Abstract
BACKGROUND AND PURPOSE: MR findings reported in conjunction with spinal dural arteriovenous fistula (SDAVF) include cord swelling, increased T2 signal within the spinal cord, and parenchymal enhancement, each of which is nonspecific. Enlarged vessels on the cord surface, the most specific MR finding, is noted in only half of SDAVF patients. Nevertheless, we have frequently observed MR peripheral hypointensity of the spinal cord in SDAVF on T2-weighted images, which is not characteristic of nonvascular or nonhemorrhagic causes of myelopathy and which has not been described in association with SDAVF. We hypothesized that peripheral cord hypointensity might reliably suggest the diagnosis of SDAVF or other causes of venous hypertensive myelopathy.
METHODS: We reviewed the MR findings in 11 consecutive cases of angiographically confirmed symptomatic SDAVF and in four cases of intracranial dural arteriovenous fistula with spinal drainage, a lesion that also causes spinal cord deficits mediated by venous hypertensive myelopathy.
RESULTS: In each case, T2 hypointensity involving the cord periphery was present. This sign has not been previously described in association with either SDAVF or other causes of venous hypertensive myelopathy. It appears, however, to be a relatively constant imaging feature of SDAVF.
CONCLUSION: In the absence of spinal hemorrhage, T2 hypointensity involving the periphery of the spinal cord suggests venous hypertensive myelopathy as a cause of spinal cord dysfunction.
Nonhemorrhagic venous hypertensive myelopathy (VHM) results from a small but clinically significant group of vascular lesions affecting the spinal cord that are characterized by arteriovenous shunting. Most often, VHM results from spinal dural arteriovenous fistulas (SDAVFs), acquired lesions in which arteriovenous shunting of blood from radicular arteries drains into the pial veins of the spinal cord (1–5). The clinical significance of VHM lies in its often confusing clinical presentation, which frequently leads to delays in diagnosis (1). Delayed diagnosis combined with the potential to cause devastating neurologic deficits, which are preventable or reversible if diagnosed and treated early, make prompt recognition of VHM essential to prevent permanent disability (6, 7).
Diagnosis of VHM constitutes a significant challenge from a radiologic standpoint. MR imaging often permits noninvasive diagnosis of VHM caused by SDAVF and has increased its recognition as an important cause of progressive myelopathy. MR findings previously reported with VHM have included cord enlargement, intramedullary enhancement, engorgement of pial veins, and increased signal intensity on T2-weighted images (8–11). We report an additional imaging sign, T2 hypointensity involving the cord periphery, which appears to be frequently present in VHM and to suggest the diagnosis reliably (Fig 1). We attempted to determine whether this sign was a frequent and reliable finding in SDAVF and how it might reflect the underlying pathophysiologic mechanisms of VHM.
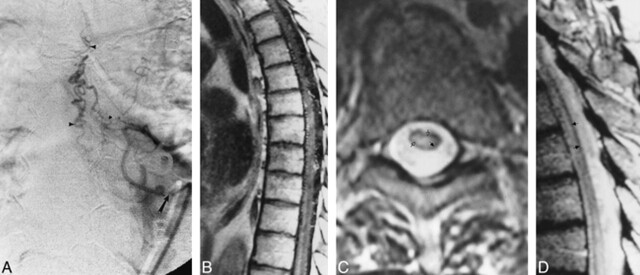
Fig 1. A, Anteroposterior view of spinal angiogram (injection of intercostal artery) shows filling of an SDAVF with pial drainage (arrowheads; arrow indicates catheter tip).
B, Sagittal contrast-enhanced T1-weighted (500/11) MR image shows enhancing enlarged pial vessels as well as intrinsic cord enhancement.
C, Axial FSE T2-weighted (4500/90) image shows peripheral hypointensity (closed arrow) with increased central cord signal (open arrows).
D, Sagittal FSE T2-weighted (2700/85eff) image shows enlarged pial vessels, central increased signal, and peripheral hypointense rim (arrows).
