Abstract
BACKGROUND AND PURPOSE: Intravascular treatment of intracranial aneurysms is a relatively new therapeutic technique and long-term controlled angiographic trials are needed to assess persistence of aneurysm occlusion. Our purpose was to evaluate the effectiveness of 3D time-of-flight (3D-TOF) MR angiography as a noninvasive screening tool in the follow-up of cerebral aneurysms treated with Guglielmi detachable coils (GDCs).
METHODS: Forty-nine patients with 50 intracranial aneurysms previously treated with GDCs were studied with both DSA and 3D-TOF MR angiography. In 14 cases, a second follow-up examination was performed, for a total of 64 aneurysms evaluated. In 25 aneurysms, both pre- and postcontrast MR angiographic studies were obtained.
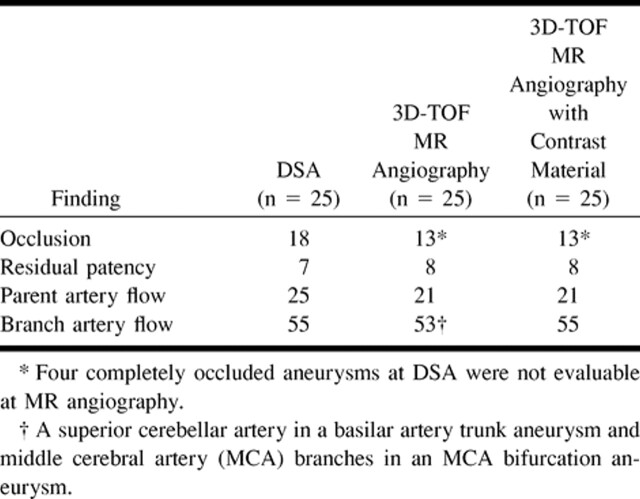
RESULTS: In seven of 64 aneurysms, the MR angiographic studies were considered to be unreliable owing to the presence of artifacts that obscured part of the parent artery and did not allow an accurate evaluation of the aneurysm neck. These seven aneurysms, however, all were shown to be completely occluded at digital subtraction angiography (DSA). In the remaining 57 aneurysms, DSA revealed complete occlusion in 39 and the presence of residual patency in 18, whereas MR angiography showed complete occlusion in 38 and residual patency in 19. Enhanced MR angiography proved to be useful in evaluating residual patency in large and giant aneurysms and in better depicting the distal branch arteries.
CONCLUSION: Although artifacts related to the presence of coils are evident on a considerable number of imaging studies, our findings indicate that MR angiography is useful in the evaluation of residual patency of cerebral aneurysms treated with GDCs and may eventually prove valuable in the follow-up of those cases in which a good initial correlation with DSA was demonstrated.
Since the first experimental and clinical studies on electrothrombosis of saccular aneurysms via the endovascular approach (1), treatment of cerebral aneurysms with Guglielmi detachable coils (GDCs) has become a widespread and routine technique in many countries. Data on the results of treatment are encouraging, especially in regard to ruptured aneurysms treated in the acute phase after subarachnoid hemorrhage; the rate of rebleeding in this group of patients is very low (2–4). Follow-up of treated aneurysms is usually performed by digital subtraction angiography (DSA). A DSA study each year for a period of approximately 3 years is empirically recommended. In cases of recanalization, retreatment is considered an option, often in the same setting. Nevertheless, DSA is an invasive and expensive procedure and, in some institutions, requires hospitalization.
Many investigators have reported on the sensitivity of MR angiography in the detection of both ruptured and unruptured cerebral aneurysms (5, 6). The high resolution of the technique, its sensitivity to flow, and its noninvasive nature make MR angiography a potentially useful tool in the identification of residual patency and recanalization of cerebral aneurysms treated with GDCs. Recently, Hartman et al (7) verified the presence of MR artifacts together with heat production and ferromagnetism of GDCs in both in vitro and in vivo studies and concluded that the coils produced minimal artifacts, with obscuration of approximately 2 to 3 mm of surrounding brain parenchyma. Derdeyn et al (8) reported promising results in their study of the clinical application of MR angiography in the follow-up of GDC-treated aneurysms; however, not all their MR angiographic examinations had DSA confirmation and the interval between MR angiography and DSA may have been, at least in some patients, long enough to allow for changes in the results.
