Abstract
BACKGROUND AND PURPOSE: Deep white matter may be the location of an internal arterial border zone. The purpose of this study was to determine whether the deep white matter was subject to a greater degree of ischemia than was the cortex among patients with chronic carotid occlusion.
METHODS: Thirty-six patients with carotid occlusion and structurally normal deep white matter were studied with positron emission tomography. Measurements of oxygen extraction fraction were made in superficial (cortical and subcortical) regions in the middle cerebral artery territory and in deep white matter (internal border zone) regions. The presence of selective ischemia of the deep white matter was assessed by the ratio of deep white matter:superficial oxygen extraction fraction. Ipsilateral hemispheric ratios among patients were assessed as a group as compared with contralateral hemispheric ratios and as compared with normal hemispheric ratios from 15 control volunteers.
RESULTS: Mean deep white matter to superficial oxygen extraction fraction ratios (±95% confidence limits) were 0.99 (±0.07), 1.01 (±0.06), and 1.02 (±0.08) for ipsilateral, contralateral, and normal hemispheres, respectively. No statistically significant difference was found between ipsilateral and contralateral (P = .691) or normal hemispheres (P = .68), nor was any statistically significant difference found when the analysis was limited to patients with increased superficial oxygen extraction fraction (n = 9). Individual deep white matter:superficial ratios were within the normal range for all patients.
CONCLUSION: Normal deep white matter among patients with carotid occlusion is not subject to a greater degree of ischemia than is the overlying cortex. It is unlikely that deep white matter infarctions observed among patients with carotid occlusion are owing to chronic selective hemodynamic compromise occurring at an internal arterial border zone.
Focal white matter lucency on CT scans or lesions of high signal intensity on T2-weighted MR images are frequently found in adult deep white matter (1, 2). Although nonspecific, there is a strong association between the presence of these lesions and atherosclerotic disease of the ipsilateral carotid artery (3). An association between infarctlike white matter lesions on MR images and a history of stroke has also been reported (2).
This evidence strongly suggests that, among patients with atherosclerotic cerebrovascular disease, these white matter lesions are the results of ischemic injury. The mechanism of ischemia is, however, uncertain. Both embolic and hemodynamic factors may be involved. High signal intensity white matter lesions may be more frequent in a cerebral hemisphere with severe hemodynamic impairment. Yamauchi et al (4) reported an association between high signal intensity centrum semiovale lesions revealed by T2-weighted MR imaging and hemodynamic compromise in the cortical territory of the middle cerebral artery in patients with carotid artery disease (4). Other investigators have reported similar findings (5, 6).
Some investigators have postulated that the deep white matter is the site of an arterial border zone and that it therefore may be more vulnerable to ischemic injury due to hemodynamic factors than is the overlying cortex (7, 8). If border zone hemodynamics is a factor in the development of the infarctlike white matter lesions, one would expect to find greater ischemia in the deep white matter than in the cortex. This selective ischemia occurs in cortical arterial border zone regions with acute hypotension (9, 10). The purpose of this study was to determine whether the deep white matter among patients with chronic carotid occlusion was subject to a greater degree of ischemia than was the overlying cortex.
Many different imaging tools can enable the identification of regional hemodynamic impairment in living humans (11). All are based on the assumption of normal responses of the cerebrovasculature to reduced perfusion pressure. Severe stenosis or occlusion of the carotid arteries or their intracranial branches may cause reduced pressure in the distal arterial system, depending on the degree of stenosis and the adequacy of collateral channels. The first reflex of the cerebrovasculature to a fall in arterial pressure is autoregulatory vasodilation. This serves to maintain cerebral blood flow. With further reductions in arterial pressure, the capacity of autoregulatory vasodilation to maintain flow is overcome and blood flow falls. In this situation, the brain increases the fraction of oxygen it extracts from the blood (oxygen extraction fraction) to maintain normal oxygen metabolism and neuronal function (Fig 1). This is known as misery perfusion or stage 2 hemodynamic compromise (12, 13). The presence of increased oxygen extraction fraction distal to an occluded carotid artery has been proven to be a powerful and independent risk factor for subsequent stroke (14). For the present study, the degree of ischemia in deep white matter and cortical regions was determined with positron emission tomographic measurements of oxygen extraction fraction.