Methods
We reviewed 1.5-T MR images of 11 consecutive patients seen at this institution with angiographically confirmed SDAVF. Each patient had clinically symptomatic VHM resulting from the SDAVF. We then analyzed imaging findings in four consecutive patients who were symptomatic from spinal drainage caused by intracranial dural arteriovenous fistulas. All patients with an intracranial dural arteriovenous fistula also had angiographic confirmation of the fistula and its drainage into the cervical spinal cord.
All patients underwent T1-weighted and fast spin-echo (FSE) T2-weighted imaging. Contrast-enhanced sagittal T1-weighted images were obtained in 10 of 11 patients with SDAVF and in all patients with intracranial dural arteriovenous fistulas. Proton density–weighted (2500/20), true T2-weighted (2500/80), and gradient-echo (117/20) images were also obtained in one patient with SDAVF and compared with the FSE images. Images were reviewed by both authors in a nonblinded fashion.
Results
In the SDAVF patients, we identified the following abnormalities on MR images: cord enlargement in six (55%) of 11; increased central cord signal on T2-weighted images in 10 (100%) of 10; visible surface vessels in 10 (91%) of 11 on T2-weighted images and in seven (70%) of 10 on contrast-enhanced images; and parenchymal cord enhancement in nine (90%) of 10 (Fig 2).
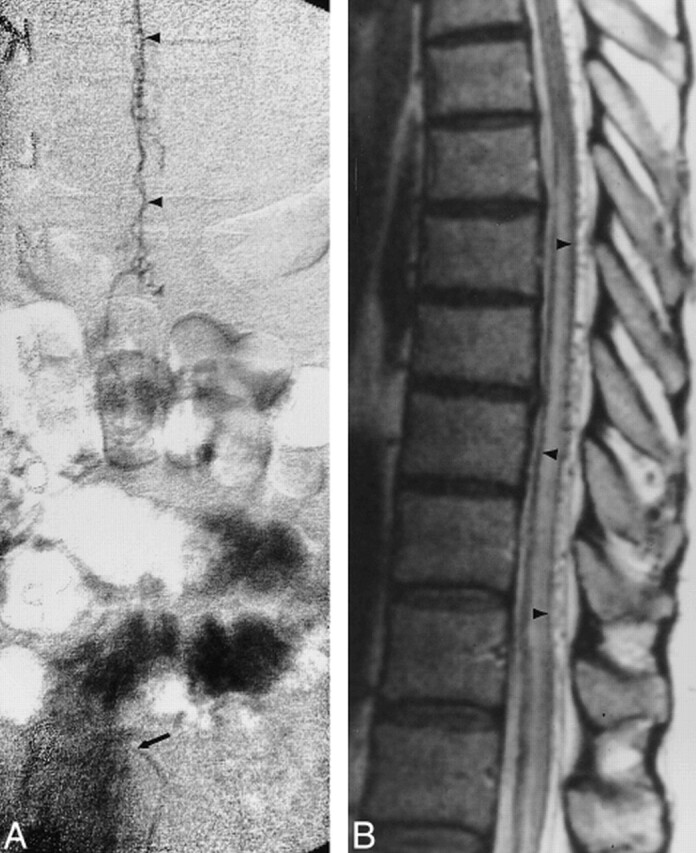
Fig 2. A, Anteroposterior view of spinal angiogram shows early filling of pial venous drainage (arrowheads) from spinal DAVF (arrow indicates catheter tip).
B, Sagittal FSE T2-weighted (5600/100eff) MR image shows enlarged pial vessels, central increased cord signal, and rim of peripheral hypointensity (arrowheads).
In addition, each patient had a rim of hypointense signal involving the periphery of the spinal cord on T2-weighted images. While sometimes subtle on FSE T2-weighted images, the finding became much more conspicuous on true T2-weighted and gradient-echo images. It was identified in patients with SDAVF originating from thoracic, lumbar, and sacral levels (Fig 3).