The aim of the present study was to verify the clinical application of MR angiography in the follow-up of cerebral aneurysms treated with GDCs and, secondarily, to ascertain in a subgroup of the same patients whether the addition of contrast material may be helpful in identifying the presence of residual slow flow within the aneurysm.
Methods
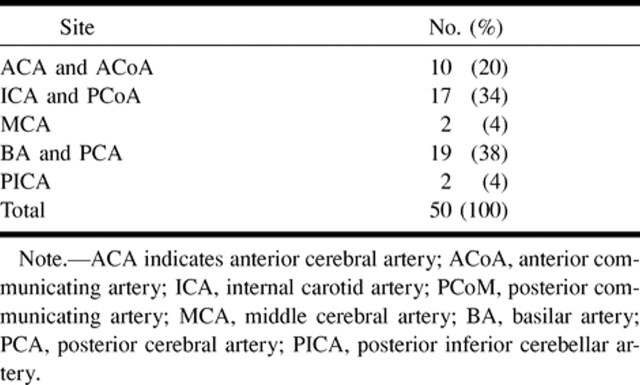
Forty-nine patients (15 men and 34 women; mean age, 53 years) harboring a total of 50 cerebral aneurysms previously treated with GDCs were entered into the study. The size and location of the aneurysms before treatment are listed in Tables 1 and 2. DSA and MR angiography were performed within 2 days of each other in all patients, and most of the MR angiograms were obtained the day before or the morning after DSA. The interval between treatment and DSA control studies ranged from 0 days (in 10 cases, the angiographic study was performed at the end of endovascular treatment) to 3 years, with a median follow-up period of 10 months. In 14 patients, a second follow-up examination was performed 1 year after the first one. In this group also, the interval between DSA and MR angiography was not greater than 2 days. Altogether, then, 64 studies were available for evaluation.
TABLE 1:
Size of the aneurysms
DSA was performed via the femoral artery and selective catheterization of the internal carotid or vertebral arteries. A Toshiba unit (Toshiba Corp., Tokyo, Japan) with a matrix of 1024 × 1024 was used for the first 54 cases and a GE Advant-X unit (General Electric Medical Systems, Milwaukee, WI) with a matrix of 1024 × 1024 was used for the last 10 cases. MR angiographic examinations were performed with a Siemens Magnetom SP 63 1.5-T unit (Siemens, Erlangen, Germany) in 45 cases and with a Siemens Vision 1.5-T unit in 19 cases. The 3D-TOF technique was performed with parameters of 43–39/8.0–6.2/1 (TR/TE/excitations) with a TONE (tilted, optimized, nonsaturating excitation) sequence, a flip angle of 20°, and magnetization transfer to acquire an axial volume centered at the known level of the treated aneurysm. The field of view (FOV) was 200 × 230 in the first 45 cases and 150 × 200 in the second group, with a matrix size of 230 × 512 and 192 × 512, respectively. The section thickness was 0.9 in both studies. The effective voxel size was 0.86 × 0.44 × 0.9 and 0.78 × 0.39 × 0.9, respectively. A presaturation slab was used in two cases that exhibited high signal within the treated aneurysm to distinguish between residual flow and methemoglobin. The saturation slab was not applied when the high signal was located at the base of the aneurysm. A second 3D-TOF MR angiographic study was obtained after injection of contrast material (0.1 mmol/kg gadopentetate dimeglumine) in 24 nonselected patients (25 aneurysms). Seventeen cases were studied with 3D-TOF sequences (nine cases, parameters = 43/8/1; eight cases, parameters = 39/6.2/1). In three of the seven cases in which artifacts were seen in association with the coils, a new technique with a very short TR/TE was used (5.0–8.2/2.1/1; flip angle, 10°; FOV, 165 × 220; matrix, 192 × 512) to reduce the artifacts and to obtain better visualization of the vessels adjacent to the aneurysm.
