Abstract
Summary: To our knowledge, the utility of coronary covered stents in the treatment of atherosclerotic carotid artery stenosis has not been defined in the English-language literature. Covered stents may prevent microembolic complications in select atherosclerotic carotid lesions, as they exclude the atherosclerotic lesion from the circulation by pressing the plaque against the vessel wall. Our early clinical experience has shown that use of these stents can be a therapeutic option in select cases of atherosclerotic stenosis of the cervical internal carotid artery.
The aim of treating atherosclerotic stenoses of the internal carotid artery (ICA) is to prevent stroke. Carotid artery stenting with percutaneous transluminal angioplasty has progressively developed in the last decade, and acceptable rates of complications (particularly in patients at high surgical risk) have been reported along with good long-term results (1–4). The most serious complication of stent placement for ICA stenosis is ipsilateral neurologic events due to a fresh acute embolus from fragmentation of the atherosclerotic lesion during stent deployment. Current stent designs may trap larger fragments, but they may not efficiently prevent microemboli because their interstitial size is too large (4, 5). Furthermore, the introduction of distal protection devices has lowered the rate of periprocedural acute ischemic complications, even in high-risk patients (6). The goal of those protection devices is to prevent cerebral embolism due to stent deployment. However, embolic complications with these systems are also reported (5).
Coronary stent grafts covered by a polytetrafluoroethylene (PTFE) membrane were introduced into the market in 1997. Cardiologists have successfully used these stents in atherosclerotic lesions of the coronary arteries (7, 8). The treatment of pseudoaneurysms of the carotid artery with covered stents is also a new concept and has been described in the English-language literature (9, 10).
We report our experience with coronary covered stents in three patients with atherosclerotic stenosis of the ICA. This technique may reduce the risk of ipsilateral embolism due to plaque disruption and also the risk associated with the use of protective devices.
Case Reports
Three patients had atherosclerotic stenosis of the proximal ICA shortly past (at least 1.5 cm) the bifurcation. All lesions were significant (ie, > 70% stenosis) as determined by using the criteria of the North American Symptomatic Carotid Endarterectomy Trial (11). All patients and their close relatives provided written informed consent.
All of the procedures were performed with the patient under conscious sedation. The patients received heparin (70–100 IU/kg body weight) followed by continuous infusion to keep the activated clotting time above twice that of the basal value. Unilateral femoral access was secured in all patients to allow the placement of a 6F guide catheter (Envoy; Cordis, Miami Lakes, FL) to the ipsilateral common carotid artery. An angiogram was obtained in the anteroposterior and lateral views to document cerebral blood flow. Under digital roadmapping, the lesion was bypassed with a 0.014-inch guidewire (Choice Extrasupport; Boston Scientific, Miami, FL), and the tip of the guidewire was placed into the distal ICA. Next, a 4-mm-diameter, 19-mm-long coronary stent graft (Jostent; Jomed Implantate, Unterschleissheim, Germany) was deployed. The stent graft consisted of two coaxially aligned, stainless steel (surgical 316 L) components that encompass a microporous PTFE membrane in a sandwich-like configuration. The premounted stent graft was available in lengths of 9, 12, 16, 19, and 26 mm. The maximum available diameter was 5 mm, and the mounted profile was 1.6 mm (7).
The procedures were performed without cerebral protection or predilation. A 4-mm-diameter stent was used in all patients, because 5-mm covered stents were not available when the procedures were performed. The patients were discharged home with a regimen of clopidogrel (75 mg/day) for 3 months and ongoing aspirin (100 mg/day).
