Abstract
BACKGROUND AND PURPOSE: Although phase-contrast MR angiography provides some information regarding hemodynamics of cerebral arteriovenous malformations (AVMs), most conventional MR angiographic techniques have not been helpful in this respect. We attempted to determine the value of MR digital subtraction angiography (DSA) in assessing AVM hemodynamics.
METHODS: We developed an MR DSA technique by combining rapid thick-section T1-weighted imaging with a bolus injection of contrast material. The temporal resolution was 0.56 to 0.61 seconds per scan. MR DSA images obtained from 14 patients with AVMs were reviewed. Anatomic depiction of each component of the AVM was rated using a four-point grading scale (excellent = 3, good = 2, fair = 1, poor = 0) to compare conventional vs MR angiograms.
RESULTS: We were able to obtain serial images in which passage of contrast material was evident within the AVM, although the sequence we used allowed images to be obtained in only one projection. The average score for feeders, nidi, and drainers was 1.6, 2.4, and 2.3, respectively, with an overall average of 2.1.
CONCLUSION: The spatial resolution of our technique may fall below the level needed for identification of small vascular components of an AVM. Additionally, the limited slab may restrict application of the technique to assessment of large or very small AVMs. MR DSA, however, can show the hemodynamics of AVMs and may serve as a supplement to conventional MR imaging in the diagnosis of cerebral AVMs.
Conventional angiography remains the standard of reference for the diagnosis of cerebral arteriovenous malformations (AVMs). Nevertheless, several MR angiographic techniques have been applied as less invasive means of diagnosis. Among them, a 3D phase-contrast technique (1–4) has been widely used, and a contrast-enhanced 3D time-of-flight (TOF) technique (5–7) has been another option. These MR angiographic techniques provide good depiction of the anatomic features of AVMs; however, they generate static images that do not allow assessment of the hemodynamics of AVMs. The status of flow through AVMs is believed to be one of the risk factors in predicting their propensity to hemorrhage (8, 9). Change of flow is also of clinical significance after endovascular or radiation therapy to evaluate the effect of treatment.
We developed an MR digital subtraction angiography (DSA) technique by combining rapid 2D T1-weighted imaging with a bolus injection of contrast material. Time-resolved contrast-enhanced 3D MR angiography is already available for the characterization of vessels of several regions (10–13); however, reports on the application of the 2D technique to the intracranial vasculature are limited (14, 15). Our 2D technique enables the acquisition of serial vascular images in a short time frame. The purpose of this study was to evaluate its usefulness in the assessment of hemodynamics of cerebral AVMs.
Methods
MR DSA images obtained from 14 patients with AVMs (seven men and seven women, 9 to 66 years old) were reviewed. The diagnosis of AVM had been established by conventional angiography (10 patients) or was suggested by MR imaging and MR angiographic findings, and later confirmed by conventional angiographic studies (four patients). Among the 14 AVMs, 10 were located in the cerebral hemisphere, two were in the cerebellum, and two were deep-seated (one in the basal ganglia and one in the thalamus).
MR DSA was performed on a 1.5-T imager using turbo fast low-angle shot (turbo-FLASH) sequences. The imaging parameters were as follows: TR/TE, 3.3–5.0/1.4–2.3; TI, 50–300; flip angle, 8–15°; imaging matrix, 80–119 × 128; section thickness, 10–20 mm; field of view, 156–250 × 220–280 mm; scanning time, 0.56–0.61 seconds per scan; and scanning interval, 0.0–0.39 seconds. Using a power injector, we administered 0.1 mmol/kg of gadopentetate dimeglumine via the antecubital vein at a rate of 2 mL/s starting 0 to 5 seconds after initiation of scanning and continuing for 30 to 40 seconds. From these serial images, we selected a mask image and subtracted it from the images that followed by using the manufacturer's standard system software incorporated in the imager.