The MR angiographic studies were evaluated after reconstruction of the single source images and application of the maximum intensity projection (MIP) reconstruction targeted on the vessel of interest, with lateral rotation obtained by using 8° increments from 180° to 270° or more when necessary. Both MIP reconstructions and single source images were obtained in all patients and used to evaluate the results.
DSA and MR angiographic studies were reviewed separately in a random fashion by two neuroradiologists, one more experienced in conventional angiography and the other in MR angiography, who were blinded to each other's results. The diagnostic value of MR angiography was assessed by determining whether patency of the parent artery was discernible sufficiently to gauge the extent of patency or occlusion of the treated aneurysm. Angiograms in which the parent artery was not satisfactorily demonstrated, throughout its length, were considered to be inadequate for evaluation of the treated aneurysm. Residual patency (or recanalization) was considered to be either present or absent at the site of the aneurysm, both at the neck and within the mesh of coils. The presence of focal high signal within the aneurysm or at its neck was interpreted as residual flow. The number of visible major branches of the parent artery was also evaluated.
Results
The results were analyzed by comparing two groups: group 1 consisted of all unenhanced MR angiographic studies (64 aneurysms) and group 2 consisted of the 25 studies obtained after administration of contrast material. Data are reported in Tables 3 and 4.
TABLE 3:
Results of group 1 (MR angiography without contrast enhancement)
Group 1: MR Angiograms Obtained without Contrast Material
Artifacts
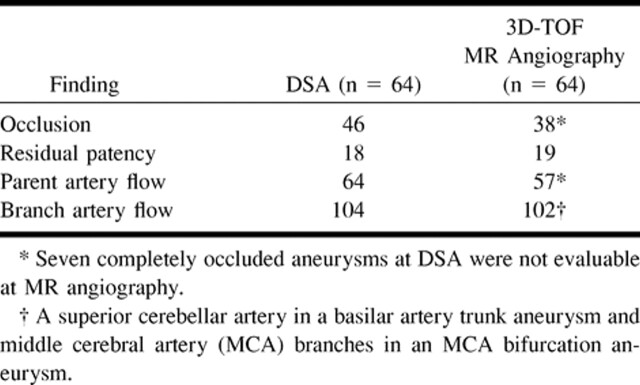
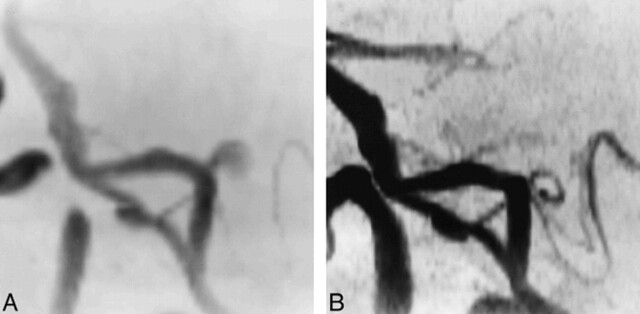
In seven (11%) of 64 aneurysms (95% CI = 4.5–21.2%), MR angiography provided no reliable information owing to the presence of significant artifacts at the neck site or on the neck itself on both source images and MIP reconstructions that prevented evaluation of the parent artery (Fig 1A and B). In the same cases, however, MR angiography was able to show patency of the adjacent branch arteries. Locations of the aneurysms were as follows: the anterior communicating artery (n = 2), the middle cerebral artery bifurcation (n = 1), the tip of the basilar artery (n = 2), the internal carotid artery at the origin of the posterior communicating artery (n = 1), and the posterior cerebral artery at the P1 segment (n = 1). Six aneurysms were small and one was large. At follow-up DSA, all seven aneurysms proved to be completely occluded. Five aneurysms were imaged with the Magnetom SP63 (with a longer TR/TE sequence) and two aneurysms were studied with both the Magnetom SP63 and the Vision imagers (with shorter TR/TE parameters). There was no significant difference in the size of the artifacts between the two examinations. In the two latter cases (small aneurysms of the basilar artery) and in a third one (a small aneurysm of the anterior communicating artery), the ultrafast technique was also applied; and despite a slight reduction in the size of the artifact, none of these studies showed the parent artery in its entirety.
