Abstract
BACKGROUND AND PURPOSE: Several anatomic abnormalities of the pituitary gland have been described as occurring in association with congenital growth hormone deficiency, including hypoplasia of the adenohypophysis, truncation of the pituitary stalk, and ectopia of the neurohypophysis. Their pathogenesis, however, is obscure. Normal pituitary development is dependent on the sequential expression of a series of ontogenetic factors. Growth hormone–releasing hormone (GHRH) is known to stimulate somatotroph proliferation, and a dwarf mouse model with a mutant GHRH receptor, the “little mouse,” has a small anterior pituitary due to hypoplasia of the somatotrophs. We recently described the human homolog of the little mouse (dwarfism of Sindh), caused by a homozygous nonsense mutation in the GHRH receptor gene in a Pakistani kindred. We investigated MR imaging characteristics to gain information regarding the potential role of GHRH in human pituitary organogenesis.
METHODS: MR images of the head were obtained of four affected male patients (age range, 22–29 years). Maximal anterior pituitary dimensions were determined from sagittal and coronal images, and pituitary volumes were estimated from cubic and ellipsoid formulae. The measurements were compared with normative values matched for age and sex.
RESULTS: The adenohypophysis was small in each of the four patients. The maximal height for the anterior pituitary was 3 mm in three patients and 2 mm in one (mean ± SD, 2.75 ± 0.5 mm), which is significantly (P < .001) less than the expected height of 5.6 ± 1.0 mm for men in this age group. Estimates of anterior pituitary volume in the patients ranged from 75 to 124 mm3 (104 ± 21 mm3), which corresponds to 35% to 52% of the normal mean volume corrected for small head size (P < .005). No other cranial abnormalities were identified.
CONCLUSION: We describe significant hypoplasia of the adenohypophysis occurring in four dwarfs with a nonsense mutation in the GHRH receptor. In addition to isolated growth hormone deficiency and severe dwarfism, affected patients have anterior pituitary hypoplasia, presumably due to somatotroph maldevelopment. Resistance to GHRH explains the hypoplasia of the adenohypophysis—a feature that contributes to growth hormone deficiency in this syndrome. This is one of the few instances in which the molecular basis of pituitary dysmorphogenesis has been identified.
The causes of congenital hypopituitarism, including its most common form, isolated growth hormone deficiency, have remained unknown in the majority of cases. MR imaging has revealed multiple anatomic abnormalities related and unrelated to the hypothalamic-pituitary axis (1–7). Hypotheses concerning the causes have focused on perinatal hypoxia/anoxia, pituitary stalk transection during breech delivery, and congenital hypoplasia of a portion of the axis (2). Among the latter, genetic causes may shed light on the pathogenesis of anatomic pituitary anomalies.
The genetic program for pituitary ontogeny has been extensively studied in the mouse. These studies have revealed the requirement for sequential expression of several transcription factors of the POU-homeodomain class, such as Rpx, Ptx-1, Otx-1, Lhx-3, Prop-1, and Pit-1, as well as the receptor for growth hormone–releasing hormone (GHRH) (8, 9). Mutations in the genes for Prop-1, Pit-1, and the GHRH receptor have been shown to be responsible for three well-characterized dwarf mouse strains that have arisen spontaneously (the Ames, Snell, and little mouse, respectively) (10–14). All three mutants have growth hormone deficiency and small pituitary glands, primarily because of deficiency in somatotrophs, which is the predominant cell type in the adenohypophysis (14). Expansion of the somatotroph lineage late in pituitary ontogeny is thought to depend on GHRH action (8), and GHRH has been shown to stimulate somatotroph proliferation in vitro (15). The little mouse, because of its dysfunctional GHRH receptor, fails to normally expand its somatotroph population, which leads to pituitary hypoplasia and growth hormone deficiency. Growth hormone deficiency is also due to the inability of the remaining somatotrophs to synthesize and secrete growth hormone normally in response to GHRH.
The human counterpart of the little mouse has been recently discovered (16, 17). The so-called “Dwarfs of Sindh,” a large, consanguineous kindred of growth hormone–deficient dwarfs in the province of Sindh, Pakistan, have severe growth hormone deficiency as a result of a nonsense mutation in the GHRH receptor gene (17, 18). Four of the affected patients recently traveled to our institution for medical studies, including MR imaging of the hypothalamic-pituitary area.
