Abstract
BACKROUND AND PURPOSE: Determining the cause of Cushing's syndrome can be difficult. Bilateral inferior petrosal sinus (IPS) sampling after ovine corticotropin–releasing hormone (oCRH) stimulation is an established technique for the differentiation of pituitary from nonpituitary sources of adrenocorticotropic hormone (ACTH) production. The purpose of this study was to review our experience to determine the sensitivity and specificity of bilateral IPS sampling.
METHODS: Between January 1990 and February 1997, 92 patients underwent 94 IPS sampling procedures. Indications for these patients with Cushing's syndrome included no discrete lesion on MR images or CT scans, a discrete lesion present on images but equivocal peripheral ACTH sampling after oCRH stimulation, and persistent Cushing's syndrome after trans-sphenoidal surgery.
RESULTS: IPS sampling yielded six false-negative results and one false-positive result, manifesting an overall sensitivity of 92.2% and a specificity of 90.0% for detection of a pituitary source of ACTH after oCRH stimulation. Microadenoma lateralization by IPS sampling after oCRH stimulation agreed with the surgical location in 70.0% of the patients. The technical success rate of initial (presampling) bilateral IPS catheterization was 93.6%. Two serious complications occurred and consisted of a venous subarachnoid hemorrhage and a lower extremity deep venous thrombosis.
CONCLUSION: Bilateral IPS sampling after oCRH stimulation is helpful in the evaluation of ACTH-dependent Cushing's syndrome. False-negative results, however, suggest that the possibility of a pituitary source must still be considered when no response to oCRH is documented. Interpetrosal ACTH gradient alone is not sufficient to lateralize the tumor reliably.
Ascertaining the cause of hypercortisolism in a patient with Cushing's syndrome can be a perplexing problem for the clinician. Two broad categories of hypercortisolism must be distinguished; those processes that are dependent on adrenocorticotropic hormone (ACTH) production and those that are not.
Measurement of plasma ACTH concentration before and after ovine corticotropin–releasing hormone (oCRH) stimulation reliably distinguishes ACTH-independent Cushing's syndrome (eg, functional adrenal tumors) from ACTH-dependent processes (1). ACTH-dependent processes comprise ACTH production from pituitary adenomas and from nonpituitary (ectopic) sources. The ideal therapy for ACTH-dependent Cushing's syndrome entails surgical removal of the ACTH-producing neoplasm. Thus, to guide appropriate intervention, one must accurately determine the source of ACTH production. Clinical history, dynamic biochemical tests (eg, dexamethasone suppression, oCRH stimulation), and CT or MR imaging of the pituitary gland aid in distinguishing the two possibilities.
Not infrequently, however, the clinical, biochemical, and imaging test results are indeterminate, resulting in uncertainty regarding the source of ACTH production. In such cases, direct sampling for ACTH in the pituitary venous effluent into the inferior petrosal sinus (IPS) before and after oCRH stimulation can help to resolve the uncertainty by accurately revealing the location of the source of ACTH production (2).
Bilateral IPS sampling after oCRH stimulation is an established technique for the differentiation of pituitary from nonpituitary sources of ACTH production (2, 3). In addition, it can help in lateralizing the pituitary microadenomas (2–4). IPS sampling can be a technically difficult technique. Oldfield et al (2) and Doppman (3) reported the largest experience to date for IPS sampling. They reported an accuracy approaching 100% in determining a pituitary source of ACTH production with no serious complication (2). A high sensitivity and specificity for IPS sampling is important, considering that the pretest probability for a pituitary ACTH source is considered to be very high (approximately 85−90%) (1). The purpose of this retrospective review was to evaluate our experience with IPS sampling, including a determination of the sensitivity and specificity of the test in our patient population.