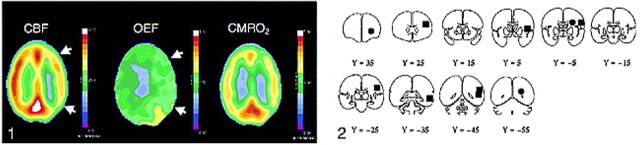
fig 1.
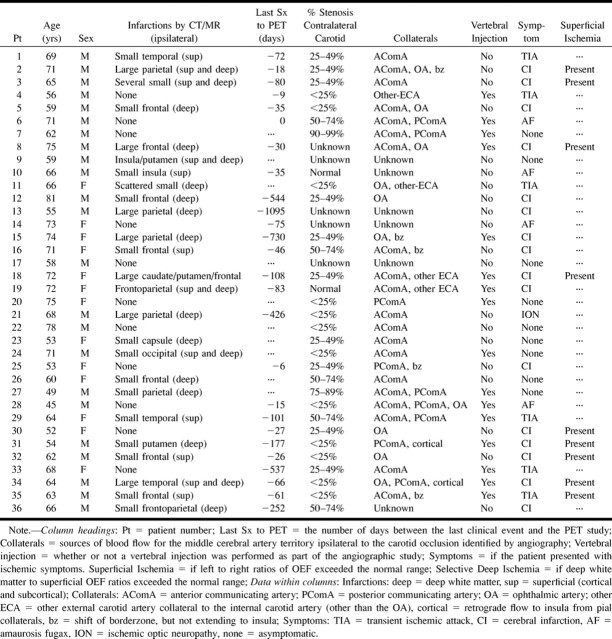
Positron emission tomographic studies show reduced cerebral blood flow (left image) and increased oxygen extraction (middle image) in the cerebral hemisphere distal to an occluded carotid artery. When autoregulatory vasodilation is insufficient to maintain normal cerebral blood flow (CBF, far-left image), CBF will fall (white arrows). The brain, however, can increase the amount of oxygen extracted from the blood (oxygen extraction fraction) (middle image, white arrows), to maintain normal oxygen metabolism and function (cerebral rate of metabolism for oxygen [CMRO2], far-right image). FIG 2. Regions of interest for superficial (black boxes) and deep white matter (black circles) oxygen extraction fraction measurements. Each region was actually a 15-mm sphere with its center in the center of the box or circle depicted in the figure. All positron emission tomographic data were converted to Talairach and Tournoux atlas space for the reproducible placement of these regions (18). The y coordinates for each coronal slice are shown beneath
Methods
Patients
The laboratory records of 117 patients with symptomatic or asymptomatic atherosclerotic carotid artery occlusion enrolled in the St. Louis Carotid Occlusion Study, a blinded prospective study of cerebral hemodynamics and ischemic stroke, were reviewed (14, 15). Ninety-six of the 117 patients had undergone complete quantitative positron emission tomographic studies. CT or MR studies were available for review for 55 of the 96 patients.
The St. Louis Carotid Occlusion Study included 18 healthy control volunteers (age range, 19−77 years; mean age, 45 years ± 18 SD) who were recruited by public advertisement for the purposes of establishing a normal range of cerebral hemodynamic and metabolic values. All underwent neurologic evaluation, MR imaging of the head, and duplex sonography of the extracranial carotid arteries. None had any of the following: 1) signs or symptoms of neurologic disease other than mild distal sensory loss in the legs that was consistent with age, 2) pathologic lesions on MR images (mild atrophy was not considered to be a pathologic finding), or 3) greater than 50% stenosis of the extracranial carotid arteries. All studies were performed under protocols approved by our institution's Human Studies Committee.
Positron Emission Tomographic Measurements of Oxygen Extraction Fraction and Image Analysis
Measurements of oxygen extraction fraction were performed on one of two scanners (ECAT 953B and ECAT EXACT HR; Siemens, Iselin, NJ) as described in previous publications (8, 9). Briefly, these measurements require three separate positron emission tomographic acquisitions after the inhalation of trace amounts of O-15-labeled carbon monoxide (for cerebral blood volume measurement), the inhalation of O-15-labeled oxygen (for oxygen extraction and consumption measurements), and the injection of O-15-labeled water (for cerebral blood flow measurements). Arterial time-activity curves from a radial artery catheter are necessary to convert the measured positron emission tomographic counts to physiologic terms. The quantitative regional map of oxygen extraction was calculated on a pixel-by-pixel basis, using data from quantitative cerebral blood flow images (to account for O-15-labeled recirculating water from tissue metabolism and to account for the delivery of oxygen to the brain) and from quantitative cerebral blood volume images (to correct for intravascular, nonextracted oxygen). These calculations also required the measurement of arterial blood oxygen content and hematocrit (16).