Methods
Four male patients, aged 22 to 29 years, whose cases have been previously described in detail (subjects 4, 5, 23, and 35 [18]) underwent MR imaging of the hypothalamic-pituitary axis. They demonstrated proportional dwarfism (mean height, 132 cm) without significant dysmorphic features. Relative microcephaly was present (mean head circumference, 49.6 cm); skull size was approximately 4 SD below the norm. Endocrine profiles corresponded to isolated growth hormone deficiency with undetectable serum growth hormone levels that did not respond to provocative stimuli (GHRH, L-dopa, or clonidine); no other endocrinopathy was identified. A representative patient is shown in Figure 1.
fig 1.

Representative patient (left; age, 25 years; height, 124 cm) affected by a homozygous nonsense mutation in the GHRH receptor, shown together with a normal-statured cousin (right; age, 22 years; height, 169 cm).
MR studies were performed with a 1.0-T system. Contiguous sagittal and coronal spin-echo T1-weighted sequences (530/17 [TR/TE], 2-mm-thick, 205 × 256 matrix) were performed before and after the IV administration of 0.1 mmol/kg gadopentetate dimeglumine. Contrast-enhanced images were obtained with fat saturation. Fat-suppressed sagittal spin-echo T1-weighted images (650/17, 2-mm-thick, 179 × 256 matrix) were also obtained without the use of IV administered contrast material.
Maximal anterior pituitary height was determined from midline sagittal images by measuring the greatest distance between the superior and inferior borders of the gland. Lateral and anteroposterior dimensions were similarly determined by measuring the greatest dimensions on the coronal and sagittal images, respectively. Estimates of pituitary volume were derived from these measurements using the cubic (length × width × height) and the ellipsoid [(length × width × height)/2] formulae (19). The first formula tends to overestimate and the latter to underestimate pituitary volume; therefore, the average of both measurements was taken as the best approximation of pituitary volume. Pituitary measurements were compared with published normal values matched for age and sex. MR images were also evaluated for the following: 1) normal or abnormal pituitary stalk, 2) normal or absent neurohypophysis, 3) midline malformations including corpus callosum agenesis or septo-optic dysplasia, 4) sellar or suprasellar masses, and 5) any other cranial abnormalities.
A statistical comparison of the patients' pituitary measurements with normative values was made using the Mann-Whitney rank sum and t tests. Data are expressed as mean ± SD unless otherwise stated.
Results
Representative MR images are shown in Figure 2. The anterior pituitary has a flattened aspect, with the gland appearing as if compressed against the sella floor. The maximum height of the adenohypophysis measured 3 mm in three of the patients and 2 mm in the fourth (2.75 ± 0.5 mm). The expected mean pituitary height for male persons in this age group is 5.6 ± 1.0 mm (20–22), which represents a statistically significant difference (P < .001). The anteroposterior length was 5 mm in three patients and 4 mm in one (4.8 ± 0.5 mm). This is also significantly smaller (P < .001) than normal reference values, which average 10.6 ± 1.1 mm (23–25). Lateral diameters were 10 mm in two patients and 11 mm in the other two; these values are within the lower normal range (23–25). Pituitary volume estimates in the four patients were 75, 99, 113, and 124 mm3 (103 ± 21 mm3), significantly smaller measures (P < .005) than the published norms (330 ± 96 mm3, using the same method of calculation [25], or 500 ± 134 mm3 when measured anatomically [26]). Even when corrected for the relatively small head size of the patients (approximately 90% of normal on a linear scale, which corresponds to a corrected mean pituitary volume of 240 mm3), the sizes of the patients' pituitary glands remain significantly decreased, ranging from 31% to 52% of the normal mean, corrected for head size (P < .005). The configuration of the abnormal gland is one of decreased height and decreased anteroposterior length, with relatively preserved lateral width. There was a significant positive correlation between pituitary volume and stature (r = 0.965, P ∼ .03), but no correlation between pituitary volume and head circumference (r = 0.098, P > .9).
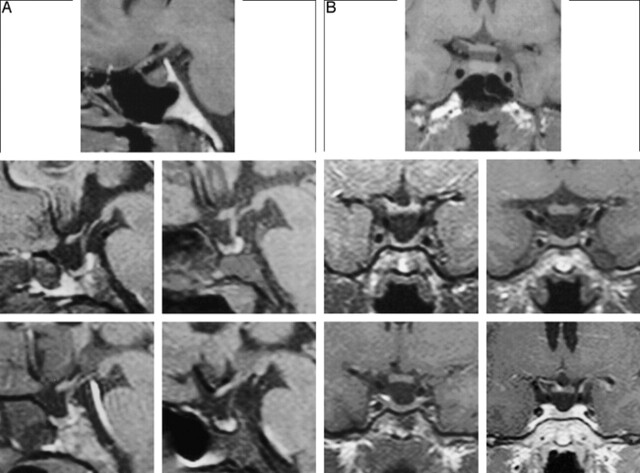
fig 2.