Case 1.—
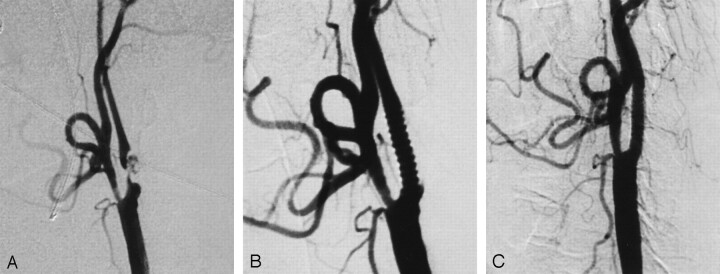
A 57-year-old, right-handed man presented with two transient ischemic attacks within 2 weeks. He had right-sided weakness, gait instability, facial asymmetry, and severe hypertension. Cranial MR examination revealed left-sided acute and subacute watershed infarctions. Digital subtraction angiography revealed 90% stenosis of the left ICA with intraplaque ulceration distal to the carotid bifurcation (Fig 1A). The stent graft was deployed across the stenotic segment of the left ICA. Final angiography showed an excellent morphologic result without residual stenosis (Fig 1B). No procedural complications occurred, and postprocedural diffusion-weighted MR imaging revealed no new lesions. The patient remained free of new neurologic signs 6 months after the intervention. Follow-up carotid angiography performed at 6 months showed a patent stent without stenosis (Fig 1C).
Fig 1.
Case 1.
A, Lateral left common carotid angiogram demonstrates severe stenosis with intraplaque ulceration in the left ICA.
B, Lateral left common carotid angiogram after treatment.
C, Follow-up angiogram obtained at 6 months shows a patent stent without stenosis.
Case 2.—
A 47-year-old man was referred for endovascular treatment of a left ICA stenosis, which was diagnosed by means of Doppler sonography. Cranial MR examination revealed a focal, acute ischemic lesion in the left occipital region. Selective carotid angiography demonstrated a 75% stenosis of the left ICA distal to the carotid bifurcation (Fig 2A). The stent graft was deployed across the stenotic segment of the left ICA (Fig 2B). Postprocedural diffusion-weighted MR examination did not demonstrate any new ischemic lesions. The patient had no procedural complication, and 3 weeks later, he underwent successful coronary artery bypass surgery. Follow-up Doppler examination at month 6 showed a patent stent without restenosis.
Fig 2.
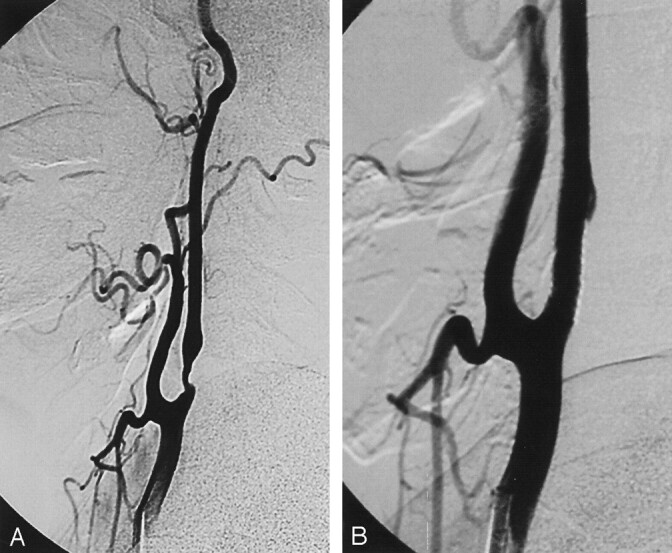
Case 2.
A, Lateral right common carotid angiogram demonstrates atherosclerotic stenosis of the left ICA distal to the bifurcation.
B, Lateral angiogram after stent placement.
Case 3.—
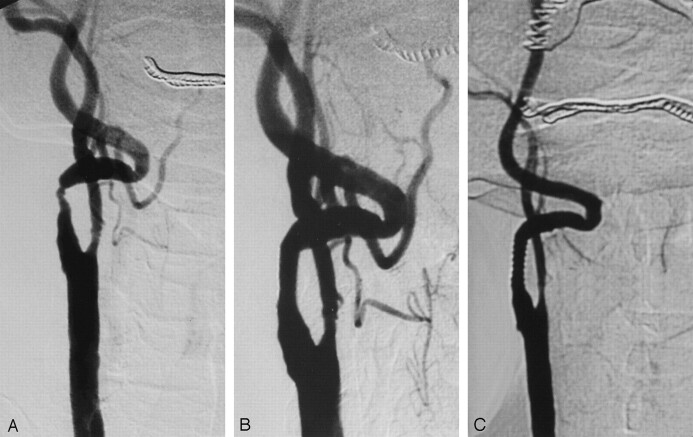
A 67-year-old, right-handed man who smoked was referred for right-sided weakness. He had several episodes of blurred vision that occurred alternately in both eyes. Diffusion-weighted MR examination revealed ischemic lesions in the right cerebellar hemisphere and in the left posterior parietal region. Carotid angiography revealed 75% stenosis of the right ICA distal to the carotid bifurcation, with severe tortuosity of the cervical segment of the right ICA and total occlusion of the left ICA (Fig 3A). The stent graft was deployed (Fig 3B) without procedural complications, and postprocedural diffusion-weighted MR imaging showed no new lesions. Six-month follow-up angiography showed a widely patent stent without restenosis (Fig 3C).