Positron emission tomographic scans were reconstructed with a ramp filter cut off at the Nyquist frequency to produce scans with resolutions of 4.9-mm (953B) or 4.3-mm (EXACT HR) full width at half-maximum. The reconstructed images were not filtered further to minimize partial volume effects from the overlying cerebral cortex on measurements in the deep white matter. All positron emission tomographic data were converted to uniform stereotactic atlas space to allow reproducible placement of regions of interest (17).
For each patient and healthy volunteer, seven spherical regions of interest, 15 mm in diameter, were placed in the territory of the middle cerebral artery in each hemisphere by using stereotactic coordinates (18). These spheres included both cortical and subcortical tissue (Fig 2). Spherical regions of interest, 15 mm in diameter, were placed in three deep white matter regions by using stereotactic coordinates: one anterolateral to the anterior horn of the lateral ventricle, one posterolateral and superior to the atrium of the lateral ventricle, and one in the centrum semiovale (7, 8). Areas of possible previous infarction were identified by a review of the cerebral rate of metabolism for oxygen images and by CT or MR examinations. Neither the regions within these areas nor the corresponding contralateral regions were used for analysis. In addition, patients and healthy volunteers with moderate enlargement of the lateral ventricles were also excluded from the analysis (because of the size constraints of the white matter regions).
Data Analysis
The mean hemispheric value of oxygen extraction fraction was separately calculated for both superficial cortical-subcortical and deep white matter regions. Left-to-right ratios of oxygen extraction fraction were then calculated for superficial regions. The patients were classified as having normal or elevated superficial oxygen extraction fraction, based on comparison with the range of ratios observed in the healthy volunteers (9). Next, ratios of ipsilateral deep white matter to ipsilateral superficial values of oxygen extraction fraction were calculated to assess selective (greater in the white matter) ischemia. These values were compared with the ratios of deep white matter:superficial oxygen extraction fraction found in control volunteers and in the hemispheres contralateral to the occlusion in the study patients (P < .05 accepted for statistical significance). In addition, this analysis was repeated for the subgroup of patients with increased oxygen extraction fraction measured in the superficial regions. Finally, individual ipsilateral deep:superficial ratios were compared with normal and contralateral values. Patients with individual ratios exceeding the range of either normal or contralateral ratios were identified as possibly having selective deep white matter ischemia.
Each of the deep white matter regions was then individually assessed. Centrum semiovale:, anterior:, and posterior deep white matter:superficial oxygen extraction fraction ratios were generated for ipsilateral, contralateral, and normal hemispheres. These ratios were analyzed in an identical manner as the averaged deep white matter ratio for all three regions described in the preceding paragraph. A Bonferroni correction was applied to the significance level for the multiple tests performed (three individual region ratios). This reduced the P value required for statistical significance to .017 (.05 per three ratios).
Results
Clinical
All three deep white matter regions were abnormal, as revealed by CT or MR imaging, in 19 of the 55 patients. Thirty-six patients had at least one normal deep white matter region on CT or MR images that was large enough for accurate positron emission tomographic measurements. Only a single region could be used for seven patients, two regions for 11 patients, and all three regions for 18 patients. The mean age of the patients was 67 years. Twenty-seven patients had previous ipsilateral ischemic symptoms, and nine patients were asymptomatic. The clinical, imaging, and angiographic data are summarized in the Table.
TABLE 1:
Clinical, imaging, angiographic, and positron emission tomographic (PET) characteristics
Two of the 18 healthy volunteers had deep white matter signal abnormalities in all three regions on the MR images and were excluded from this analysis. One additional volunteer had generalized atrophy and enlargement of the lateral ventricles, which precluded placement of the three white matter regions. All three regions were used for the remaining 15 healthy control volunteers.