Fat-suppressed T1-weighted MR images of four adult male dwarfs show the pituitary area affected by a mutation in the GHRH receptor. An MR study of a normal 25-year-old man is shown at the top for comparison. The four lower panels show, in sequence (upper left to lower right), MR images of four patients (ages, 22, 27, 27, and 29 years, respectively). Note the hypoplastic anterior pituitary and the normal posterior lobe. As a result of adenohypophyseal hypoplasia, the neurohypophyseal “bright spots” appear very prominent. The MR image of the patient shown in figure 1 is on the upper left.
A, Sagittal views. All images were obtained without the administration of contrast material.
B, Coronal views. The coronal image on the lower right was obtained after the administration of contrast material; all others were obtained without the administration of contrast material.
The pituitary stalk was present in each patient and had a normal shape without thinning or truncation. The neurohypophysis (posterior pituitary bright spot) was present in normal position and size. No midline abnormalities were present. Specifically, no patient had signs of corpus callosum agenesis, septo-optic dysplasia, sellar/suprasellar mass, or Chiari I malformation.
Other craniofacial features, as assessed by MR imaging, were normal. We found no evidence of facial hypoplasia, deformities of the nasal bridge, or other abnormalities of cranial configuration that are typically associated with other types of growth hormone deficiency or growth hormone resistance.
Discussion
The MR findings described provide evidence for significant hypoplasia of the anterior pituitary in a newly described syndrome of genetic GHRH receptor deficiency. Affected patients have a homozygous nonsense mutation (Glu50→Stop) in the extracellular domain of the GHRH receptor, which completely inactivates the receptor, leading to severe growth hormone deficiency, dwarfism, and, as shown herein, pituitary hypoplasia. Our MR findings are in agreement with the hypoplastic pituitaries present in the little mouse, a growth hormone–deficient dwarf mouse that bears a missense mutation resulting in a dysfunctional GHRH receptor that cannot bind GHRH (12, 13, 27). Because GHRH is critical for terminal differentiation and proliferative expansion of the somatotroph cells late in pituitary ontogeny (8), the pituitary gland of the little mouse contains only one-fourth the normal complement of somatotrophs, whereas lactotrophs, thyrotrophs, gonadotrophs, and corticotrophs are normal in number (14). Because the somatotroph is the most predominant cell type in the pituitary gland, accounting for 50% to 60% of cell mass, this lack of development results in a significant reduction of pituitary size (13, 28). Our present studies show that the same seems to occur in humans with GHRH resistance. In humans, as in mice, somatotrophs account for approximately half the anterior pituitary cell mass (29). Estimates of pituitary volume in our patients are consistent with the loss of this cellular complement, with pituitary glands of approximately half the normal size. Our results are also consistent with those of a recent report by Netchine et al (30), who described MR findings in two members (9 and 10 years old) of a family from Sri Lanka affected with GHRH resistance. The similarity of our MR results and theirs, both in terms of pituitary size and configuration, is striking. Of interest is our finding of a correlation between statural height and pituitary size, perhaps suggesting that greater somatotroph deficits result in more severe growth hormone deficiency. Nonetheless, this speculation needs to be viewed with caution, because it is based on only four patients.
In contrast to the small pituitary size, the volume of the bony sella turcica appears relatively normal, with a depressed diaphragm sellae and a prominent suprasellar cistern. This suggests that the early development of the pituitary and sella may have been concordant, with later regression of pituitary size or failure of pituitary growth to keep up with skull development. Although there is no direct evidence for this speculative possibility in humans, it is interesting to note that, in the little mouse, pituitary size is normal at birth but progressively deviates from the norm during the postnatal period, when GHRH-dependent somatotroph proliferation should normally occur (31). A similar developmental history may have contributed to the disparity between sellar and pituitary size in our adult patients. Available, although limited, information regarding sellar size in isolated growth hormone deficiency of non-tumorous origin indicates that the sella tends to be small (1, 32, 33). Because terminal somatotroph expansion is GHRH-dependent, it is possible that the pituitary/sellar size disparity shown in our patients may be useful for differentiating this type of pituitary hypoplasia from those of other origins. Additional studies comparing pituitary/sellar size ratios in the various causes of primary pituitary hypoplasia are required to clarify this issue.