We compared MR DSA images with conventional angiograms in 10 patients. In the remaining four patients, MR DSA images were compared with MR angiograms, because conventional angiograms obtained at other institutions were not available for comparison. MR angiography was carried out using contrast-enhanced 3D TOF sequences. Anatomic depiction of each component of the AVM was rated by consensus of two radiologists using a four-point grading scale (excellent = 3, good = 2, fair = 1, poor = 0). Grade 3 was scored when MR DSA depicted each component equally on conventional and MR angiograms, grade 2 when some details were absent on MR DSA images, grade 1 when MR DSA depicted only a part of each component of the AVM, and grade 0 when no part of any component was visible. The two radiologists independently reviewed images and scored them. When their ratings did not agree, a final judgment was reached by consensus. Interobserver concordance at the initial review was evaluated by the κ test.
Results
At the initial assessment of feeders, nidi, and drainers, the two observers' ratings agreed in 11 (79%), 11 (79%), and 12 (86%) cases, respectively, with κ values of 0.74 (substantial reproducibility), 0.64 (substantial reproducibility), and 0.77 (substantial reproducibility), respectively.
In each case, we were able to obtain serial images in which passage of contrast material was seen within the AVM as well as in the surrounding normal vessels. Feeders, nidi, and drainers within the scanned section were identified separately. Arteries, however, frequently remained visible when veins were depicted. The average score for feeders, nidi, and drainers was 1.6, 2.4, and 2.3, respectively, with an overall average of 2.1.
Representative cases are illustrated in Figures 1 through 3.
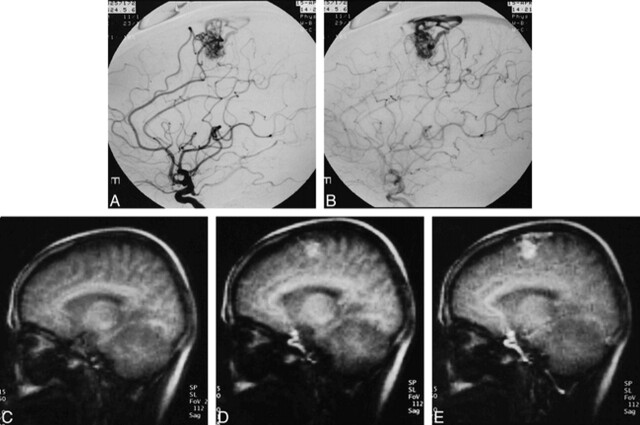
fig 1.
48-year-old woman with left frontal AVM.
A and B, Conventional angiograms show an AVM fed by branches of the anterior cerebral artery. It drains into the superior sagittal sinus via several draining veins.
C–G, Original images of MR DSA (5/2.3/1) in the sagittal plane. C is a mask image. Scans for D, E, F, and G were initiated 1.22 seconds, 2.44 seconds, 4.27 seconds, and 7.93 seconds after C, respectively. Scanning time for each image was 0.61 seconds.(cont'd) →
Discussion
Three-dimensional phase-contrast MR angiography with a proper setting for velocity encoding has been widely used in the evaluation of cerebral AVMs (1–4). This technique can provide anatomic information as well as details on flow direction and velocity. Other methods used to assess AVMs effectively include the 2D phase-contrast technique with variable velocity encodings and a 3D TOF technique with or without multiple overlapping thin-slab acquisition. It has been reported that administration of contrast material is useful for visualizing AVMs with both phase-contrast and TOF techniques (3, 5–7, 16); however, these methods provide only static images of AVMs. Our goal in this study was to depict the hemodynamics of AVMs by using 2D turbo-FLASH sequences, which provide excellent temporal resolution.