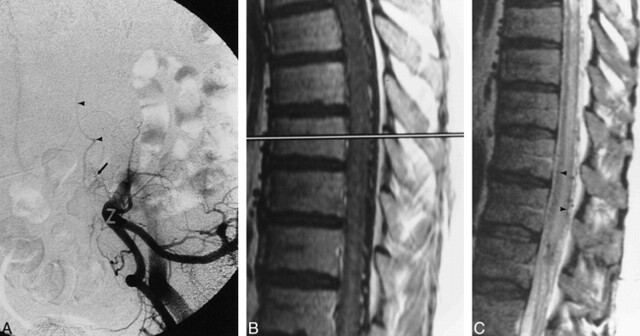
Fig 3. A, Anteroposterior subtracted view of early arterial-phase injection of the left hypogastric artery shows dural AVF (arrow) with sacral feeding vessels. Arrowheads signify draining veins.
B, Sagittal contrast-enhanced T1-weighted (400/14) MR image shows intraparenchymal cord enhancement as well as enhancement of enlarged vessels on the cord surface.
C, Sagittal FSE T2-weighted (4400/100eff) image shows multiple flow voids on the cord surface, increased signal intensity within the central cord, as well as a rim of peripheral hypointensity (arrowheads).
In the patients with intracranial dural arteriovenous fistulas, MR findings of cord swelling, increased T2 signal involving the central portion of the spinal cord, and cord parenchymal enhancement were present in each case. Abnormal vessels were visible on FSE T2-weighted images in two (50%) of the four patients and on contrast-enhanced images in 100% of patients. A rim of decreased signal involving the periphery of the spinal cord was also present on FSE T2-weighted images in each patient (Fig 4).
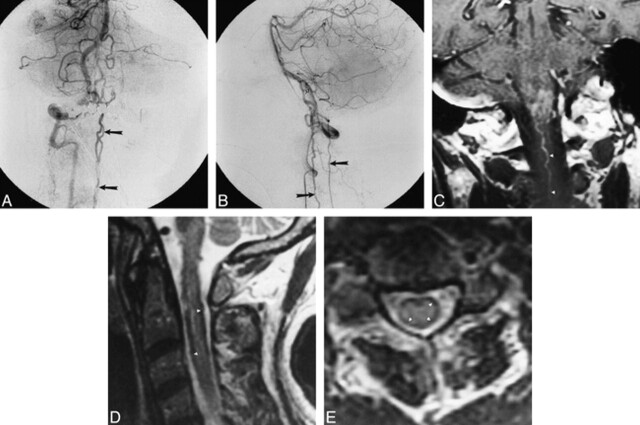
Fig 4. A and B, Late arterial-phase anteroposterior (A) and lateral (B) right vertebral artery injection shows filling of dural arteriovenous fistula (open arrow, B) originating from intradural vertebral artery. Drainage along cervical spinal cord is visible in both views (closed arrows).
C, Coronal contrast-enhanced MR image shows intraaxial cervicomedullary enhancement and enlarged pial cervical vein (arrowheads).
D and E, Sagittal (3733/100eff) (D) and axial (4000/100eff) (E) T2-weighted images of cervical cord show subpial hypointensity (arrowheads).
Discussion
SDAVFs are the most common type of spinal vascular abnormality. These acquired lesions represent arteriovenous shunts involving a small area of dura with afferent supply from a radicular artery. Arterialized drainage from the SDAVF flows into the pial veins of the spinal cord, causing enlargement and engorgement of the cord venous system. The resultant VHM is responsible for the progressive neurologic deficits caused by the lesions. Hemorrhage is not associated with SDAVF (1–5).
A number of investigators have examined MR imaging findings in VHM resulting from SDAVF. Reported findings include cord swelling, enlarged pial veins, increased signal intensity on T2-weighted images, and enhancement of the cord parenchyma (8–11). In the largest published study, Gilbertson et al (10) examined MR findings in 66 patients with SDAVF and reported increased T2 signal in the cord (100%), contrast enhancement (88%), cord enlargement (45%), and flow voids on the cord surface (on 35% of T1-weighted images and on 45% of T2-weighted images).
The most constantly reported MR finding has been increased signal in the spinal cord parenchyma on T2-weighted images. This finding is, however, extremely nonspecific and may result from infection, inflammatory disorders, demyelinating disease, vasculitis, cord neoplasm, and a host of other sources of spinal cord dysfunction.