Methods
We conducted a retrospective analysis of 94 IPS sampling procedures for ACTH that was performed in 92 patients between January 1990 and February 1997. At our institution, only a select patient population with ACTH-dependent Cushing's syndrome undergoes IPS sampling for ACTH. Indications for IPS sampling include the following: 1) no discrete pituitary lesion identified by imaging or equivocal imaging results; 2) a discrete lesion identified by imaging but peripheral ACTH sampling findings equivocal after oCRH stimulation; 3) persistent Cushing's syndrome after trans-sphenoidal surgery; and 4) the clinician's requirement of resolution of discrepancies between the clinical picture, biochemistry, and imaging findings. Our study population consisted of 70 female and 22 male patients of ages ranging from 13 to 75 years (mean age, 44.5 years). Eighteen (19.6%) patients had undergone previous trans-sphenoidal surgery. In one patient, a catheter could not be inserted in either IPS; thus, internal jugular vein sampling was performed. This patient was subsequently excluded from further analysis.
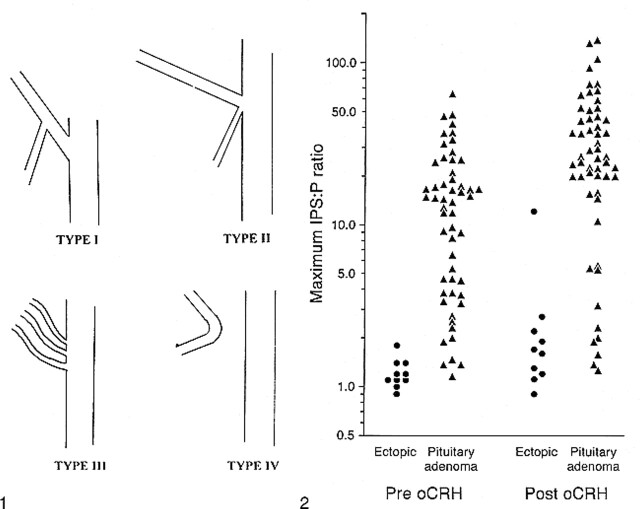
The charts of the remaining 91 patients were reviewed to determine imaging, surgical, and pathologic findings, technical details of the procedure, and subsequent clinical outcomes. Angiographic films obtained during IPS sampling were available for review for 89 patients (91 procedures). The angiographic studies were not available for two patients, who were thus excluded from the technical portion of the study. Pre- and post-sampling catheter position, level of contrast material reflux, and variant, bilateral anatomy of the IPS were recorded. Anatomic variants of the IPS were classified into four patterns based on a modification of the original classification presented by Shiu et al (5) (Fig 1).
fig 1.
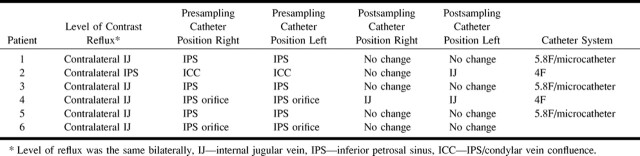
Modified classification of IPS anatomic variants. Type I consists of an IPS anastomosing with the internal jugular vein. The anterior condylar vein is absent or joins the IPS at a defined origin. The short segment of vein from the point of this anastomosis to the internal jugular vein is termed the inferior condylar confluence. Type II consists of a common origin of the IPS and anterior condylar vein with the internal jugular vein. Types III and IV remain as originally described by Shiu et al (5) (ie, an IPS consisting of several small channels communicating with the internal jugular vein and an IPS that communicates with the anterior condylar vein and not the internal jugular vein, respectively).fig 2. Maximum IPS-to-peripheral-blood ACTH ratios before and after oCRH administration in 65 procedures in which confirmation of the ACTH source was obtained
IPS catheterization was performed in a manner that was a modification of the technique reported by Miller and Doppman (4). All patients or their parents completed an informed consent form, approved by our institutional review board, before the procedure was performed. After catheterization of the femoral veins was performed, 4000 U of heparin was administered. Before 1992, a 4F 45° head catheter was used (30 procedures). Nearly all of the IPS sampling procedures after that date were performed using a coaxial technique that included 5.8F catheters advanced into the internal jugular veins with a Tracker 18 (Target Therapeutics, Freemont, CA) microcatheter positioned in each IPS (61 procedures). Sampling required 3 mL of blood, and samples were obtained without difficulty through the microcatheters in approximately 75 seconds. Eighty-nine percent of all catheterizations were performed through bilateral femoral vein punctures, 7% through bilateral internal jugular vein punctures, 3% through a mixed femoral vein and internal jugular vein approach, and 1% with both catheters placed in the left femoral vein. After placement of the catheters in the IPS, catheter tip position was confirmed with a hand injection of contrast material and digital subtraction techniques. Simultaneous sampling of the IPS and peripheral veins was performed 5 and 1 minute(s) before oCRH stimulation (1 μg/kg; maximum, 100 μg) and 2, 5, and 10 minutes after oCRH stimulation. Digital subtraction venography was performed at the end of IPS sampling to document catheter position. A technically successful catheterization was defined as bilateral IPS catheterization in which the catheters did not move into the internal jugular veins during the course of IPS sampling.