Although they are not understood completely, the hemodynamics of AVMs are considered to be a risk factor for AVM hemorrhage, along with a history of bleeding, large size, deep venous drainage, and intranidal aneurysm (8, 9, 17). In relation to the hemodynamics of AVMs, it has been reported that feeding artery pressure is greater in a ruptured AVM than in an unruptured AVM (8, 9). Meanwhile, large and high-flow AVMs are reported to be frequently accompanied by perioperative hyperemic complications (18–23). Therefore, assessment of flow plays an important part in the radiologic diagnosis of AVMs. In addition to characterizing flow dynamics, we hoped to be able to depict fine vessels with the use of the subtraction technique. Although the temporal resolution did not exceed that of conventional angiography, it was possible to identify the flow of contrast material through each component of the AVM. Thus, our results suggest that this application can be used to ensure the diagnosis of an AVM suggested at conventional MR imaging and MR angiography.
As compared with phase-contrast MR angiography, which can provide information about blood flow of AVMs (eg, flow speed and direction), our technique is advantageous in that AVM hemodynamics can be assessed visually. To our knowledge, there have been no reports of a technique similar to ours in which 3D data acquisition was used to evaluate intracranial AVMs or other vascular lesions. At present, the scan time per frame in 3D MR DSA limits its ability to assess physiologic information concerning AVM hemodynamics. Although not included in the present series, the use of subtraction seems to be quite effective in cases associated with hematoma. This is because misleading signals from hematoma are deleted and only those from vessels are displayed. MR DSA can also be of use in the follow-up period after embolization or radiosurgery of AVMs. Blood flow through an AVM can decrease after such therapies, with no significant anatomic change visible at angiography. We consider our technique to be valuable in the follow-up of hemodynamic changes because it is less invasive than conventional angiography.
Nevertheless, the technique has several limitations. First, spatial resolution is still inadequate, as evidenced by the low-average score for feeders, which are generally smaller than nidi and drainers. This concern may be a trade-off, considering the size of the imaging matrix in the phase-encoding direction. Application of more rapid imaging techniques, such as the partial Fourier method, could be effective in this regard. Second, the section thickness we used was 10 to 20 mm, which was inadequate to cover relatively large AVMs (Fig 3). But when thick sections are used, overlapping vessels may hamper visualization of the AVM's details. Third, in our experience, arteries such as the carotid siphon frequently remained visible when veins were depicted (Fig 1). This probably happened because the contrast material was not injected in an adequate bolus. We assume that this could make the evaluation of hemodynamics with our technique difficult as compared with the use of conventional angiography. We consider further investigations necessary to evaluate the optimal dose and rate of contrast material injection. Fourth, it took more than 20 minutes to generate one series of MR DSA images, because we had to perform subtraction by commanding the system for each frame. We expect that postprocessing can be achieved more easily and in a more timely manner in the near future, as new software becomes available.
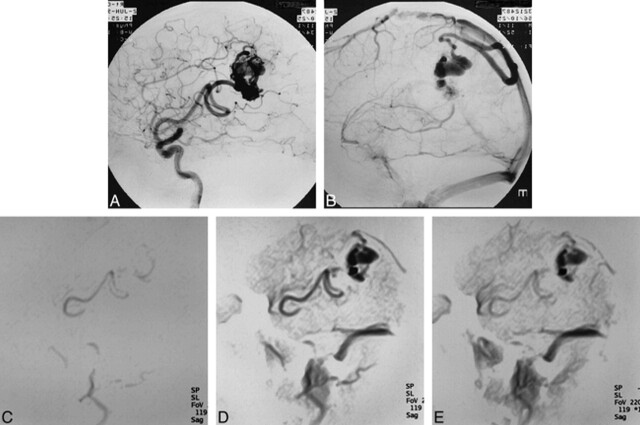
fig 3.
66-year-old woman with right parietal AVM.
A and B, Conventional angiograms show an AVM fed by the angular artery draining into the superior sagittal sinus via an enlarged cortical vein.
C–E, MR DSA images (5/2.3/1) in the sagittal plane clearly show the nidus and drainer. Part of the feeder just proximal to the nidus is not visible because it is outside the scanned section (feeder = 2, nidus = 3, drainer = 3).