fig 1.
Small anterior communicating artery aneurysm treated with GDCs. The presence of an artifact does not permit evaluation of the aneurysm neck.
A, 3D-TOF MR angiographic source image (39/6.2/1) shows a region of absence of signal related to the presence of coils in the site of the treated aneurysm. The A2 segment of both anterior cerebral arteries is not visible.
B, Corresponding targeted MIP reconstruction shows the gap in vascular signal at the site of the treated aneurysm and at the level of the origin of both A2 segments.
C, Targeted MIP reconstruction from the ultrashort-TE MR angiographic sequence (5/2.1/1) shows the origin of both A2 segments, although the anterior communicating artery is still not visible.
Reliable cases
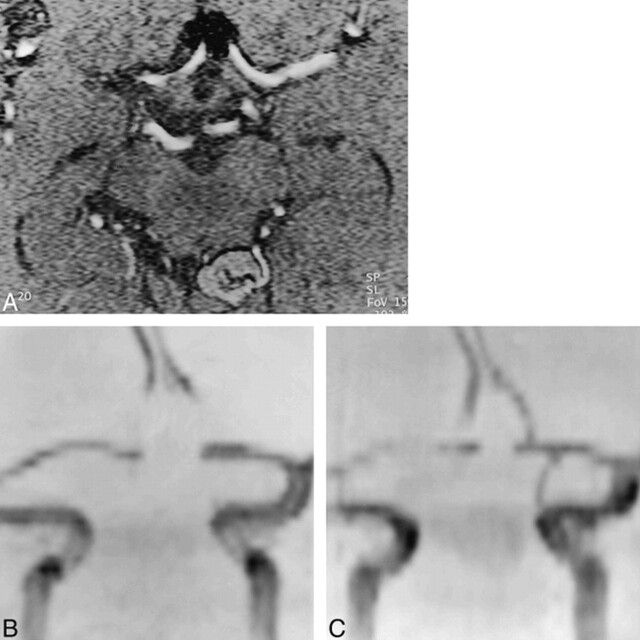
In the remaining 57 cases, MR angiography satisfactorily showed the vascular anatomy of the parent artery and of the aneurysm. DSA revealed complete occlusion in 39 cases (68%) and the presence of residual patency or partial recanalization in 18 cases (32%) (four giant, seven large, and seven small aneurysms), while MR angiography showed complete occlusion in 38 cases (67%) and incomplete occlusion in 19 cases (33%). The sensitivity of MR angiography as compared with DSA (the standard of reference) was 97% (95% CI = 86.5–99.9%) and specificity was 100% (95% CI = 81.5–100%); the positive predictive value was 100% (95% CI = 90.7–100%) and the negative predictive value was 94.7% (95% CI = 74–99.9%). Different results between the two studies were obtained in only one case, a small basilar tip aneurysm. The small aneurysm was considered to be completely occluded at DSA, whereas MR angiography, performed 24 hours later, showed the presence of high signal at the base of the aneurysm both on source images and on MIP reconstructions (Fig 2A–C). The reviewer interpreted this data as being suggestive of aneurysmal recanalization, although a presaturation slab was not applied to exclude the presence of subacute methemoglobin. At the second follow-up, performed 6 months later, MR angiography and DSA provided similar results, each showing incomplete occlusion of the aneurysmal malformation (Fig 2D). In the remaining cases, DSA and MR angiography provided similar results as to the degree of residual patency (or recanalization) (Fig 3); in one case (a large basilar tip aneurysm), MR angiography slightly overestimated the size of residual patency. In 17 of 19 cases of incomplete occlusion, MR angiography showed the residual patency as high signal at the aneurysm neck; in the other two cases, the high signal was between the mesh of coils. In these last two cases, the presence of a subacute thrombus of methemoglobin was ruled out by acquiring another sequence with a presaturation slab, which showed the disappearance of the high signal and of the residual flow.
fig 2.