All plasma ACTH determinations were made at our institution. Plasma ACTH was measured using an extraction radioimmunoassay in which the antibody was generated against synthetic ACTH (1−24 amino acids) (6). Intra- and interassay coefficients of variation were 7.6% and 10.5%, respectively.
For the determination of a pituitary source of ACTH production, the ratio of IPS to peripheral vein established by Oldfield et al (2) was used. The ratio was calculated from the simultaneous IPS and peripheral venous samples obtained before and after oCRH administration, and the highest ratio was used. Pituitary-dependent Cushing's syndrome was diagnosed with a baseline central-to-peripheral ratio of 2.0 or a post-oCRH ratio of 3.0 or both. As determined by Oldfield et al (2), an interpetrosal gradient of 1.4 was used as a predictor of the lateralization of a pituitary microadenoma.
Pathologic and clinical criteria for the establishment of the source of ACTH production were similar to those described by Oldfield et al (2). The diagnosis of a pituitary source of ACTH was established if histologic examination of the pituitary surgical specimen confirmed the presence of tumor (39 patients) with resultant postoperative normalization of cortisol or hypocortisolemia. In addition, a pituitary source of ACTH was diagnosed if resection of a portion of the pituitary gland (14 patients) or irradiation after resection (one patient) resulted in normalization or hypocortisolemia despite the lack of histologic identification of a tumor. The diagnosis of an ectopic source was confirmed by histologic specimen (nine patients). Ectopic sources consisted of six patients with bronchial carcinoid, two patients with ACTH-producing adrenal pheochromocytomas, and one patient with a metastatic “neuroendocrine” tumor.
Serum cortisol levels did not normalize in 10 patients with pituitary-dependent Cushing's syndrome despite the finding of a pituitary adenoma confirmed surgically (n=5), histologically (n=4), or after radiotherapy following trans-sphenoidal surgery (n=1). In 10 patients, the diagnosis was clinically thought to be ectopic ACTH syndrome, but repeated imaging failed to reveal a tumor. The source of ACTH excess remains unknown in 13 patients.
Results
ACTH Sampling of IPS
Confirmation of a pituitary or ectopic source of ACTH production was available for 63 patients (65 procedures). Figure 2 shows the measured ACTH IPS:peripheral ratios for both ectopic and pituitary sources of these 65 procedures. The mean baseline ACTH IPS:peripheral ratio for sampling procedures with a pituitary source was 15.2 and with an ectopic source was 1.1. The mean, post-oCRH stimulation ACTH IPS:peripheral ratio for sampling procedures with an ectopic source was 2.7 (this was elevated because of a false-positive result; without it, the mean was 1.6) and with a pituitary source was 33.6. The mean time to the maximum ratio after oCRH stimulation was 3.6 minutes for pituitary sources of ACTH.