Hemodynamic
No statistically significant difference was found between the ipsilateral ratios and the contralateral (P = .69) or normal (P = .68) ratios for all 36 patients. The mean (±95% confidence intervals) deep white matter:superficial ratio for oxygen extraction fraction measured in the hemispheres ipsilateral to the carotid occlusion (0.987 ± 0.067) was less than that measured in the contralateral hemispheres (1.010 ± 0.057) and in the normal hemispheres of the control volunteers (1.017 ± 0.077). Plots of these ratios are shown in Figure 3. The range of deep white matter:superficial ratios of oxygen extraction fraction for all 36 patients was 0.583 to 1.491. The range of these ratios contralateral to the occlusion was 0.431 to 1.370, and the range in the healthy control volunteers was 0.673 to 1.545. Two patients (patients 3 and 8) had ipsilateral deep white matter:superficial ratios that were beyond the range found in contralateral hemispheres, but both ratios were within the range found in the healthy volunteers.
fig 3.
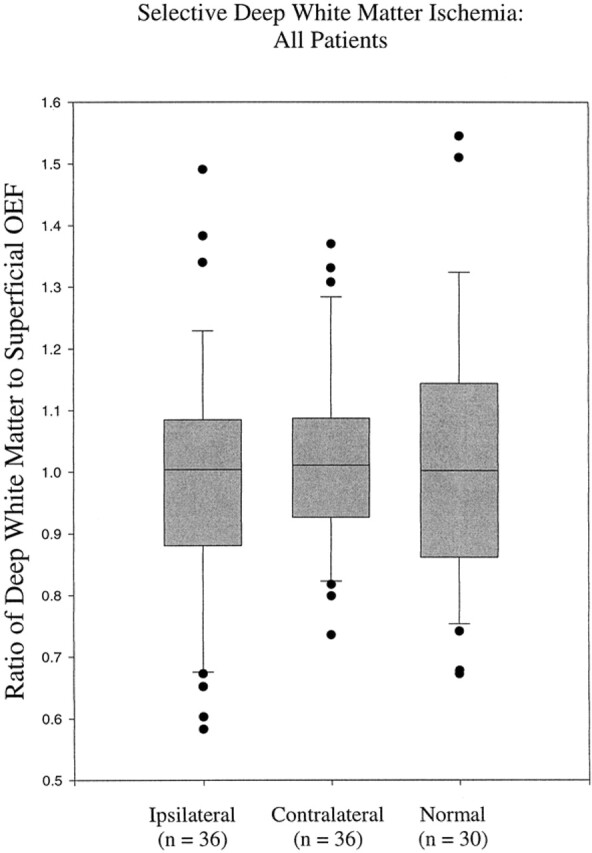
Range of ipsilateral deep white matter:superficial oxygen extraction fraction ratios for all patients (n = 36) compared with values from their contralateral hemispheres (n = 36) and from healthy volunteers (15 volunteers, 30 hemispheres) is very similar. The ratio of deep white matter:superficial oxygen extraction fraction is shown on the y axis and ratios of the three groups of volunteers are shown on the x axis. The boxes indicate the 95% confidence limits for the oxygen extraction fraction ratios. The lines within the boxes indicate the median values, and the bars above and below the boxes indicate the 99% confidence limits. Patients with oxygen extraction fraction ratios beyond the 99% confidence limits are represented with black circles.
The mean difference between ipsilateral and contralateral deep white matter:superficial oxygen extraction fraction ratios was 0.028. The 95% confidence interval for the difference of the means was −0.106 to 0.162. The mean difference between ipsilateral and normal control ratios was 0.030. The 95% confidence interval for the difference was −0.081 to 0.143.
The range of left-to-right oxygen extraction fraction ratios in the superficial regions in the healthy control volunteers was 0.823 to 1.139. Nine of the 36 patients were identified as having increased oxygen extraction fraction in the superficial regions, based on individual ratios outside of this normal range. An example of one patient with increased oxygen extraction fraction is shown in Figure 4. The mean deep white matter:superficial oxygen extraction fraction ratio in this subgroup was 0.983 (±0.21). No statistically significant difference was found between the ipsilateral ratios for the nine patients with increased superficial oxygen extraction fraction and the contralateral (P = .56) or normal (P = .69) ratios (Fig 5).