Small basilar tip aneurysm found to be incompletely occluded at MR angiography and completely occluded at DSA follow-up after treatment with GDCs.
A, DSA study before treatment shows the presence of a small basilar tip aneurysm.
B, The aneurysm was judged to be completely occluded at the first follow-up DSA study.
C, On the first follow-up 3D-TOF MR angiogram (39/6.2/1), a small residual patency was detected.
D, Residual patency of the aneurysm was confirmed at the second DSA follow-up (6 months after the first follow-up study).
fig 3.
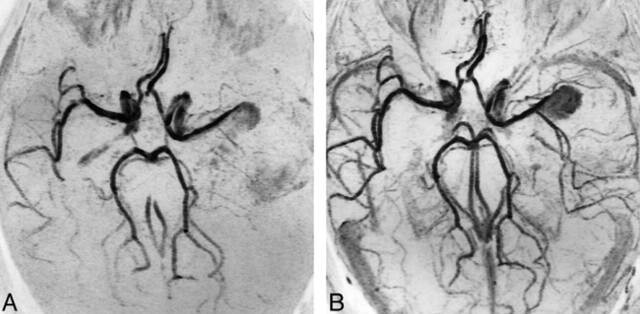
Small basilar trunk aneurysm incompletely occluded after treatment with GDCs, as demonstrated at the first follow-up examination. The patient was retreated, with complete occlusion of the aneurysm noted at 1-year follow-up study.
A, DSA shows the presence of residual patency of the treated aneurysm near the neck.
B, 3D-TOF MR angiogram (43/8/1) shows residual patency with the same extent as seen at DSA, despite the presence of a vascular signal gap, related to the coils, at the P1 segment of the left posterior cerebral artery.
Group 2: MR Angiograms Obtained with Contrast Material
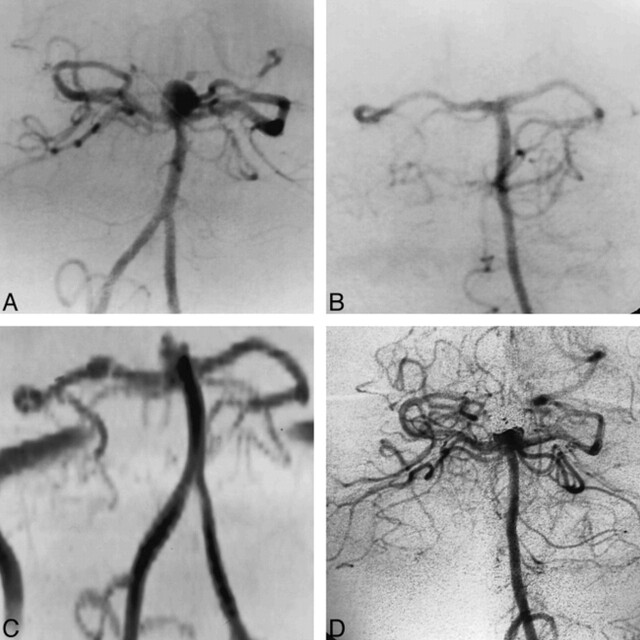
Four of the 25 treated aneurysms studied with both unenhanced and enhanced MR angiography were among the group of seven in which MR angiography was not reliable owing to the presence of artifacts. The addition of contrast material did not improve the quality of these studies, and the artifacts on the parent artery remained visible. In the remaining 21 aneurysms (three giant, six large, and 12 small), contrast injection did not modify the judgment formulated on the basis of the unen-hanced studies of the 12 small aneurysms. On the other hand, for the six large and three giant aneurysms, the addition of contrast material, which shortened T1 and reduced saturation, helped identify regions of slow flow, providing better definition of the aneurysm (Fig 4). Moreover, in two cases, the enhanced MR angiographic studies showed the presence of branch arteries not visible at basal examination (Table 4).
fig 4.