A baseline ACTH IPS:peripheral ratio of 2.0 denoted a pituitary source of ACTH secretion (2). Using this ratio and excluding those patients in whom the catheter was noted to be in the internal jugular vein, either unilaterally or bilaterally on the post-test images, the baseline sensitivity was 92.2%, specificity was 100%, and accuracy was 93.4% (n = 61 procedures). After oCRH stimulation, an ACTH IPS:peripheral ratio of 3.0 denoted a pituitary source of ACTH secretion (2). With this ratio, the post-oCRH stimulation sensitivity was 92.2%, specificity was 90%, and accuracy was 91.8%.
Forty-one patients who had confirmation of a pituitary or ectopic source of ACTH underwent catheterization with the 5.8F/coaxial microcatheter system. This system reflects the technique of the test as it is performed currently. For this subset of patients, the pre-oCRH stimulation sensitivity was 91.2%, specificity was 100%, and accuracy was 92.7%. The post-oCRH stimulation sensitivity was 91.2%, specificity was 85.7%, and accuracy was 90.2%.
Only nine patients who had undergone previous surgery had confirmation of the source of ACTH production. Among these nine patients, there was one false-negative result both before and after ACTH stimulation.
Six IPS sampling procedures rendered false-negative results (classifying a pituitary source as an ectopic source of ACTH secretion) after oCRH stimulation. Four of these procedures yielded false-negative results both before and after oCRH stimulation. All patients had type I anatomy bilaterally.
Each of the six post-oCRH false-negative results showed a blunted and delayed time course when ACTH concentration from both petrosal sinuses was plotted as a function of time. In half of the cases, the response did not exceed the significance ratio during the course of the assay. In the remainder, there was a parallel increase in peripheral ACTH concentration with a magnitude near that of the IPS samples. Only one patient had undergone previous trans-sphenoidal surgery. All patients had type I IPS anatomy bilaterally. In two patients (not included in calculation of sensitivities and specificities), the catheters were noted to be in the internal jugular vein unilaterally and bilaterally, respectively. The technical factors of the IPS catheterization in these patients are presented in the Table. In one patient, a false-positive test result was identified after oCRH stimulation. This case has been discussed previously (7).
Technical factors of inferior petrosal sinus catheterization of six patients with false-negative sampling results
Lateralization
In 35 patients, the side of the pituitary adenoma was established at surgery. Using an interpetrosal ACTH ratio of 1.4 or greater, lateralization by IPS sampling after oCRH stimulation agreed with the surgical location in 70% of patients, was opposite in 11%, and nonlateralizing in the remainder. Baseline interpetrosal ACTH gradients agreed with the surgical location in 67% and were opposite the surgical location in 19% of the patients. An insufficient number of patients had confirmation of tumor location to determine accuracy of lateralization in this subset of patients.
Technical Success
Bilateral catheterization of the IPS with no displacement of the catheters into one or both internal jugular veins during subsequent sampling was considered a technical success. Using these criteria, 82 (87%) of the 94 procedures were technically successful. In only one patient (1.1% of procedures), a catheter could not initially be inserted in the IPS bilaterally. In this patient, no IPS orifice in the internal jugular vein was found on the right and a left internal jugular vein was not identified. The anatomic classification of the IPS in this patient remains unknown. In five patients, only unilateral catheterization of an IPS was successful. One patient had Shiu type IV anatomy, which precluded IPS catheterization. An additional patient had a duplicated channel paralleling the internal jugular vein and connecting to the left IPS. Catheterization of the IPS could not be accomplished; thus, sampling was performed in the internal jugular veins. Unilateral placement of a catheter was noted (during a retrospective review of the angiograms) in the remaining three patients' basilar plexus (8). Thus, the technical success rate of the initial (presampling) bilateral IPS catheterization was 93.6%. The remaining six procedures were considered to be technical failures because, after initial successful catheter placement into the IPS, the catheters were displaced into the internal jugular veins bilaterally in three and unilaterally in three procedures during the course of venous sampling. In these six patients, IPS sampling was performed with the angled 45° 4F catheters. No catheter was displaced into the internal jugular vein after ACTH sampling when the 5.8F/microcatheter system was used.