The MR feature considered most specific for VHM, that of enlarged pial veins, has been reported in less than half the confirmed cases. Our finding of visible spinal vessels in 10 of 11 patients represents an improvement in sensitivity. This may reflect advancements in MR techniques since the publication of previous studies or may simply reflect the smaller number of patients in our group. Nevertheless, hypothesizing the diagnosis of SDAVF on the basis of noninvasive imaging findings remains difficult and requires spinal angiography for confirmation.
A feature we observed in all our patients with SDAVF was peripheral hypointensity of the spinal cord on T2-weighted images (Fig 1). This imaging feature has not been previously reported in association with SDAVF or other nonhemorrhagic spinal cord disorders and may be subtle, particularly on FSE images, which minimize susceptibility differences. Nevertheless, the finding was present on gradient-echo as well as on true T2-weighted sequences, both of which considerably increase the conspicuity of peripheral hypointensity over FSE sequences. The sign was present in each patient symptomatic with angiographically confirmed SDAVF and might reflect the presence of VHM.
We also examined patients with intracranial dural arteriovenous fistulas with spinal venous drainage identical to that seen in patients with SDAVF in whom VHM developed (12–14). We confirmed peripheral T2 hypointensity involving the spinal cord in each of four symptomatic patients with spinal drainage from intracranial dural arteriovenous fistulas (Fig 4), which supports the conclusion that the sign is associated with the diagnosis of VHM regardless of the inciting lesion.
Pathologic changes found in VHM include pial venous engorgement, parenchymal edema, ischemia, and venous infarction (4, 15–18). These features underlie the most commonly identified MR findings, including enlarged surface vessels, cord swelling, and increased T2 signal within the cord. None of these pathologic findings would be expected to decrease spinal cord parenchymal signal on T2-weighted images. We hypothesized that the observed T2 shortening is a result of slow flow of blood containing deoxyhemoglobin within a distended spinal cord capillary and venous system.
Pathologic studies of spinal venous hypertension have identified dilatation involving the pial veins on the cord surface that extends retrogradely into the venous system within the cord parenchyma. The surface hypointensity occurs where the effects of venous dilatation and slow flow would be expected to be most prominent. The finding was present in each patient but was more conspicuous in some than in others. The variable conspicuity of the sign may be related to the degree of venous hypertension. Each of our patients had relatively severe spinal cord dysfunction as evidenced by a symptomatic course of at least 1 year and fixed neurologic deficits resulting from the cord VHM.
Differences in T2 weighting alter the conspicuity of the sign. The use of FSE sequences minimizes susceptibility differences relative to true T2-weighted images (19, 20). Use of true T2-weighted and gradient-echo sequences results in increased conspicuity of the finding (Fig 5).
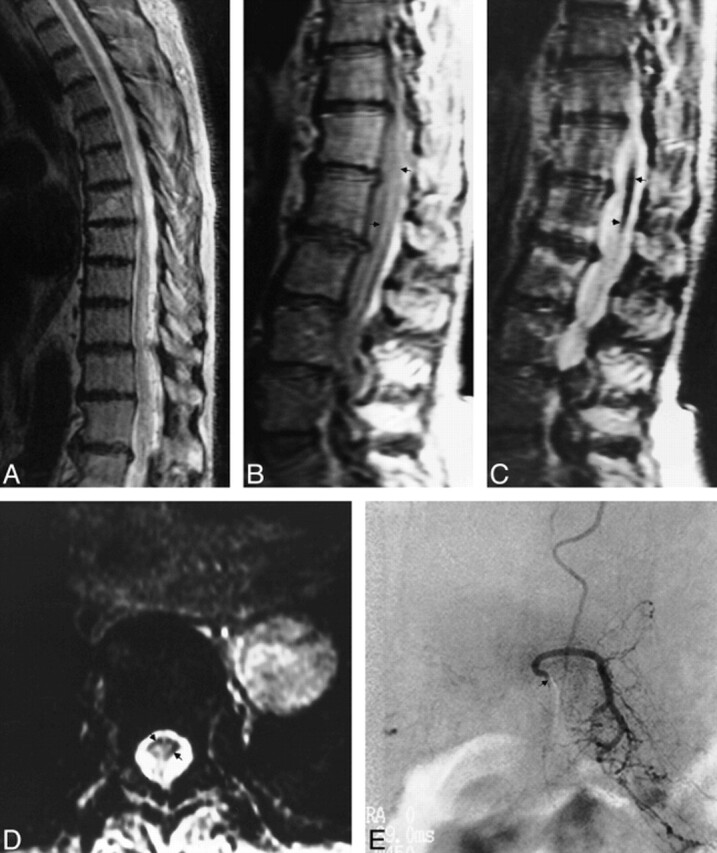
Fig 5. A, Sagittal FSE T2-weighted (5417/98) MR image shows increased central cord signal with subtle peripheral hypointensity.