Fig 3.
Case 3.
A, Right common carotid angiogram shows severe stenosis and tortuosity of the right ICA.
B, Control angiogram after stent placement.
C, Follow-up angiogram obtained at 6 months demonstrates a patent stent without stenosis.
Discussion
The search for the ideal stent for atherosclerotic lesions of the carotid artery is ongoing. Theron et al (6) first described cerebral protection during carotid angioplasty and stent placement. Currently, three temporary cerebral protection systems are available: balloon occlusion, filtration baskets, and flow reversal in the ICA. Although these systems provide a considerable degree of protection, as shown in in vitro and preliminary clinical studies, distal embolization might still occur, even with protection (5, 12, 13). Histopathologic analysis of embolized debris collected in protective filters during carotid stent implantation show that the debris consists of material (featuring atheroembolism) displaced as a consequence of squeezing an atheromatous plaque and rupture occurring during stent placement (especially during stent postdilation) (14). Covered stents may prevent microembolic complications during their placement in select atherosclerotic carotid lesions, as these stents exclude the atherosclerotic lesion from the circulation by pressing the plaque against the vessel wall.
PTFE has been in use for a long time in surgical vascular prostheses. Long-term patency of up to 12 years has been reported (7, 15). Several reports have documented the safety and efficacy of stent grafts covered with PTFE or other materials in the treatment of coronary and carotid artery aneurysms, perforations, arteriovenous fistulas, and pseudoaneurysms (9, 10, 16–19). Sovik et al (8) reported their experience with the elective placement of covered stents in native coronary arteries of 50 patients with atherosclerotic lesions. Their restenosis rates were similar to those of bare stents at 6-month follow-up. In their study, a higher rate of subacute occlusion of stent grafts was also reported, and they advised continued treatment with Plavix to 3 months. Therefore, we also continued clopidogrel treatment for 3 months in our three patients.
Implantation of a covered stent to treat an atherosclerotic carotid stenosis is based on the idea that the stent graft may prevent the material of soft lesions and thrombus from embolizing to the distal circulation and that it may prevent the atherosclerotic plaque material from prolapsing between the stent struts into the lumen (8). Preprocedural and postprocedural diffusion-weighted MR images were obtained in all these three patients, and no new embolic lesions were detected after the procedure. Possible embolus release may occur during the passage of the covered stent through the stenosis. However, embolus release can also occur during the passage of a closed filter device through the stenosis (14). Covered stents have a crossing profile of 1.6 mm, which is slightly larger than currently available low-profile filter devices. Carotid stent placement with distal protection involving filters is a complex procedure requiring multiple exchange steps. The main advantages of covered stent implantation without distal protection, compared with stent placement with protection, are its simplicity and cost savings. Also, in patients with a severely tortuous cervical ICA that precludes the safe placement of a filter device without causing vasospasm (as in case 3), flexible covered stents may again be considered an option for endovascular treatment. Currently, the major limitation of covered stent placement to treat ICA stenosis is the maximally available diameter of the covered stents, which is 5.0 mm. Because of this limitation, we implanted covered stents in patients with small ICAs. Larger-diameter, monorail covered stents are required. Also, the use of covered stents is limited to lesions at least 1 cm distal to the carotid bifurcation to avoid covering the origin of the external carotid artery.
Conclusion
Our early clinical experience has shown that the use of coronary covered stents can be considered a therapeutic option for select cases of atherosclerotic cervical ICA stenosis. Prospective studies with longer follow-up are needed to determine the clinical effectiveness and long-term patency of covered stents in the carotid artery.