Ours is one of the first studies in which a molecular basis for pituitary hypoplasia in humans has been shown. Most cases of congenital growth hormone deficiency are idiopathic, although many potential causative factors have been postulated. Perinatal asphyxia/hypoxia, breech delivery with transection of the pituitary stalk, congenital hypo/dysplasia of the hypothalamic-pituitary axis, parasellar radiation, hydrocephalus, and parasellar masses have been suggested as potential causes (2). Reported imaging findings by either CT or MR imaging include normalcy, ectopic or absent neurohypophysis, abnormal pituitary stalk (thinned, truncated, or absent), hypoplastic or absent adenohypophysis, midline malformations, corpus callosum agenesis, septo-optic dysplasia, sellar/parasellar masses, and Chiari I malformation (1–7). In none of these cases is the cause of maldevelopment known with certainty. A causative link has been confirmed in only two syndromes: genetic GHRH receptor deficiency (the present report) and genetic septo-optic dysplasia, which was shown in one family to be due to a defect in the Rpx (also known as Hesx1) transcription factor (34).
Several studies have focused on the MR findings of growth hormone deficiency. Emphasizing the diversity of potential causes, imaging findings are variable. Pituitary height in all 49 patients studied by Arrigo et al (35) were 2 SDs below the mean normal value (an inclusion criterion). Seventeen of the patients also had pituitary stalk interruption, and one had septo-optic dysplasia. Kornreich et al (36) recently studied 21 patients with isolated growth hormone deficiency. The adenohypophysis was normal in 13 and small or absent in eight. The stalk was absent, thinned, or truncated in 20, the posterior pituitary was ectopic or nonvisualized in 18, and the more severe abnormalities were predictive of panhypopituitarism. Nagel et al (37) studied 21 patients with isolated growth hormone deficiency, six of whom had signs of isolated adenohypophyseal hypoplasia. Twelve patients had ectopia of the neurohypophysis, two were normal, and one had septo-optic dysplasia. In a large series presented by Bozzola et al (38), 46 of 72 patients with isolated growth hormone deficiency had pituitary heights 2 SDs below the norm. Five MR studies showed the stalk to be absent, and eight showed neurohypophyseal ectopia. Pellini et al (3) described the imaging findings of 18 children with isolated growth hormone deficiency. Total gland volume was 2 SDs below the mean value for age-matched control subjects. The pituitary stalk was normal in 10 children and truncated or thinned in eight. The children with abnormal stalks had signs of ectopic neurohypophysis. Marwaha et al (33) investigated 22 children with isolated growth hormone deficiency and reported small adenohypophyses in 17, stalk thinning in six, and associated brain anomalies in five. Maghnie et al (39) studied 29 patients with isolated growth hormone deficiency and found the adenohypophysis to be reduced in size or hypoplastic in 19 and normal in 10. The pituitary stalk was hypoplastic in nine of the patients, all of whom also had ectopia of the neurohypophysis. Two patients had a Chiari I malformation. It seems from this survey that anatomic abnormalities of the pituitary gland are common in cases of growth hormone deficiency. The descriptive and diverse nature of the literature, however, makes it difficult to derive pathogenetic insights into the causes of pituitary maldevelopment. With increasing knowledge of the factors responsible for pituitary-hypothalamic organogenesis in conjunction with the elucidation of genetic syndromes, such as the one reported herein, our understanding of these abnormalities at the molecular level is likely to improve in the near future.
Conclusion
We describe consistent adenohypophyseal hypoplasia occurring in four dwarfs affected by a nonsense mutation in the GHRH receptor gene. GHRH is important for pituitary organogenesis and development of somatotroph cells in the mouse; it appears from the present study that the same is true in humans. Because of GHRH receptor deficiency, the effects of GHRH, namely growth hormone release and promotion of somatotroph proliferation, are nullified with resultant dwarfism and predictable hypoplasia of the anterior pituitary. The absence of associated imaging abnormalities may help to distinguish these patients from others of short stature before genetic testing is performed.
Footnotes
This work was supported by National Institutes of Health grant MO1-RR00048 and a travel grant from Pharmacia and Upjohn, was presented in part at the 81st Annual Meeting of the Endocrine Society, San Diego, CA, 1999, and has appeared in abstract form (Program of the 81st Annual Meeting of the Endocrine Society, 1999, p 401).