B and C, Sagittal proton density–weighted (2500/20) (B) and true T2-weighted (2500/80) (C) images show decrease in cord signal intensity (arrows).
D, Axial gradient-echo (117/20) image shows increased conspicuity of peripheral hypointensity (arrows).
E, Anteroposterior view of left L1 injection shows filling of dural arteriovenous fistula (arrow indicates catheter tip).
A second but less likely mechanism for T2 shortening is accumulation of paramagnetic substances within spinal cord parenchyma affected by venous hypertension. Staining for such compounds, including those containing both iron and calcium, has been confirmed in studies of VHM (4, 5, 15, 17, 18, 21). Nevertheless, the hypointensity seen in our patients with VHM was not nearly as marked and was more stereotyped than has been reported from paramagnetic effects. None of our patients had hypointensity visible on T1-weighted images. In addition, staining indicated that paramagnetic substances were usually distributed uniformly throughout the spinal cord parenchyma. VHM-associated hypointensity, in contrast, demonstrates exquisite localization to the pial surface. These features suggest that paramagnetic deposition probably played a minor role, if any, in the T2 hypointensity seen in our patients.
Hemorrhage, a phenomenon well known to result in T2 shortening, may result from other spinal vascular lesions, such as spinal cord arteriovenous malformations or cavernous malformations, but has not been reported in conjunction with SDAVF. None of our patients had a clinical or radiologic picture that would have suggested spinal hemorrhage. Although not specifically mentioned, the sign may be identified in previously published cases of SDAVF. It may be seen on the FSE T2-weighted image in the study by Bowen et al (8) of MR angiography in SDAVF. Willinsky et al (22), in the investigation of posttreatment MR findings in SDAVF, provided additional evidence for both the presence and origin of peripheral hypointensity. In their study, peripheral hypointensity was seen on the pretreatment T2-weighted MR image but was not evident on the study done 17 months after treatment. The resolution of the sign further supports the conclusion that the hypointensity is due to deoxygenated blood in distended pial veins, which return to a more normal size after obliteration of the fistula.
Conclusion
Our review of 11 patients with SDAVF and symptomatic VHM reveals that T2 signal loss involving the peripheral portions of the spinal cord is a constant finding. The identical finding in four patients with intracranial dural arteriovenous fistula with spinal drainage, a disorder that also causes symptomatic VHM, suggests that peripheral T2 hypointensity is a reliable marker for the diagnosis of VHM. This MR finding has not been previously reported and, in the presence of increased central cord signal, may suggest a more specific diagnosis of VHM, the most common vascular mechanism of spinal cord dysfunction.
Footnotes
Address reprint requests to Robert W. Hurst MD.