Giant middle cerebral artery bifurcation aneurysm partially treated with incomplete occlusion verified at the 6-month follow-up examination.
A, MIP reconstruction from basal 3D-TOF MR angiogram (43/8/1) shows flow within the portion of the aneurysm seen previously; the slow flow is responsible for low signal of both the aneurysm and the middle cerebral artery branches.
B, Enhanced 3D-TOF MR angiogram (43/8/1) better depicts the partially occluded aneurysm and the patency of the middle cerebral artery branches.
TABLE 4:
Results of group 2 (MR angiography with contrast material)
Discussion
Endovascular treatment of cerebral aneurysms has become a well-established procedure worldwide (9). Viñuela and coworkers (4) demonstrated the safety of GDC treatment in the acute phase of ruptured cerebral aneurysms of the anterior and posterior circulation. Nonetheless, long-term follow-up data concerning rate of rebleeding and recanalization in large series of GDC-treated aneurysms are still needed. In this regard, DSA follow-up examinations are recommended. Because DSA is invasive, expensive, and often requires hospitalization, a noninvasive, rapid, less expensive, reliable examination would be of great value.
GDCs are associated with significant beam-hardening artifacts on CT scans, whereas signal loss caused by platinum coils is minimal on MR studies (7). Derdeyn et al (8) described their preliminary experience with MR angiographic follow-up of GDC-treated aneurysms. Their conclusion, although cautious, is optimistic, suggesting that MR angiography be performed in some clinical situations, such as in patients who cannot tolerate DSA, as a long-term follow-up examination, and in the acute setting as a complement to conventional MR studies. Their results were somewhat different from ours, despite similarly positive conclusions. Of a total of 26 studies in which both MR angiography and DSA were performed, these authors reported three false-negative examinations (in which recanalization or residual patency was demonstrated at DSA but not at MR angiography) and two false-positive examinations (in which the presence of artifacts or hematoma was interpreted as flow at MR angiography). In our series, with a total of 64 aneurysms studied, we had no false-negative results: all cases of residual patency and recanalization were well demonstrated at MR angiography and along the same extent as seen at DSA, even in small aneurysms with very little recanalization. In one case of recanalization evident on the second follow-up DSA examination, MR angiography slightly overestimated the size of the residual patency, probably because of the volumetric acquisition of this technique vs the bidimensional one of DSA as well as its depiction of multiple projections, which is sometimes difficult with DSA. The only instance of a false-positive MR angiographic finding was that in which high signal was seen at the base of the aneurysm on the first follow-up study and was consequently interpreted as aneurysmal recanalization. Even though the presence of methemoglobin could not be excluded, as a presaturation slab was not applied, this result suggests a reduction of coil compaction at the aneurysm neck. This case turned out to be positive for recanalization at the second follow-up DSA examination. Otherwise, all complete occlusions depicted at DSA were equally demonstrated at MR angiography.
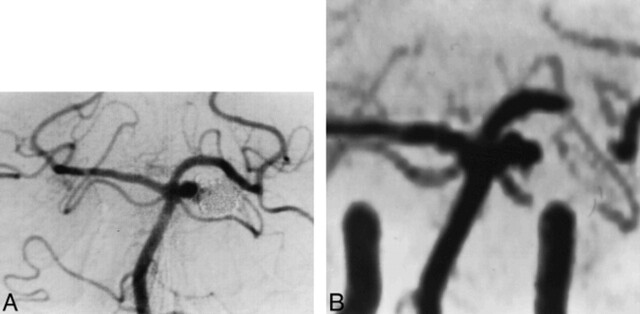
The patency of branch arteries was discernible in all but two cases, probably because of saturation effects, which can eliminate signal from slow flow or small vessels. In one case of a small aneurysm located at the origin of the posterior inferior cerebellar artery (PICA), complete occlusion of the aneurysm reestablished full perfusion of the PICA, which was well seen at follow-up MR angiography (Fig 5).
fig 5.