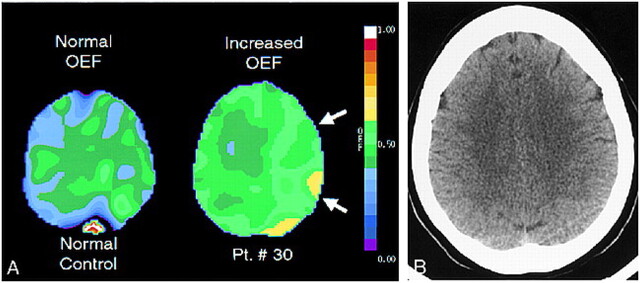
fig 4.
Positron emission tomographic and CT scans.
A, Example of subjects with normal (left) and increased (right, white arrows) oxygen extraction fraction on positron emission tomographic sections at the level of the centrum semiovale. Left and right on positron emission tomographic scans are reversed relative to CT or MR convention. Note the uniformity of oxygen extraction fraction values across the image on the left, compared with the hemispheric increase in oxygen extraction fraction seen on the image on the right. Oxygen extraction fraction values in the centrum semiovale are not quantitatively or qualitatively greater than those in the more superficial regions (white arrows).
B, Corresponding CT scan of patient 30 (Table 1). This patient presented with two episodes of profound left-sided weakness that occurred 10 days apart. These symptoms had nearly completely resolved except for a mild pronator drift at the time of the CT and positron emission tomographic examinations. Angiography showed complete occlusion of the right internal carotid artery at its origin and moderate 25% to 50% stenosis of the contralateral carotid siphon.
fig 5.
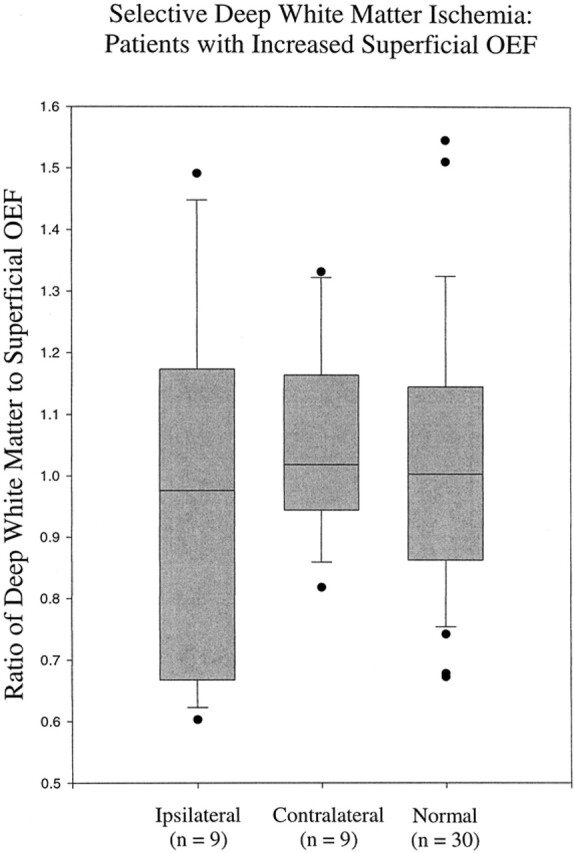
Range of deep white matter:superficial oxygen extraction fraction ratios for the nine patients with high oxygen extraction fraction in superficial regions compared with their contralateral hemispheres and with normal hemispheres is similar. Two patients had deep white matter:superficial oxygen extraction fraction ratios beyond the range seen in the contralateral hemispheres but within the normal range. As with figure 2, the ratio of deep white matter:superficial oxygen extraction fraction is shown on the y axis and the ratios of the three groups of volunteers are shown on the x axis. The boxes indicate the 95% confidence limits for the oxygen extraction fraction ratios. The lines within the boxes indicate the median values, and the bars above and below the boxes indicate the 99% confidence limits. Patients with oxygen extraction fraction values beyond the 99% confidence limits are represented with black circles.
