Abstract
Summary: The imaging and pathologic features of three cases of nonlaryngeal neuroendocrine carcinoma of the head and neck are described. Neuroendocrine carcinomas represent malignant epithelial neuroendocrine neoplasms and are classified as three types: typical carcinoid (well differentiated), atypical carcinoid (moderately differentiated), and small cell neuroendocrine (poorly differentiated) carcinomas. The CT and MR imaging features of these tumors are nonspecific. Paranasal sinus neuroendocrine carcinomas showed expansion and destruction of the sinus, whereas metastatic neuroendocrine carcinomas to an intraparotid lymph node presented as a circumscribed parotid mass on CT scans.
Neuroendocrine neoplasms have an epithelial or a neural origin (1). The true neuroendocrine tumors are those derived from the neurons and appropriate associated structures, such as the paraganglia of the peripheral nervous system (1). The term neuroendocrine carcinoma encompasses a spectrum of malignant epithelial neuroendocrine neoplasms, ranging from atypical or well-differentiated to high-grade neoplasms (2, 3). Although most neuroendocrine carcinomas are found in the lung and alimentary tract and its appendages, they have been found in almost every organ in the body (4). There are a number of reports on neuroendocrine tumors of the larynx in the literature (5–7), but we found no comprehensive reports on nonlaryngeal neuroendocrine carcinomas. We present the radiologic and pathologic features of three cases of nonlaryngeal neuroendocrine carcinomas of the head and neck.
Case Reports
Case 1
A 48-year-old Caucasian man was found to have a sphenoid sinus mass during routine CT of the head after minor trauma (Fig 1). The mass was seen to expand the sphenoid sinus and erode its walls. MR imaging was subsequently performed and showed an expansile sphenoid sinus mass, which was hypointense on T1-weighted images and heterogeneously hyperintense on T2-weighted images (Fig 1). The mass showed minimal heterogeneous enhancement after the IV administration of contrast material. The mass also extended inferiorly into the nasopharynx. The floor of the sella appeared to be intact, and the pituitary gland was uninvolved.
fig 1.
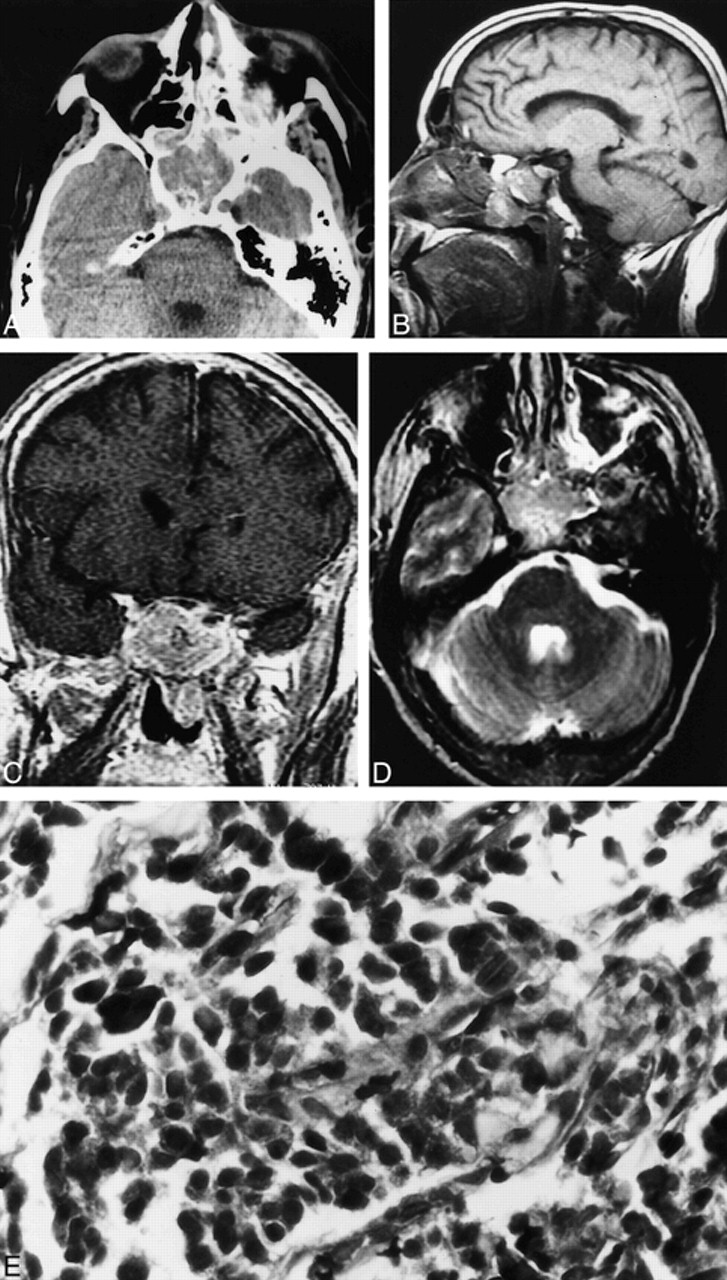
Case 1. A, Axial unenhanced CT scan shows a soft-tissue density mass in the sphenoid sinus. The mass is seen to expand the sinus.
B, Sagittal T1-weighted MR image (600/16/1 [TR/TE/excitations]) shows the nasopharyngeal component of the sphenoid mass. Note that the floor of the sella appears intact.