References
- 1.Stankovic G, Liistro F, Moshiri S, et al. Carotid artery stenting in the first 100 consecutive patients: results and follow up. Heart 2002;88:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357:1729–1737 [PubMed] [Google Scholar]
- 3.Dietz A, Berkefeld J, Theron JG, et al. Endovascular treatment of symptomatic carotid stenosis using stent placement: long-term follow-up of patients with a balanced surgical risk/benefit ratio. Stroke 2001;32:1855–1859 [DOI] [PubMed] [Google Scholar]
- 4.Phatouros CC, Higashida RT, Malek AM, et al. Carotid artery stent placement for atherosclerotic disease: rationale, technique, and current status. Radiology 2000;217:26–41 [DOI] [PubMed] [Google Scholar]
- 5.Theron J, Guimaraens L, Coskun O, Sola T, Martin JB, Rufenacht DA. Complications of carotid angioplasty and stenting. Neurosurg Focus 1998;5:1–19 [DOI] [PubMed] [Google Scholar]
- 6.Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis with protected balloon angioplasty and stent placement. Radiology 1996;201:627–636 [DOI] [PubMed] [Google Scholar]
- 7.Elsner M, Auch-Schwelk W, Britten M, Walter DH, Schachinger V, Zeiher AM. Coronary stents grafts covered by a polytetrafluoroethylene membrane. Am J Cardiol 1999;84:335–338 [DOI] [PubMed] [Google Scholar]
- 8.Sovik E, Klow NE, Brekke M, Stavnes S. Elective placement of covered stents in native coronary arteries. Acta Radiol. 2003;44:294–301 [DOI] [PubMed] [Google Scholar]
- 9.Scavee V, De Wispelaere JF, Mormont E, Coulier B, Trigaux JP, Schoevaerdts JC. Pseudoaneurysm of the internal carotid artery: treatment with a covered stent. Cardiovasc Intervent Radiol. 2001;24:283–285 [DOI] [PubMed] [Google Scholar]
- 10.Ellis PK, Kennedy PT, Barros AAB. Successful exclusion of a high internal carotid pseudoaneurysm using the wallgraft endoprosthesis. Cardiovasc Intervent Radiol 2002;25:68–69 [DOI] [PubMed] [Google Scholar]
- 11.North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke 1991;22:711–720 [DOI] [PubMed] [Google Scholar]
- 12.Terada T, Tsuura M, Matsumoto H, et al. Results of endovascular treatment of internal carotid artery stenoses with a newly developed balloon protection catheter. Neurosurgery 2003;53:617–623 [DOI] [PubMed] [Google Scholar]
- 13.Schlüter M, Tübler T, Mathey DG, Schofer J. Feasibility and efficacy of balloon-based neuroprotection during carotid artery stenting in a single-center setting. J Am Coll Cardiol 2002;40:890–895 [DOI] [PubMed] [Google Scholar]
- 14.Angelini A, Reimers B, Barbera MD, et al. Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke 2002;33:456–461 [DOI] [PubMed] [Google Scholar]
- 15.McLarty AJ, Philips MR, Holmes DRJ, Schaff HV. Aortocoronary bypass grafting with expanded polytetrafluoroethylene: 12-year patency. Ann Thorac Surg 1998;65:1442–1444 [DOI] [PubMed] [Google Scholar]
- 16.Elsner M, Zeiher AM. Perforation and rupture of coronary arteries [in German]. Herz 1998;23:311–318 [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee D, Roffi M, Bajzer C, Yadav JS. Endovascular treatment of carotid artery aneurysms with covered stents. Circulation 2001;104:2995. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay DW, McAuliffe W. Traumatic pseudoaneurysm and high flow arteriovenous fistula involving internal jugular vein and common carotid artery: treatment with covered stent and embolization. Australas Radiol 2003;47:177–180 [DOI] [PubMed] [Google Scholar]
- 19.Colombo A, Toh A, Di Mario C, et al. Successful closure of a coronary vessel rupture with a vein graft stent: case report. Cathet Cardiovasc Diagn 1996;38:172–174 [DOI] [PubMed] [Google Scholar]