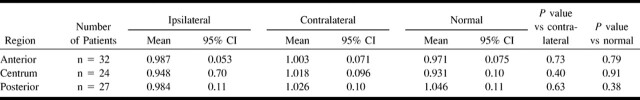
No significant difference was observed between the ratios of each of the individual ipsilateral deep white matter regions and the contralateral or normal values (Table 2). Ipsilateral deep white matter:superficial ratios for each of the three regions were within the normal range for all patients.
TABLE 2:
Individual deep white matter regions
Discussion
Deep white matter infarctions are common among patients with atherosclerotic cerebrovascular disease and have been associated with previous ischemic symptoms and with hemodynamic impairment of the overlying cerebral cortex (2–6). The cause of these lesions is unknown. Some investigators have suggested that they are caused by chronic, selective (greater in the white matter than in the cortex) ischemia secondary to a possible internal arterial border zone (7, 8). The purpose of the present investigation was to determine whether the deep white matter of patients with atherosclerotic carotid artery occlusion was subject to a greater degree of ischemia than was the overlying cortex.
No evidence for selective deep white matter ischemia was identified in this group of 36 patients with carotid occlusion or in the subgroup of nine patients with increased oxygen extraction fraction measured in the superficial regions of the middle cerebral artery territory. These data suggest that the cause of deep white matter lesions occurring among patients with hemodynamically significant cerebrovascular disease is not chronic selective border zone ischemia at an internal arterial border zone. Rather, the association of deep white matter infarctions with atherosclerotic cerebrovascular disease reported by previous investigators could result from embolic factors alone. In addition, the increased frequency of deep white matter infarctions seen with hemodynamic compromise of the superficial cortex could be attributed an increased risk for stroke due to hemodynamic factors affecting both superficial and deep tissue equally. Increased oxygen extraction fraction measured in superficial regions within the middle cerebral artery territory in patients with carotid occlusion is associated with both previous and subsequent stroke (14, 15).
Evidence for acute selective hemodynamic compromise of the deep white matter is also lacking. White matter infarctions have not been observed in neuropathologic studies of acute hypotension in higher-order primates (10, 19, 20) or in examination of human brains after systemic hypotension (21). Mendelow et al (20) created 90% stenosis (n = 5) or occlusion (n = 11) of the common carotid artery in adult baboons 30 minutes before inducing profound hemorrhagic hypotension for 30 minutes. A neuropathologic examination revealed varying degrees of ischemic injury to cortical tissue at the arterial border zone but no abnormalities in deeper tissue.
In addition, the anatomic evidence for an internal border zone is controversial. The concept of an internal border zone was first proposed by Zulch (7), based on gross pathologic observations of patterns of ischemic infarction. van den Bergh (22) subsequently reported the anatomic demonstration of an internal border zone in the centrum semiovale. He observed a centrifugal system of penetrating arteries from the surface of the lateral ventricles, creating a border zone 1 cm from the ventricular surface. It is, however, possible that these were medullary veins filled by a continuous injection of contrast medium from the arterial side and that they were mistakenly interpreted as arteries (23).
The detailed anatomic studies presented by Moody et al (23) determined that the deep white matter of the centrum semiovale was supplied by long terminal arteries with relatively little potential for collateral supply, as compared with other vascular territories in the brain. They observed, as have others (24), that there is a continuous capillary system in both gray and white matter, which is a potential source of collateral flow. They identified several different patterns of arterial supply, including multiple arteriolar sources from widely separate pial arteries and the phenomena of interdigitation, in which arteriolar territories from different pial arteries overlap or interdigitate. Both of these features were absent in the centrum semiovale, which was supplied by long, single arterioles from a single pial artery. Whether this arterial anatomy renders the deep white matter more vulnerable to hemodynamic than embolic factors is unknown.
The indicator of hemodynamic compromise used in this study was oxygen extraction fraction. The assessment of hemodynamic impairment in living humans is complicated and has been the subject of a recent review (11). Several neuroimaging methods are currently available for the indirect assessment of the hemodynamic effect of atherosclerotic stenosis or occlusion on the distal cerebrovasculature. These methods rely on different underlying physiologic mechanisms from which the presence of reduced perfusion pressure is inferred. Two basic categories of hemodynamic impairment can be assessed with these techniques: stage 1, in which autoregulatory vasodilation secondary to reduced perfusion pressure is inferred by the measurement of either increased blood volume or an impaired blood flow response to a vasodilatory stimulus; and stage 2, in which increased oxygen extraction fraction is noninvasively but directly measured (11). The correlation of different stage 1 methods with each other and with stage 2 techniques is variable (11). An advantage of positron emission tomographic measurements of oxygen extraction fraction for the determination of hemodynamic impairment is that they currently have the strongest empirical evidence as predictors of stroke risk (11, 14).