Address reprint requests to G. Baumann, MD, 303 E. Chicago Avenue, Chicago, IL 60611.
References
- 1.Kelly WM, Kucharczyk W, Kucharczyk J, et al. Posterior pituitary ectopia: an MR feature of pituitary dwarfism. AJNR Am J Neuroradiol 1988;9:453-460 [PMC free article] [PubMed] [Google Scholar]
- 2.Koroiwa T, Okabe Y, Hasuo K, Yasumori K, Mizushima A, Masuda K. MR imaging of pituitary dwarfism. AJNR Am J Neuroradiol 1991;12:161-164 [PMC free article] [PubMed] [Google Scholar]
- 3.Pellini C, diNatale B, De Angelis R, et al. Growth hormone deficiency in children: role of magnetic resonance imaging in assessing aetiopathogenesis and prognosis in idiopathic hypopituitarism. Eur J Pediatr 1990;149:536-541 [DOI] [PubMed] [Google Scholar]
- 4.Arygropoulo M, Perignon F, Brauner R, Brunelle F. Magnetic resonance imaging in the diagnosis of growth hormone deficiency. J Pediatr 1992;120:886-891 [DOI] [PubMed] [Google Scholar]
- 5.Elster AD. Modern imaging of the pituitary. Radiology 1993;184:1-14 [DOI] [PubMed] [Google Scholar]
- 6.Zucchini S, di Natale B, Ambrosetto P, De Angelis R, Cacciari E, Chiumello G. Role of magnetic resonance imaging in hypothalamic-pituitary disorders. Horm Res 1995;44[suppl]:8-14 [DOI] [PubMed] [Google Scholar]
- 7.Adamsbaum C, Chaussain JL. Diagnostic strategies in pediatric imaging. Horm Res 1996;46:165-169 [DOI] [PubMed] [Google Scholar]
- 8.Treier M, Gleiberman AS, O'Connell SM, et al. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 1998;12:1691-1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parks JS, Adess ME, Brown MR. Genes regulating hypothalamic and pituitary development. Acta Paediatr Suppl 1997;423:28-32 [DOI] [PubMed] [Google Scholar]
- 10.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 1996;384:327-333 [DOI] [PubMed] [Google Scholar]
- 11.Li S, Crenshaw EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain pit-1. Nature 1990;347:528-533 [DOI] [PubMed] [Google Scholar]
- 12.Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo K. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet 1993;4:227-232 [DOI] [PubMed] [Google Scholar]
- 13.Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature 1993;364:208-213 [DOI] [PubMed] [Google Scholar]
- 14.Wilson DB, Wyatt DP, Gadler RM, Baker CA. Quantitative aspects of growth hormone cell maturation in the normal and little mutant mouse. Acta Anat (Basel) 1988;131:150-155 [DOI] [PubMed] [Google Scholar]
- 15.Billestrup N, Swanson LW, Vale W. Growth hormone-releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A 1986;83:6854-6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wajnrajch MP, Gertner JM, Harbison MD, Chua SC Jr, Leibel RL. Nonsense mutation in the human growth hormone-releasing hormone receptor causes growth failure analogous to the little (lit) mouse. Nat Genet 1996;12:88-90 [DOI] [PubMed] [Google Scholar]
- 17.Baumann G, Maheshwari H. The dwarfs of Sindh: severe growth hormone (GH) deficiency caused by a mutation in the GH-releasing hormone receptor gene. Acta Paediatr Suppl 1997;423:33-38 [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari HG, Silverman BL, Dupuis J, Baumann G. Phenotype and genetic analysis of a syndrome caused by an inactivating mutation in the growth hormone releasing hormone receptor: dwarfism of Sindh. J Clin Endocrinol Metab 1998;83:4065-4074 [DOI] [PubMed] [Google Scholar]
- 19.Di Chiro G, Nelson KB. The volume of the sella turcica. AJR Am J Roentgenol 1962;87:989-1008 [PubMed] [Google Scholar]
- 20.Tsunoda A, Okuda O, Sato K. MR height of the pituitary gland as a function of age and sex: especially physiological hypertrophy in adolescence and in climacterium. AJNR Am J Neuroradiol 1997;18:551-554 [PMC free article] [PubMed] [Google Scholar]