References
- 1.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine: clinical features and surgical results in 55 cases. J Neurosurg 1984;60:238-247 [DOI] [PubMed] [Google Scholar]
- 2.Muraszko K, Oldfield E. Vascular malformations of the spinal cord and dura. Neurosurg Clin North Am 1990;1:631-652 [PubMed] [Google Scholar]
- 3.Morgan M, Marsh W. Management of spinal dural arteriovenous malformations. J Neurosurg 1989;70:832-836 [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Kim R, Choi B. Nontraumatic ischemic myelopathy: a review of 25 cases. Paraplegia 1988;26:262-268 [DOI] [PubMed] [Google Scholar]
- 5.Hurst R, Kenyon L, Lavi E, Raps E, Marcotte P. Spinal dural arteriovenous fistula: the pathology of venous hypertensive myelopathy. Neurology 1995;45:1309-1313 [DOI] [PubMed] [Google Scholar]
- 6.Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery 1997;40:675-682 [DOI] [PubMed] [Google Scholar]
- 7.Tacconi L, Lopez I, Symon L. Outcome and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas: a long term study. Br J Neurosurg 1997;11:298-305 [DOI] [PubMed] [Google Scholar]
- 8.Bowen B, Fraser K, Kochan J, Pattany P, Green B, Quencer R. Spinal dural arteriovenous fistulas: evaluation with MR angiography. AJNR Am J Neuroradiol 1995;16:2029-2043 [PMC free article] [PubMed] [Google Scholar]
- 9.DeMarco J, Dillon W, Halbach V, Tsuruda J. Dural arteriovenous fistulas: evaluation with MR imaging. Radiology 1990;175:193-199 [DOI] [PubMed] [Google Scholar]
- 10.Gilbertson J, Miller G, Goldman M, Marsh W. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol 1995;16:2049-2057 [PMC free article] [PubMed] [Google Scholar]
- 11.Isu T, Iwasaki Y, Akino M, Koyanagi I, Abe H. Magnetic resonance imaging in cases of spinal dural arteriovenous fistula. Neurosurgery 1989;24:919-923 [DOI] [PubMed] [Google Scholar]
- 12.Brunereau L, Gobin Y, Meder J, Cognard C, Tubiana J, Merland J. Intracranial dural arteriovenous fistulas with spinal venous drainage: relation between clinical presentation and angiographic findings. AJNR Am J Neuroradiol 1996;17:241-249 [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Chen C, Lin T. Enhanced cervical MRI in identifying intracranial dural arteriovenous fistulae with spinal perimedullary venous drainage. Neuroradiology 1998;40:393-397 [DOI] [PubMed] [Google Scholar]
- 14.Hahnel S, Jensen O, Geletnekyu K. MR appearance of an intracranial dural arteriovenous fistula leading to cervical myelopathy. Neurology 1998;51:1131-1135 [DOI] [PubMed] [Google Scholar]
- 15.Adams J, Duchen L. Greenfield's Neuropathology.. New York: Oxford University Press; 1992:1107-1110 [Google Scholar]
- 16.Hurst R, Hackney D, Goldberg H, Davis R. Reversible arteriovenous malformation-induced venous hypertension as a cause of reversible neurological deficits. Neurosurgery 1992;30:422-425 [DOI] [PubMed] [Google Scholar]
- 17.Kim R. Necrotizing myelopathy. AJNR Am J Neuroradiol 1991;12:1084-1086 [PMC free article] [PubMed] [Google Scholar]
- 18.Jellinger K. Pathology of Spinal Vascular Malformations and Vascular Tumors.. Berlin: Springer; 1978:18-44 [Google Scholar]
- 19.Gomori J, Grossman R, Goldberg H, Zimmerman R, Bilaniuk L. Intracranial hematomas: imaging by high-field MR. Radiology 1985;157:87-93 [DOI] [PubMed] [Google Scholar]
- 20.Thulborn K, Waterton J, Matthews P, Radda G. Oxygenation dependence of the transverse relaxation time of water proteins in whole blood at high field. Biochem Biophys Acta 1982;714:265-270 [DOI] [PubMed] [Google Scholar]
- 21.Hughes J. Disorders of the Spine and Spinal Cord.. New York: Wiley; 1990:799-806 [Google Scholar]
- 22.Willensky R, terBrugge K, Montanera W, Mikulis D, Wallace C. Posttreatment MR findings in spinal dural arteriovenous malformations. AJNR Am J Neuroradiol 1995;16:2063-2071 [PMC free article] [PubMed] [Google Scholar]