Six patients had unilateral hypoplastic IPS, and one patient with previous pituitary apoplexy had bilateral hypoplastic IPS. The catheter could not be advanced distal to the IPS/condylar confluence in two patients by using the 4F catheter system. The catheter tip was placed in the IPS origin in two patients by using the 5.8F microcatheter system but could be placed in the region of the genu or horizontal segment in the remainder. In one patient, a 4F system was replaced with a microcatheter system to perform successful catheterization of the hypoplastic IPS.
Initially, in the majority of the patients, the catheter tip was located in the IPS bilaterally before ACTH sampling (Table). In 12.1% of procedures, the catheter was located in the cavernous sinus on either side (bilaterally in 6%). Initial catheter placement depended on the catheter system used. The 4F catheter was less compliant, and manipulations into the IPS could be difficult with that system. In many cases, the catheter was positioned only as far as the IPS origin or IPS/condylar vein confluence before ACTH sampling because of difficulty in advancing the catheter further. This was not a factor in placement of the 5.8F microcatheter system. Thus, the catheter tip was at the IPS origin in 15.6% and at the IPS/condylar vein confluence in 50.5% of all IPS catheterizations with the 4F catheter system compared with 5.1% and 1.7%, respectively, for the 5.8F microcatheter system.
Complications
Two major complications occurred in two patients. One patient suffered a venous subarachnoid hemorrhage during the course of the procedure. A second patient, who underwent catheterization by a femoral vein approach, developed a deep venous thrombosis during the periprocedural period. The deep venous thrombosis was appropriately treated with anticoagulants, and no further complications occurred. Minor complications consisted of two small groin hematomas and one small cervical hematoma, which occurred after catheterization performed by a direct internal jugular vein approach. The cervical hematoma was small and resolved without symptoms other than local swelling and pain.
The patient with venous subarachnoid hemorrhage experienced elevated arterial blood pressure, headache, and confusion during the procedure. Three additional patients (total of 4.2%) experienced headaches during or after the procedure but had no sequelae. Other subjective complaints observed included nausea in 2.1% of patients, neck pain in 2.1%, and chest discomfort in 1%. These patients did not go on to develop adverse reactions.
Discussion
The treatment of ACTH-dependent Cushing's syndrome, whether from a pituitary or ectopic source, is surgical removal of the neoplasm. Accurate localization using clinical and biochemical means is important to avoid subjecting the patient to unnecessary surgery without the possibility of cure. The pretest probability of a pituitary source of ACTH (Cushing's disease) is estimated to be approximately 90% (1) in unselected patients presenting with ACTH-dependent Cushing's syndrome. With such a high pretest probability, it is important that the diagnostic test used have very high sensitivity and specificity. Noninvasive means of localizing the source of ACTH production include clinical presentation, the dexamethasone suppression test, the peripheral oCRH stimulation test, and radiographic imaging of the pituitary (either by MR imaging or CT) (1). Some researchers estimate the accuracy of the dexamethasone suppression test to be approximately 80% (9). The sensitivity and specificity of oCRH stimulation and peripheral venous sampling of ACTH is estimated to be 93% and 100% (1, 10), respectively, which is only slightly greater than the pretest probability of pituitary ACTH production. Complete reliance on imaging studies of the pituitary, whether it be on CT or MR imaging, is problematic in that there is a wide range of sensitivities reported for MR and CT examination of the pituitary in detecting microadenomas, ranging from 65% to 94% (11–13). Thus, imaging does not reveal all adenomas. In an autopsy series of unselected cases, incidental pituitary lesions were reported to have occurred in 6.1% of the patients (14), and in an additional MR study, the findings indicated that approximately 10% of the normal adult population had abnormalities compatible with pituitary microadenomas, which were asymptomatic (15). These observations raise uncertainty regarding whether a pituitary abnormality detected by imaging is the source of ACTH production or whether it may be an incidental finding.