C, Coronal contrast-enhanced T1-weighted MR image (433/17/1) shows mild heterogeneous enhancement of the sphenoid sinus mass.
D, Axial fast spin-echo T2-weighted MR image (3250/95/3) shows the sphenoid sinus mass to be heterogeneously hyperintense.
E, Neuroendocrine carcinoma of the sphenoid sinus consisting of small pleomorphic cells exhibiting crowded hyperchromatic nuclei (hematoxylin and eosin stain; original magnification, ×40).
Endoscope-guided biopsy of the nasopharyngeal portion of the mass was performed. A histologic examination revealed a well-differentiated neuroendocrine carcinoma. Microscopic examination showed infiltrating trabecular cords and perisinusoidal organoid nests of polygonal cells exhibiting elliptical nuclei with finely dispersed chromatin, inconspicuous nucleoli, faintly granular eosinophilic cytoplasm, and poorly defined cell borders. There was mild cytologic atypia without any mitotic activity. No well-formed rosette or neurofibrillary matrix was identified. The tumor was strongly positive for cytokeratin (AE 1/3), chromogranin, and neuron-specific enolase. At the time of this writing, the patient was being treated at a different hospital, and the details of the treatment were not available.
Case 2
A 52-year-old Caucasian man presented with a 3-month history of toothache and left facial swelling. He was treated with antibiotics for almost 2 months without resolution. A biopsy was then performed along with extraction of the involved tooth. A histologic examination revealed a poorly differentiated (small cell) neuroendocrine carcinoma.
Microscopic examination showed nests of pleomorphic epithelial cells with irregular, hyperchromatic, spindled nuclei, scant cytoplasm, numerous mitotic figures, central necrosis, and dense fibrous stroma. Immunohistochemical stains showed the tumor to be strongly positive for neuron-specific enolase and faintly positive for cytokeratin and chromogranin.
CT showed a soft-tissue mass expanding and eroding the left maxillary sinus (Fig 2). Medially, the mass extended into the nasal cavity and the nasopharynx. The left half of the hard palate and ethmoid air cells were destroyed. There was also erosion of the floor of the sphenoid sinus with extension into the sinus. Superiorly, the floor of the left orbit was eroded with intraorbital extension. Anteriorly, the mass extended into the adjacent subcutaneous soft tissues of the maxillary region. There was no evidence of calcification in the mass. No lymphadenopathy was present at this time in the neck.
fig 2.