- 21.Wiener SN, Rzeszotarski MS, Droege RT, Pearlstein AE, Shafron M. Measurement of pituitary gland height with MR imaging. AJNR Am J Neuroradiol 1985;6:717-722 [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M, Takashima T, Kadoya M, et al. Height of normal pituitary gland on MR imaging: age and sex differentiation. J Comput Assist Tomogr 1990;14:36-39 [DOI] [PubMed] [Google Scholar]
- 23.Doraiswamy PM, Potts JM, Axelson DA, et al. MR assessment of pituitary gland morphology in healthy volunteers: age- and gender-related differences. AJNR Am J Neuroradiol 1992;13:1295-1299 [PMC free article] [PubMed] [Google Scholar]
- 24.Glaser B, Sheinfeld M, Benmair J, Kaplan N. Magnetic resonance imaging of the pituitary gland. Clin Radiol 1986;37:9-14 [DOI] [PubMed] [Google Scholar]
- 25.Lurie SN, Doraiswamy PM, Husain MM, et al. In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab 1990;71:505-508 [DOI] [PubMed] [Google Scholar]
- 26.McLachlan MSF, Williams ED, Fortt RW, Doyle FH. Estimation of pituitary gland dimensions from radiographs of the sella turcica. Br J Radiol 1968;41:323-330 [DOI] [PubMed] [Google Scholar]
- 27.Kajkowski EM, Price LA, Pausch MH, Young KH, Ozenberger BA. Investigation of growth hormone releasing hormone receptor structure and activity using yeast expression technologies. J Recept Signal Transduct Res 1997;17:293-303 [DOI] [PubMed] [Google Scholar]
- 28.Eicher EM, Beamer WG. Inherited ateliotic dwarfism in mice: characteristics of the mutation, little, on chromosome 6. J Hered 1976;67:87-91 [DOI] [PubMed] [Google Scholar]
- 29.Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In Wilson JD, Foster D, Kronenberg HM, Larsen PR, eds. Williams' Textbook of Endocrinology. 9th ed, Philadelphia: WB Saunders; 1998: 249-340
- 30.Netchine I, Talon P, Dastot F, Vitaux F, Goossens M, Amselem S. Extensive phenotypic analysis of a family with growth hormone deficiency caused by a mutation in the GH-releasing hormone receptor gene. J Clin Endocrinol Metab 1998;83:432-436 [DOI] [PubMed] [Google Scholar]
- 31.Wilson D, Wyatt DP. Immunofluorescent analysis of somatotroph distribution in the adenohypophysis of developing lit/lit mice. J Anat 1988;156:51-59 [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SP, Wolpert SM, Sadeghi-Nejad A, Senior B. Value of computed tomographic scanning in patients with growth hormone deficiency. Pediatrics 1986;78:601-605 [PubMed] [Google Scholar]
- 33.Marwaha R, Menon PSN, Jena A, Pant C, Sethi AK, Sapra ML. Hypothalamo-pituitary axis by magnetic resonance imaging in isolated growth hormone deficiency patients born by normal delivery. J Clin Endocrinol Metab 1992;74:654-659 [DOI] [PubMed] [Google Scholar]
- 34.Dattani MT, Martinez-Barbera JP, Thomas PQ, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet 1998;19:125-133 [DOI] [PubMed] [Google Scholar]
- 35.Arrigo T, De Luca F, Maghnie M, et al. Relationships between neuroradiological and clinical features in apparently idiopathic hypopituitarism. Eur J Endocrinol 1998;139:84-88 [DOI] [PubMed] [Google Scholar]
- 36.Kornreich L, Horev G, Lazar L, Schwarz M, Sulkes J, Pertzelan A. MR findings in growth hormone deficiency: correlation with severity of hypopituitarism. AJNR Am J Neuroradiol 1998;19:1495-1499 [PMC free article] [PubMed] [Google Scholar]
- 37.Nagel BHP, Palmbach M, Petersen D, Ranke MB. Magnetic resonance images of 91 children with different causes of short stature: pituitary size reflects growth hormone secretion. Eur J Pediatr 1997;156:758-769 [DOI] [PubMed] [Google Scholar]
- 38.Bozzola M, Adamsbaum C, Biscaldi I, et al. Role of magnetic resonance imaging in the diagnosis and prognosis of growth hormone deficiency. Clin Endocrinol 1996;45:21-26 [PubMed] [Google Scholar]
- 39.Maghnie M, Triulzi F, Larizza D, et al. Hypothalamic-pituitary dwarfism: comparison between MR imaging and CT findings. Pediatr Radiol 1990;20:229-235 [DOI] [PubMed] [Google Scholar]