IPS sampling of ACTH after oCRH stimulation is an established invasive technique that can also be used for distinguishing pituitary from ectopic sources of ACTH production. Many experts consider IPS sampling to be the most reliable study for this purpose. Oldfield et al (2) published the largest experience with this technique, reporting a sensitivity and specificity of 100% for detection of a pituitary source of ACTH. This exceeds the estimated pretest probability of a pituitary source in patients with ACTH-dependent Cushing's syndrome, making this test, in theory, the most reliable diagnostic test. Recently, cavernous sinus sampling has also been reported to be highly accurate in differentiating pituitary from ectopic sources of ACTH production (16).
In our series, we experienced six false-negative results and one false-positive result. In two procedures, the catheter position had changed after the procedure into the internal jugular vein, and this could, in theory, be responsible for the false-negative results in these two patients. Thus, these patients were not included in the sensitivity or specificity calculations. Overall, the sensitivity was 92.2% and the specificity was 90% for detection of a pituitary source of ACTH after oCRH stimulation. For the subset of patients in whom the 5F coaxial microcatheter system was used, which reflects the current technique, the sensitivity was 91.2% and the specificity was 85.7% for detection of a pituitary source of ACTH after oCRH stimulation. These values approximate the estimated pretest probability for a pituitary source of ACTH production in patients presenting with Cushing's syndrome. The pretest probability for a pituitary ACTH source, however, is unknown in patients with normal CT and MR imaging findings. Since the initial investigation by Oldfield et al in 1991 (2), others have also reported false-negative results for IPS sampling after oCRH administration (17, 18). Most recently, Lopez et al (19) reported one false-negative result in a series of 24 procedures. The presence of false-negative results suggests that the lack of a significant gradient after oCRH stimulation does not entirely exclude Cushing's disease in favor of ectopic sources, as pointed out by Lopez et al (19).
The false-negative results obtained in our study are likely multifactorial and may be attributed to technical factors, anatomic variation, or intrinsic properties of tumor ACTH secretion. In all six cases, there was a response to oCRH, albeit blunted. In two of these patients, the catheters were found to have slipped into the internal jugular vein at the end of sampling. Thus, we can attribute technical factors (eg, sampling from internal jugular vein with subsequent dilution of effluent) as the likely cause of the false-negative results in these patients. One patient had undergone two previous trans-sphenoidal operations. It is possible that this patient's altered venous anatomy resulted in the false-negative finding. In the remaining patients, rises in the concentration ACTH in the peripheral venous samples resulted in IPS:peripheral ratios of less than 3.0. In two of these patients, the pre-oCRH ratio was greater than 2.0 (leading to correct localization of the tumor); however, the ratio after oCRH administration was less than 3.0, resulting in a false-negative test. Whether these findings were because of altered anatomic drainage or a property of tumor ACTH secretion is unknown. Error in the immunoassay or sampling handling could also be considered; however, no sample handling errors were documented. The single false-positive result occurred in a patient who was not hypercortisolemic at the time of IPS sampling. This patient's case was the subject of a previous article (7), and the condition was attributed to periodic hormonogenesis of the patient's autopsy-proven bronchial carcinoid. This case emphasizes the importance of sampling only when the patient is hypercortisolemic.
Bilateral IPS sampling has been advocated as a potential method of localizing a pituitary microadenoma by using the interpetrosal gradient of ACTH (2). After oCRH stimulation and using an interpetrosal gradient of 1.4, IPS sampling for ACTH correlated with the surgical location of the pituitary lesion in 70% of the cases. This is in agreement with the results presented by Oldfield et al (2) and Lopez et al (19), who found correlation in 72% and 66.7% of their cases, respectively. Using cavernous sinus sampling, Graham et al (16) reported a higher correlation of 83%. In our study, 11% of the tests indicated that tumor location was on opposite side of the pituitary; our study was inconclusive regarding the remainder. We agree with Lopez et al (19) that the interpetrosal gradient should not be relied on as the sole determinant in anatomic location of a pituitary source of ACTH secretion. It may be useful in confirming that a questionable area on an image of the pituitary is the site of tumor, but this was not specifically evaluated in this study.