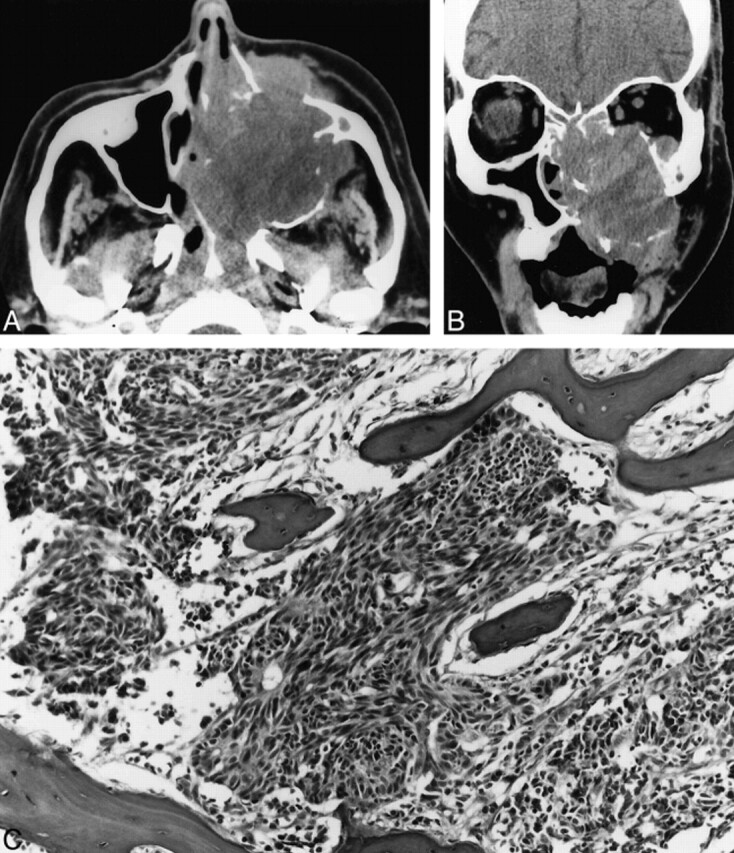
Case 2. A, Axial unenhanced CT scan shows a large expansile maxillary sinus mass with extension into the nasal cavity, the nasopharynx, the infratemporal fossa, and the subcutaneous tissues of the left maxillary region.
B, Coronal unenhanced CT scan shows a large expansile mass in the left maxillary sinus that erodes the sinus walls and extends into the nasal cavity, left orbit, and subcutaneous soft tissues of the maxillary region.
C, Poorly differentiated neuroendocrine carcinoma infiltrating maxillary bone. The tumor consists of small pleomorphic spindled cells with hyperchromatic nuclei. Focal rosette formation is noted (hematoxylin and eosin stain; original magnification, ×10).
The patient initially underwent chemotherapy with VP-16 and cisplatin and local radiotherapy, resulting in significant reduction of the tumor size. A left radical maxillectomy with bilateral modified radical neck dissection was subsequently performed, 10 months from the time of tumor detection. The cervical lymph nodes showed metastases to multiple nodes bilaterally in levels I through V. The patient did apparently well for 2 months after surgery and then developed hepatosplenomegaly with evidence of liver metastases. He died as a result of the disease shortly thereafter.