We used unfiltered images and smaller regions of interest in the present study than in the original analysis of the St. Louis Carotid Occlusion Study data, in which each 18-mm spherical region of interest included both cortical and adjacent subcortical tissue (15). Smaller regions of interest and the use of unfiltered images ensured that oxygen extraction fraction measurements in the deep white matter were not affected by partial volume averaging from the overlying superficial structures. The consequence of these two factors is greater noise (random variability) in the positron emission tomographic measurements. The broader range of normal values for the left-to-right superficial oxygen extraction fraction ratios in the present study (0.823−1.139) as compared with the two previously published analyses (0.914−1.082) is due to these two factors.
We used ratios of deep white matter:superficial oxygen extraction fraction to identify selective deep white matter ischemia for two reasons. First, quantitative values of oxygen extraction fraction are relatively uniform throughout the brain of a healthy volunteer but may vary considerably among healthy volunteers. The use of ratios is less sensitive to intersubject variability in baseline oxygen extraction fraction. Second, absolute values or hemispheric (side-to-side) ratios of deep white matter oxygen extraction fraction might simply reflect general changes throughout the hemisphere, not selective white matter abnormalities. We used t tests to assess differences between groups. The 95% confidence intervals for the difference of the means were broad; however, no individual ipsilateral ratio exceeded the range found in healthy control volunteers.
The imaging selection criterion (structurally normal white matter) used in the present study requires discussion. We excluded patients with white matter infarctlike lesions for the following reasons. First, oxygen extraction fraction can be increased in areas of infarction for reasons other than ongoing ischemia. When blood flow is reduced to a greater degree than tissue metabolism, oxygen extraction fraction is increased. This phenomenon can be observed in areas of subacute and chronic infarction (25). Second, once infarction has occurred, the metabolic needs of the tissue are reduced. As blood flow and tissue metabolism are closely matched, less blood flow will be required. A region with severe ischemia (increased oxygen extraction fraction) before infarction may no longer be ischemic after infarction.
This selection criterion could affect our results and conclusions only if normal white matter regions (as revealed by imaging) are functionally or anatomically different (no internal border zone) from those with white matter infarctions. The possibility of this anatomic variability within and between patients cannot be excluded. We, however, studied a total of 83 of 108 regions in 36 of 55 patients with available CT or MR studies and found no evidence for selective white matter ischemia in any individual patient or region.
Conclusion
In summary, we found no evidence for chronic selective hemodynamic impairment in structurally normal deep white matter among patients with carotid occlusion. Experimental evidence suggests that acute drops in perfusion pressure occurring at the time of the carotid occlusion are also an unlikely cause of deep white matter infarction. These data suggest that deep white matter infarction among patients with carotid occlusion are not caused by selective hemodynamic compromise due to an internal arterial border zone.
Footnotes
Supported by National Institutes of Health grants NS02029 and NS28947.
Presented at the Annual Meeting of the American Society of Neuroradiology, San Diego, CA, May 27, 1999.
Address reprint requests Colin P. Derdeyn, MD, 510 South Kingshighway Boulevard, St. Louis, MO 63110.