Initial bilateral catheterization of the IPS was successful in 93.6% of procedures, which compares with the 93.8% success rate reported by Lopez et al (19), but is less than the 99% success rate reported by Oldfield et al (2). The main impediment to initial catheterization success was anatomic (ie, failure of one or both IPS to have an identifiable connection with the jugular vein). In three patients, however, a retrospective review of the films during this study revealed catheterization of a parallel venous system, which was not the main drainage route from the cavernous sinuses. This emphasizes careful analysis of the pretest IPS venogram to scrutinize the level and path of contrast material reflux to the contralateral side (4, 8). Additionally, in six procedures, one or both catheters slipped into the internal jugular vein. This was strictly dependent on the catheter system used and occurred only with the stiffer 4F catheters, which we no longer use. The importance of postsampling venography is emphasized by these cases, in which catheter position can, at least in theory, alter the reliability of the test results. Cases performed with microcatheters and high-quality road-mapping fluoroscopic techniques resulted in accurate catheter placement without postsampling catheter displacement.
Complications from IPS sampling are infrequent (19, 20). The reported complications include transient brain stem symptoms, infarction, or pontine hemorrhage (20–22). Two (2.2%) patients in our series experienced major complications. Our sole neurologic complication consisted of a venous subarachnoid hemorrhage, which is described in detail elsewhere (23). We could not confidently identify what caused this event, although, most certainly, placement of a microcatheter perforated a vein. The second was a patient who developed deep venous thrombosis, which was treated successfully. Several patients were identified with subjective complaints of nausea, neck pain, or headache, with no sequelae. Other investigators (20) have described similar findings and have warned that these findings may be harbingers of impending neurologic complications.
Conclusion
IPS sampling is a technically challenging procedure that is the closest to a diagnostic standard of reference for the evaluation of ACTH-dependent Cushing's syndrome (2). As the test is used in our institution, the pretest probability of a pituitary source of ACTH production may not be as high as that estimated for an unselected population of patients with ACTH-dependent Cushing's syndrome. Thus, the lower sensitivity and specificity we report compared with those findings reported by Oldfield et al (2) and Graham et al (16) are still adequate for the evaluation of ACTH-dependent Cushing's syndrome in our select patient population. The false-negative results we report, however, suggest that the possibility of a pituitary source must still be considered among patients with blunted responses or relatively large increases in peripheral ACTH after oCRH stimulation. In addition, regarding lateralization of pituitary microadenomas, the interpetrosal ACTH gradient alone is not sufficient for lateralizing the tumor reliably.
Acknowledgments
We gratefully acknowledge Cindy Rausch for assistance with manuscript preparation.
Footnotes
Address reprint requests to John Huston III, MD, Mayo Foundation, 200 1st Street SW, Rochester, MN 55905.
References
- 1.Findling JW. Differential diagnosis of Cushing's syndrome. Endocrinologist 1996;7:17S-23S [Google Scholar]
- 2.Oldfield OH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotopin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med 1991;325:897-905 [DOI] [PubMed] [Google Scholar]
- 3.Doppman JL. Petrosal sinus sampling and corticotrophin-releasing hormone in Cushing's syndrome. Endocrinologist 1997;7:24 S 29 S [Google Scholar]
- 4.Miller DL, Doppman JL. Petrosal sinus sampling: technique and rationale. Radiology 1991;178:37-47 [DOI] [PubMed] [Google Scholar]