Case 3
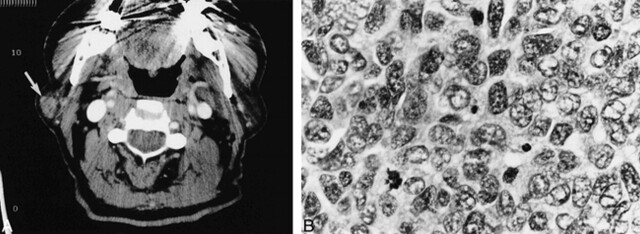
A 64-year-old Caucasian man presented with a 3-month painless mass in the inferior auricular region on the right side. CT revealed a well-circumscribed, minimally enhancing 1.5-cm mass in the superficial lobe of the right parotid gland (Fig 3). There was no lymphadenopathy in the neck. A histopathologic examination of the resected mass revealed the mass to be an enlarged intraparotid lymph node with nests of pleomorphic cells exhibiting nuclear atypia and frequent mitoses. The tumor also showed positive immunoperoxidase staining for cytokeratin (AE 1/3), chromogranin, and neuron-specific enolase. The additional presence of punctate perinuclear cytokeratin staining was suggestive of metastatic Merkel cell carcinoma (poorly differentiated neuroendocrine carcinoma of the skin). The primary was, however, not identified despite an extensive dermatologic examination. Additionally, the results of CT of the chest and abdomen were unremarkable. The patient underwent six cycles of chemotherapy of VP-16 and cisplatin and local irradiation therapy. He is being followed up closely, and there is no evidence of recurrence.
fig 3.
Case 3. A, Axial contrast-enhanced CT scan shows a mildly enhancing circumscribed mass (arrow) in the lateral aspect of the superficial lobe of the right parotid gland.
B, Metastatic poorly differentiated neuroendocrine carcinoma to an intraparotid lymph node. The tumor cells display pleomorphic vesicular nuclei with finely dispersed chromatin, scant cytoplasm, and a high mitotic rate (hematoxylin and eosin stain; original magnification, ×40).
Discussion
Neuroendocrine tumors encompass a broad spectrum of neoplasms. They are of two types, depending on whether they have a neural or an epithelial origin (4). Those of neural origin represent the paragangliomas, and those of epithelial origin are the neuroendocrine carcinomas. Paragangliomas of the head and neck are well known and are not considered in this report. The cell of origin in cases of neuroendocrine carcinomas is not clearly known. Some think that they are derived from endocrine cells of the dispersed neuroendocrine system, and others theorize that they arise from pluripotential stem cells that are capable of dual epithelial and endocrine differentiation (8).
The neuroendocrine carcinomas are further classified into three subtypes: typical carcinoid tumor (well-differentiated neuroendocrine carcinoma), atypical carcinoid tumor (moderately differentiated neuroendocrine carcinoma), and small cell carcinoma (poorly differentiated neuroendocrine carcinoma) (2, 3). Histologically, typical carcinoid tumor shows uniform and small cells organized in organoid or trabecular architecture. The cells are typically devoid of cellular pleomorphism, mitoses, or necrosis (9). In contrast, the atypical carcinoid tumor and small cell carcinoma manifest cellular pleomorphism, nuclear atypia, frequent mitoses, and necrosis. The clinical behavior of these tumors correlates with their histologic characteristics. Although the typical carcinoid tumors are, for the most part, benign, the atypical carcinoid and small cell neuroendocrine carcinomas can metastasize by lymphatic and hematogenous routes. The small cell neuroendocrine carcinomas are the most aggressive of the three types and frequently manifest metastases at the time of tumor discovery (10). Both the typical and atypical carcinoids are usually nonfunctional (1). The cells of the neuroendocrine carcinomas show characteristic immunoreactivity for markers of epithelial and neuroendocrine differentiation, such as cytokeratin (AE 1/3), chromogranin, and neuron-specific enolase. They also contain argyrophilic cytoplasmic granules and are shown by electron microscopy to have membrane-bound neurosecretory granules (9).
Although there is abundant literature on laryngeal neuroendocrine tumors (5–7), to our knowledge, there are no comprehensive reports on nonlaryngeal neuroendocrine carcinomas of the head and neck. The laryngeal neuroendocrine tumors have an overall male predilection (10), and the same seems to be true of nonlaryngeal neuroendocrine carcinomas of the head and neck. All three of our patients were Caucasian men; there is, however, no reported increased risk for Caucasians. In the larynx, the most common neuroendocrine tumor is the atypical carcinoid (11). Two of our cases were of poorly differentiated neuroendocrine carcinomas (small cell type), whereas one was of a well-differentiated carcinoma.