Conclusion
The MR DSA technique we developed has limited value at present in depicting the anatomic details of cerebral AVMs. It, however, can depict AVM hemodynamics. We believe that MR DSA could be a supplement to conventional MR imaging in patients with cerebral AVMs at both initial diagnosis and at follow-up after therapy.
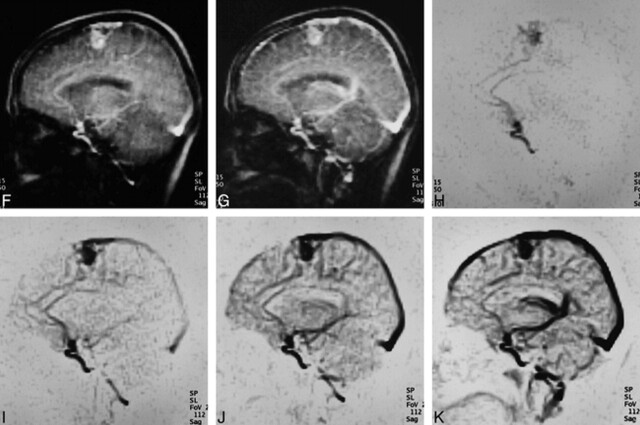
fig 1.
← (cont'd) H–K, Gray-scale–reversed MR DSA images of H, I, J, and K correspond to source images of D, E, F, and G, respectively. Demonstration of each component of the AVM was graded as excellent (feeder = 3, nidus = 3, drainer = 3). Note the carotid siphon remains visible when major veins and dural sinuses are shown (K)
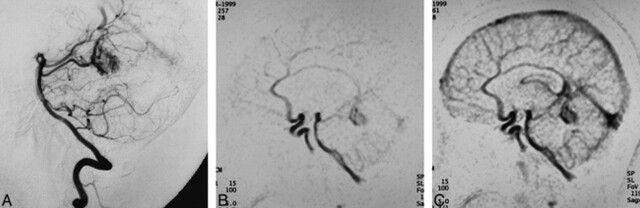
fig 2.
24-year-old woman with cerebellar vermian AVM.
A, Conventional angiogram shows a nidus of an AVM fed by the superior cerebellar artery draining to the vein of Galen.
B and C, MR DSA images (5/2.3/1) in the sagittal plane clearly show each component of the AVM (feeder = 3, nidus = 3, drainer = 3).
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, San Diego, May 1999.
References
- 1.Huston J III, Rufenacht DA, Ehman RL, Wiebers DO. Intracranial aneurysms and vascular malformations: comparison of time-of-flight and phase-contrast MR angiography. Radiology 1991;181:721-730 [DOI] [PubMed] [Google Scholar]
- 2.Marks MP, Pelc MJ, Ross MR, Enzmann DR. Determination of cerebral blood flow with a phase-contrast cine MR imaging technique: evaluation of normal subjects and patients with arteriovenous malformation. Radiology 1992;182:467-476 [DOI] [PubMed] [Google Scholar]
- 3.Turski P, Korosec F. Phase contrast angiography. In: Anderson CM, Edelman R, Turski P, eds. Clinical Magnetic Resonance Angiography. New York: Raven; 1993:43-72 [Google Scholar]
- 4.Pant B, Sumida M, Arita K, Tominaga A, Ikawa F, Kurisu K. Usefulness of three dimensional phase contrast MR angiography on arteriovenous malformations. Neurosurg Rev 1997;20:171-176 [DOI] [PubMed] [Google Scholar]
- 5.Marchal G, Michiels J, Bosmans H, Van Hecke P. Contrast-enhanced MRA of the brain. J Comput Assist Tomogr 1992;16:25-29 [DOI] [PubMed] [Google Scholar]
- 6.Runge VM, Kirsch JE, Lee C. Contrast-enhanced MR angiography. J Magn Reson Imaging 1993;3:233-239 [DOI] [PubMed] [Google Scholar]
- 7.Jung HW, Chang KH, Choi DS, Han MH, Han MC. Contrast-enhanced MR angiography for the diagnosis of intracranial vascular diseases: optimal dose of gadopentetate dimeglumine. AJR Am J Roentgenol 1995;165:1251-1255 [DOI] [PubMed] [Google Scholar]
- 8.Kader A, Young WL, Pile-Spellman J, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery 1994;34:801-808 [DOI] [PubMed] [Google Scholar]