- 5.Shiu PC, Hanafee WN, Wilson GH, Rand RW. Cavernous sinus venography. AJR Am J Roentgenol 1968;104:57-62 [DOI] [PubMed] [Google Scholar]
- 6.Kao PC, Jiang NS, Carpenter PC. Human corticotropin (ACTH) radioimmunoassay with synthetic 1–24 ACTH. Clin Chem 1979;25:1267-1273 [PubMed] [Google Scholar]
- 7.Yoshihiro Y, Dudley D, Nippoldt T, Young W, Huston J, Parisi J. False positive inferior petrosal sampling in the diagnosis of Cushing's disease. J Neurosurg 1995;83:1087-1091 [DOI] [PubMed] [Google Scholar]
- 8.Doppman JL, Krudy AG, Girton ME, Oldfield EH. Basilar venous plexus of the posterior fossa: a potential source of error in petrosal sinus sampling. Radiology 1985;155:375-378 [DOI] [PubMed] [Google Scholar]
- 9.Findling JW, Kehoe ME, Shaker JL, et al. Routine inferior petrosal sinus sampling in the differential diagnosis of adrenocorticotropin (ACTH)-dependent Cushing's syndrome: early recognition of occult ectopic ACTH syndrome. J Clin Endocrinol Metab 1986;73:408-413 [DOI] [PubMed] [Google Scholar]
- 10.Nieman LK, Oldfield EH, Welsey R, et al. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab 1993;77:1308-1312 [DOI] [PubMed] [Google Scholar]
- 11.Colombo N, Loli P, Vignati F, Scialfa G. MR of corticotropin-secreting pituitary microadenomas. AJNR Am J Neuroradiol 1994;15:1591-1595 [PMC free article] [PubMed] [Google Scholar]
- 12.Stadnik T, Spruyt D, van Binst A, Luypaert R, d'Haens J, Osteaux M. Pituitary microadenomas: diagnosis with dynamic serial CT, conventional CT and T1-weighted MR imaging before and after injection of gadolinium. Eur J Radiol 1994;18:191-198 [DOI] [PubMed] [Google Scholar]
- 13.Bartynski WS, Lin L. Dynamic and conventional spin-echo MR of pituitary microlesions. AJNR Am J Neuroradiol 1997;18:965-972 [PMC free article] [PubMed] [Google Scholar]
- 14.Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology 1994;193:161-164 [DOI] [PubMed] [Google Scholar]
- 15.Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994;120:817-820 [DOI] [PubMed] [Google Scholar]
- 16.Graham KE, Samuels MH, Nesbit GM, et al. Cavernous sinus sampling is highly accurate in distinguishing Cushing's disease from ectopic adrenocorticotropin syndrome and in predicting intrapituitary tumor location. J Clin Endocrinol Metab 1999;84:1602-1610 [DOI] [PubMed] [Google Scholar]
- 17.Boscaro M, Rampazzo A, Paoletta A, et al. Selective venous sampling in the differential diagnosis of ACTH-dependent Cushing's syndrome. Neuroendocrinology 1992;55:264-268 [DOI] [PubMed] [Google Scholar]
- 18.McNally PG, Bolia A, Absalom SR, Falconer-Smith J, Howlett TA. Preliminary observations using endocrine markers of pituitary venous dilution during bilateral simultaneous inferior petrosal sinus catheterization in Cushing's syndrome: is combined CRF and TRH stimulation of value? Clin Endocrinol (Oxf) 1993;39:681-686 [DOI] [PubMed] [Google Scholar]
- 19.Lopez J, Barcelo T, Lucas F, et al. Petrosal sinus sampling for diagnosis of Cushing's disease: evidence of false negative results. Clin Endocrinol (Oxf) 1996;45:146-156 [DOI] [PubMed] [Google Scholar]
- 20.Miller DL, Doppman JL, Peterman SB, Nieman LK, Oldfield EH, Chang R. Neurologic complications of petrosal sinus sampling. Radiology 1992;185:143-147 [DOI] [PubMed] [Google Scholar]
- 21.Sturrock ND, Jeffcoate WJ. A neurological complication of inferior petrosal sinus sampling during investigation for Cushing's disease: a case report. J Neurol Neurosurg Psychiatry 1997;62:527-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyer H, Frisch H, Fahlbusch R, et al. Raymond's syndrome following petrosalsinus sampling. Acta Neurochir (Wein) 1994;131:157-159 [DOI] [PubMed] [Google Scholar]
- 23.Bonelli FS, Huston J, Meyer FB, Carpenter PC. Venous subarachnoid hemorrhage after inferior petrosal sinus sampling for adrenocorticotropic hormone. AJNR Am J Neuroradiol 1999;20:306-307 [PMC free article] [PubMed] [Google Scholar]