References
- 1.George AE, de Leon MJ, Gentes CI, et al. Leukoencephalopathy in normal and pathologic aging: 1. CT of brain lucencies. AJNR Am J Neuroradiol 1985;7:561-566 [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan RN, Wells SW, Miller TJ, et al. Infarct-like lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly: data from the Cardiovascular Health Study. Radiology 1997;202:47-54 [DOI] [PubMed] [Google Scholar]
- 3.Waterston JA, Brown MM, Butler P, Swash M. Small deep cerebral infarcts associated with occlusive internal carotid artery disease: a hemodynamic phenomenon? Arch Neurol 1990;47:953-957 [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi H, Fukuyama H, Yamaguchi S, Miyoshi T, Kimura J, Konishi J. High-intensity area in the deep white matter indicating hemodynamic compromise in internal carotid artery occlusive disorders. Arch Neurol 1991;48:1067-1071 [DOI] [PubMed] [Google Scholar]
- 5.Weiller C, Ringelstein EB, Reiche W, Buell U. Clinical and hemodynamic aspects of low-flow infarcts. Stroke 1991;22:1117-1123 [DOI] [PubMed] [Google Scholar]
- 6.Krapf H, Widder B, Skalej M. Small rosary-like infarctions in the centrum semiovale suggest hemodynamic failure. AJNR Am J Neuroradiol 1998;19:1479-1484 [PMC free article] [PubMed] [Google Scholar]
- 7.Zulch KJ. Uber die entstehung und lokalization der hirn-infarkte. Zentralbl Neurochir 1961;21:158-178 [PubMed] [Google Scholar]
- 8.Wodarz R. Watershed infarctions and computed tomography: a topographical study in cases with stenosis or occlusion of the carotid artery. Neuroradiology 1980;19:245-248 [DOI] [PubMed] [Google Scholar]
- 9.Carpenter DA, Grubb RL Jr, Powers WJ. Border zone hemodynamics in cerebrovascular disease. Neurology 1990;40:1587-1592 [DOI] [PubMed] [Google Scholar]
- 10.Brierley JB, Excell BJ. The effects of profound systemic hypotension upon the brain of M. Rhesus: physiological and pathological observations. Brain 1966;89:269-298 [DOI] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Grubb RL Jr, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology 1999;53:251-259 [DOI] [PubMed] [Google Scholar]
- 12.Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal “misery perfusion syndrome” by extra-intracranial artery bypass in hemodynamic cerebral ischemia: a case study with 0–15 positron emission tomography. Stroke 1981;12:454-459 [DOI] [PubMed] [Google Scholar]
- 13.Powers WJ, Press GA, Grubb RL Jr, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Int Med 1987;106:27-35 [DOI] [PubMed] [Google Scholar]
- 14.Grubb RL Jr, Derdeyn CP, Fritsch SM, et al. The importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055-1060 [DOI] [PubMed] [Google Scholar]
- 15.Derdeyn CP, Yundt KD, Videen TO, Grubb RL Jr, Carpenter DA, Powers WJ. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 1998;29:754-758 [DOI] [PubMed] [Google Scholar]
- 16.Mintun MA, Raichle ME, Martin WRW, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med 1984;25:177-187 [PubMed] [Google Scholar]
- 17.Fox PT, Perlmutter JS, Raichle ME. A stereotactic method of anatomical localization for positron emission tomography. J Comput Assist Tomogr 1985;9:141-153 [DOI] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain.. New York: Thieme Medical Publishers; 1988
- 19.Graham DI, Mendelow AD, Tuor U, Fitch W. Neuropathologic consequences of internal carotid artery occlusion and hemorrhagic hypotension in baboons. Stroke 1990;21:428-434 [DOI] [PubMed] [Google Scholar]
- 20.Mendelow AD, Graham DI, Tuor UI, Fitch A. The hemodynamic effects of internal carotid artery stenosis and occlusion. J Neurosurg 1987;66:755-763 [DOI] [PubMed] [Google Scholar]
- 21.Adams JH, Brierley JB, Connor RCJ, Treip C. The effects of systemic hypotension upon the human brain: clinical and neuropathologic observations in 11 cases. Brain 1966;89:235-268 [DOI] [PubMed] [Google Scholar]
- 22.van den Bergh R. Centrifugal elements in the vascular pattern of the deep intracerebral blood supply. Angiology 1969;20:88-94 [DOI] [PubMed] [Google Scholar]
- 23.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygen deficiency: an anatomic study. AJNR Am J Neuroradiol 1990;11:431-439 [PMC free article] [PubMed] [Google Scholar]
- 24.van der Zwan A, Hillen B, Tulleken CAF, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg 1992;77:927-940 [DOI] [PubMed] [Google Scholar]
- 25.Heiss W-D, Graf R, Weinhard K, et al. Dynamic penumbra demonstrated by sequential multi-tracer PET after middle cerebral artery occlusion in cats. J Cereb Blood Flow Metab 1994;14:892-902 [DOI] [PubMed] [Google Scholar]