Small aneurysm at the origin of the left PICA, which was completely occluded after treatment with GDCs.
A, 3D-TOF MR angiogram (39/6.2/1) targeted MIP reconstruction shows the aneurysm before treatment.
B, 3D-TOF MR angiogram (39/6.2/1) targeted MIP reconstruction 6 months after treatment shows complete occlusion of the aneurysm and reperfusion of the left PICA.
In a subgroup of patients, we repeated MR angiography after injection of contrast material to limit the effect of saturation. We did not modify the 3D-TOF sequence parameters so that we could verify the isolated effect of the contrast agent, although a slight reduction in FOV could have had a beneficial effect on resolution. The use of contrast-enhanced conventional sequences does not seem to be indicated for small aneurysms nor, most likely, for aneurysms located near the base of the skull, where enhancement of veins can interfere with evaluation of adjacent arterial vessels. Nevertheless, in giant and large aneurysms, the extent of recanalization is more evident when its limits are well portrayed. Moreover, in two cases, the use of contrast material revealed the presence of branch arteries not visible on unenhanced MR studies. Both these cases were giant aneurysms, in which flow steal is more prominent and slow flow in the branch arteries is subsumed by saturation effects on unenhanced 3D-TOF MR angiograms.
In seven (10.9%) of our cases, the presence of significant artifacts precluded evaluation of the entire parent artery and, as a consequence, the aneurysm neck. In an attempt to understand why artifacts were present only in these cases and not in the entire group, we analyzed different aspects of our study design, including the anatomic characteristics of the aneurysm (site, dimension, morphology); the characteristics of the GDCs used for treatment (number, type); and the characteristics of the MR imaging sequence that could interfere with both magnetic susceptibility (TE, voxel size) and flow saturation (slab position with respect to position of the aneurysm). The results of this analysis are as follows.
Anatomic Characteristics
All seven aneurysms were representative of the entire series: they were not located in a particular site, they did not differ in shape from the other 57 cases, and all were small, except for one large basilar artery aneurysm.
GDC Characteristics
The seven aneurysms did not differ from the other 57 with respect to the number and type of coils used to treat them. The only aspect of the GDCs we could not verify was the date of their manufacture and, consequently, their delivery method, which can lead to the release of small metallic particles together with the coils.
MR Imaging Sequence Characteristics
All cases were studied with a 3D-TOF sequence, although 45 examinations were performed on a Magnetom SP 63 (Siemens) unit (with a longer TR/TE sequence) and 19 examinations were performed on a Vision (Siemens) unit (with a shorter TR/TE sequence), which also resulted in a small difference in the effective voxel size. Five of the examinations containing artifacts were obtained with the first set of parameters and two were acquired with the shorter TR/TE sequence. Because magnetic susceptibility corresponds to both TE value and voxel size, we can conjecture that the use of a shorter TE and a smaller voxel may have reduced the size of the artifacts. However, if we consider the two groups separately, we find that the percentage of artifacts is not significantly different between them: 11.1% (5/45) in the first group and 10.5% (2/19) in the second group. Moreover, in two cases in which both sequences were performed, no significant difference in artifact size was found. In the same two cases (small aneurysms of the basilar artery) and in a third one (a small aneurysm of the anterior communicating artery) an ultrafast technique was applied, similar to the one described by Gonner et al (10). Yet, despite the slight reduction in the size of the artifacts, in our cases the image quality was worse than with the technique of reference in relation to overall signal reduction; and the parent artery was still not entirely evident (Fig 1C).