- 9.Spetzler RF, Hargraves RW, McCormick PW, Zarbramski JM, Flom RA, Zimmerman RA. Relationship of perfusion pressure and size to the risk of hemorrhage from arteriovenous malformations. J Neurosurg 1992;76:918-923 [DOI] [PubMed] [Google Scholar]
- 10.Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med 1996;36:345-351 [DOI] [PubMed] [Google Scholar]
- 11.Levy RA, Prince MR. Arterial-phase three-dimensional contrast-enhanced MR angiography of the carotid arteries. AJR Am J Roentgenol 1996;167:211-215 [DOI] [PubMed] [Google Scholar]
- 12.Prince MR, Chenevert TL, Foo TKF, Londy FJ, Ward JS, Maki JH. Contrast-enhanced abdominal MR angiography: optimization of imaging delay time by automating the detection of contrast material arrival in the aorta. Radiology 1997;203:109-114 [DOI] [PubMed] [Google Scholar]
- 13.Foo TKF, Saranathan M, Prince MR, Chenevert TL. Automated detection of bolus arrival and initiation of data acquisition in fast, three-dimensional, gadolinium-enhanced MR angiography. Radiology 1997;203:275-280 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Johnston DL, Breen JF, et al. Dynamic MR digital subtraction angiography using contrast enhancement, fast data acquisition, and complex subtraction. Magn Reson Med 1996;36:551-556 [DOI] [PubMed] [Google Scholar]
- 15.Hori M, Aoki S, Yoshikawa T, et al. Evaluation of two-dimensional thick-slice MR DSA: preliminary study [in Japanese]. Nippon Acta Radiol 1999;59:203-205 [PubMed] [Google Scholar]
- 16.Kesava PP, Turski PA. Magnetic resonance angiography of vascular malformations. Neuroimaging Clin North Am 1998;8:349-370 [PubMed] [Google Scholar]
- 17.Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D. Factors that predict the bleeding risk of cerebral arteriovenous malformations. Stroke 1996;27:1-6 [DOI] [PubMed] [Google Scholar]
- 18.Drake CG. Cerebral arteriovenous malformations: considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg 1979;26:145-208 [DOI] [PubMed] [Google Scholar]
- 19.Wilson CB, U HS, Domingue J. Microsurgical treatment of intracranial vascular malformations. J Neurosurg 1979;51:446-454 [DOI] [PubMed] [Google Scholar]
- 20.Mullan S, Brown FD, Partonas NJ. Hyperemic and ischemic problems of surgical treatment of arteriovenous malformations. J Neurosurg 1979;51:757-764 [DOI] [PubMed] [Google Scholar]
- 21.Bonnal J, Born JD, Hans P. One-stage excision of high-flow arteriovenous malformations. J Neurosurg 1980;52:705-708 [DOI] [PubMed] [Google Scholar]
- 22.Day AL, Friedman WA, Sypert GW, Mickle JP. Successful treatment of the normal perfusion breakthrough syndrome. Neurosurgery 1982;11:625-630 [DOI] [PubMed] [Google Scholar]
- 23.Pasquilin A, Barone G, Cioffi F, Rosta L, Scienza R, Da Pian R. The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 1991;28:370-379 [DOI] [PubMed] [Google Scholar]