There are no reports in the radiologic literature of nonlaryngeal neuroendocrine carcinomas in the head and neck. The paranasal sinus neuroendocrine carcinomas in both of our cases showed no distinctive CT or MR imaging characteristics. Both, however, appeared to expand as well as erode the sinus walls. The presence of expansion of the sinus, rather than just destruction, could be a useful indicator that the tumor represents something other than the more usual squamous cell carcinoma. The metastatic parotid neuroendocrine tumor appeared to be circumscribed on the CT scan, and both at surgery and by histopathologic examination, the margins around the mass were seen to be clear. Again, no distinctive CT features of the mass were appreciated to differentiate this lesion from the more common salivary gland neoplasms.
Conclusion
Nonlaryngeal neuroendocrine carcinomas of the head and neck are rare tumors. In the head and neck, the paranasal sinuses seem to be a more common site of involvement. Differentiation from the more common squamous cell carcinoma is important for management and prognostic purposes. The neuroendocrine carcinomas involving the maxillary and sphenoid sinuses showed expansion and erosion of the sinuses, whereas the metastatic intraparotid lymph node presented as a nonspecific circumscribed parotid mass lesion.
Footnotes
Address reprint requests to Prabhakar P. Kesava, MD, Department of Radiology, University of Texas Health Science Center, 7703 Floyd Curl Drive, San Antonio, TX 78284.
References
- 1.Batsakis JG, El-Naggar AK, Luna MA. Neuroendocrine tumors of the larynx. Ann Otol Rhinol Laryngol 1992;101:710-714 [DOI] [PubMed] [Google Scholar]
- 2.Shanmugaratnam K, Sobin LH, Barnes L, et al. Histological Typing of Tumors of the Upper Respiratory Tract and Ear, World Health Organization (International Histological Classification of Tumors). 2nd ed. New York: Springer-Verlag; 1991
- 3.Wenig BM, Hyams VJ, Heffner DK. Moderately differentiated neuroendocrine carcinoma of the larynx. Cancer 1998;62:2658-2676 [DOI] [PubMed] [Google Scholar]
- 4.Erlandson RA, Nesland JM. Tumors of the endocrine/neuroendocrine system. Ultrastruct Pathol 1994;18:149-170 [DOI] [PubMed] [Google Scholar]
- 5.El-Nagger AK, Batsakis JG. Carcinoid tumor of the larynx: a critical review of the literature. ORL J Otorhinolaryngol Relat Spec 1991;53:188-193 [DOI] [PubMed] [Google Scholar]
- 6.El-Nagger AK. Laryngeal neuroendocrine carcinoma: victims of semantics. Arch Pathol Lab Med 1992;116:237-238 [PubMed] [Google Scholar]
- 7.Ferlito A, Rosai J. Terminology and classification of neuroendocrine neoplasms of the larynx. ORL J Otorhinolaryngol Relat Spec 1991;53:185-187 [DOI] [PubMed] [Google Scholar]
- 8.Wenig BM, Gnepp DR. The spectrum of neuroendocrine carcinomas of the larynx. Semin Diagn Pathol 1989;6:329-350 [PubMed] [Google Scholar]
- 9.David SM, Haskins KK. Pathological quiz case 2. Arch Otolaryngol Head Neck Surg 1998;124:219-2229485118 [Google Scholar]
- 10.Woodruff JM, Senie RT. Atypical carcinoid tumor of the larynx: a critical review of the literature. ORL Otorhinolaryngol Relat Spec 1991;53:194-209 [DOI] [PubMed] [Google Scholar]
- 11.Ferlito A, Milroy CM, Wenig BM, Barnes L, Silver CE. Laryngeal paraganglioma versus atypical carcinoid tumor. Ann Otol Rhinol Laryngol 1995;104:78-83 [DOI] [PubMed] [Google Scholar]