Another aspect worth considering is the effect of saturation, which contributes to signal reduction. This effect can be worse at the border of the volume of interest (VOI) and when the flow tends to be parallel with the major axis of the VOI. In all our cases, the volume was centered exactly on the treated aneurysm, which never happened to be on the periphery of the VOI. The orientation of the parent artery could play a role, particularly in the case of anterior communicating artery aneurysms, in which the anterior communicating artery is often parallel to the volume of acquisition. In the four patients in whom artifacts were seen who were also examined with contrast-enhanced 3D-TOF MR angiography to reduce saturation effects, the use of contrast material did not modify the results nor help to show the parent artery better.
Finally, we analyzed whether a signal pile-up defect in MIP reconstruction could have produced the artifacts seen in the seven cases. Examination of the single source images did not add any information to the MIP reconstructions, either concerning the neck of the aneurysm or the parent artery.
Although the use of a shorter TE and a reduction of the voxel dimension can certainly reduce the size of an artifact, it seems that in certain cases an accumulation of characteristics, such as local anatomy, saturation effects, and, most likely, the metallic material used for embolization, can play a role in image quality. Nevertheless, in the vast majority of our cases, MR examinations were of diagnostic value, showing recanalization of the treated aneurysm as satisfactorily as DSA did.
Conclusion
We conclude that 3D-TOF MR angiography is useful as a follow-up technique in evaluating GDC-treated aneurysms in those cases in which good correlation with DSA had been demonstrated at discharge. The 3D acquisition and the ability to obtain multiple projections enable visualization, in some cases, of regions of residual flow, especially within the coils, whereas with DSA, visualization may be impeded, as the coils can hide the flow.
TABLE 2:
Site of the aneurysms
Footnotes
Presented in part at the annual meeting of the American Society of Neuroradiology, Toronto, May 1997.
Address reprint requests to Dr. Nicoletta Anzalone, Scientific Institute H San Raffaele, Via Olgettina 60, 20132 Milan, Italy.
References
- 1.Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach, 1: electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1-7 [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach, 2: preliminary clinical experience. J Neurosurg 1991;75:8-14 [DOI] [PubMed] [Google Scholar]
- 3.Graves VB, Strother CM, Duff TA, Perl J. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640-648 [DOI] [PubMed] [Google Scholar]
- 4.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475-482 [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Masaryk TJ, Modic MT, Ruggieri PM, Haacke EM, Selman WR. Intracranial aneurysm: evaluation by MR angiography. AJNR Am J Neuroradiol 1990;11:449-456 [PMC free article] [PubMed] [Google Scholar]
- 6.Schuierer G, Huk WJ, Laub G. Magnetic resonance angiography of intracranial aneurysms: comparison with intra-arterial digital subtraction angiography. Neuroradiology 1992;35:50-54 [DOI] [PubMed] [Google Scholar]
- 7.Hartman J, Nguyen T, Larsen D, Teitelbaum GP. MR artifacts, heat production, and ferromagnetism of Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:497-501 [PMC free article] [PubMed] [Google Scholar]
- 8.Derdeyn CP, Graves VB, Turski PA, Masaryk AM, Strither CM. MR angiography of saccular aneurysms after treatment with Guglielmi detachable coils: preliminary experience. AJNR Am J Neuroradiol 1997;18:279-286 [PMC free article] [PubMed] [Google Scholar]
- 9.Richling B, Bavinski G, Gross C, Gruber A, Killer M. Early clinical outcome of patients with ruptured cerebral aneurysms treated by endovascular (GDC) or microsurgical techniques: a single center experience. Intervent Neuroradiol 1995;1:19-27 [DOI] [PubMed] [Google Scholar]
- 10.Gonner F, Remonda L, Nicoli G, Baumgartner W, Schroth G. MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1324-1328 [PMC free article] [PubMed] [Google Scholar]