosurg 1998;88:670–678 [DOI] [PubMed] [Google Scholar]
- 8.Saeki N, Uchino Y, Murai H, et al. MR study on edema-like change along the optic tract in patients with pituitary region tumors. AJNR Am J Neuroradiol 2003;24:336–342 [PMC free article] [PubMed] [Google Scholar]
- 9.Rennels M, Gregory TF, Blaumanis O, Fujimoto K, Grady PA. Evidence of a paravascular fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain to the subarachnoid space. Brain Res 1985;326:47–63 [DOI] [PubMed] [Google Scholar]
- 10.Weller RO, Kida S, Zhang E. Pathways of fluid drainage from the brain-morphological aspects and immunological significance in rat and man. Brain Pathol 1992;2:277–284 [DOI] [PubMed] [Google Scholar]
- 11.Tamraz JC, Outin-Tamraz C, Saban R. MR imaging anatomy of the optic pathways. Radiol Clin North Am 1999;37:1–36 [DOI] [PubMed] [Google Scholar]
- 12.Horton JC, Landau K, Maeder P, Hoyt WF. Magnetic resonance imaging of the human lateral geniculate body. Arch Neurol 1990;47:1201–1206 [DOI] [PubMed] [Google Scholar]
- 13.DeArmond S, Fusco MM, Dewey MM. Structure of the Human Brain. New York: Oxford Press;1976. :26
- 14.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego: Academic Press;1997. :160–216
- 15.Nagahata M, Hosoya T, Kayama T, Yamaguchi K. Edema along the optic tract: useful MR finding for the diagnosis of craniopharyngiomas. AJNR Am J Neuroradiol 1998;19:1753–1757 [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JD, Ironside JW. Raised intracranial pressure, edema and hydrocephalus. In: Graham DI, Lantos PL, eds. Greenfield’s Neuropathology. Vol 1. 6th ed. London: Arnold;1997. :157–195 [Google Scholar]