Abstract
Summary: We describe our experience with follow-up MR imaging and MR spectroscopy in two patients after embolization of meningiomas without subsequent surgery. In both patients there was a marked reduction in tumor size and a decrease in contrast enhancement associated with spectroscopic signs of fatty degeneration. We did not observe regrowth; however, in one patient with incomplete angiographic devascularization, residual vital tumor tissue was seen at follow-up examinations.
Surgery is the treatment of choice for intracranial meningiomas. In selected patients, however, surgery may be withheld because of age, clinical condition, multiple or asymptomatic meningiomas, or problematic location. For these patients, various therapeutic regimens have been suggested, including partial resection, chemotherapy, or radiotherapy. We describe our experience with the embolization of meningiomas without subsequent surgery.
Patients and Technique
In the two patients who are the subjects of this report, diagnostic angiography was performed via a transfemoral approach under local anesthesia. A microcatheter system (FasTracker 10 or 18, Target Therapeutics, Fremont, CA) was placed in the feeding branches of the external carotid artery, and trisacryl gelatin microspheres of 100 to 300 μm (Embospheres, Biosphere Medical, Marlborough, MA), which had been mixed with a nonionic contrast medium (Imeron 250, Bracco-Byk Gulden, Konstanz, Germany), were injected slowly through the microcatheter under fluoroscopic control until stagnation of the intratumoral perfusion was accomplished. If technically feasible, this procedure was repeated for all tumor-supplying vessels. Neither patient had embolization of the branches of the internal carotid artery.
MR imaging and MR spectroscopy were performed on a 1.5-T unit (Magnetom Vision, Siemens, Erlangen, Germany) before embolization and within 24 hours after embolization. The protocol was repeated at 4 weeks, 4 months, and 8 months after embolization, and continued every 6 months. MR imaging included T1- and T2-weighted images before and after intravenous administration of contrast material. Tumor size and extent of nonenhancing portions of the tumor were evaluated on a workstation using postprocessing software (Virtuoso, Siemens). The spectroscopy protocol consisted of single-voxel point-resolved spectroscopy (PRESS) (1500/135,270/128 [TR/TE/excitations]) with a voxel size of 20 mm3. Spectral postprocessing included 4k zero-filling, gaussian apodization, Fourier transformation, water reference processing, frequency shift correction, as well as phase and baseline correction. Peak integral values were determined by a fit curve algorithm at 3.0 ppm for creatine (Cr), 3.2 ppm for choline-containing compounds (Cho), 1.35 for lactate, and 2.0 ppm for N-acetylaspartate (NAA). Peak integral values were normalized to the internal Cr peak.
Patient 1
A 46-year-old man presented with slowly progressing psychiatric disorders. Neurologic examination did not show a focal neurologic deficit. MR images revealed multiple intracranial meningiomas, three of them large (>15 cm3) (one right frontal, one right frontobasal, and one on the right-sided medial sphenoid wing) and five of them small (<10 cm3). Intraarterial angiography showed an external carotid artery supply of the frontal and frontobasal meningiomas by branches of the right middle meningeal artery (Fig 1A), which were completely devascularized. The meningioma of the medial sphenoid wing had an exclusive internal carotid artery blood supply and was not embolized. Despite extensive angiographic devascularization, there was no significant reduction of contrast enhancement of the frontal and frontobasal meningiomas 12 hours after embolization (Fig 1B). MR spectroscopy at that time, however, showed a marked lactate doublet at 1.35 ppm, which was not present before embolization. Owing to its extensive mass effect, the large frontal meningioma was removed first. At surgery, the tumor was strongly vascularized but there were also large areas of intratumoral necrosis. Histologically, the meningioma was classified as grade 1 (World Health Organization categorization scheme).
fig 1.
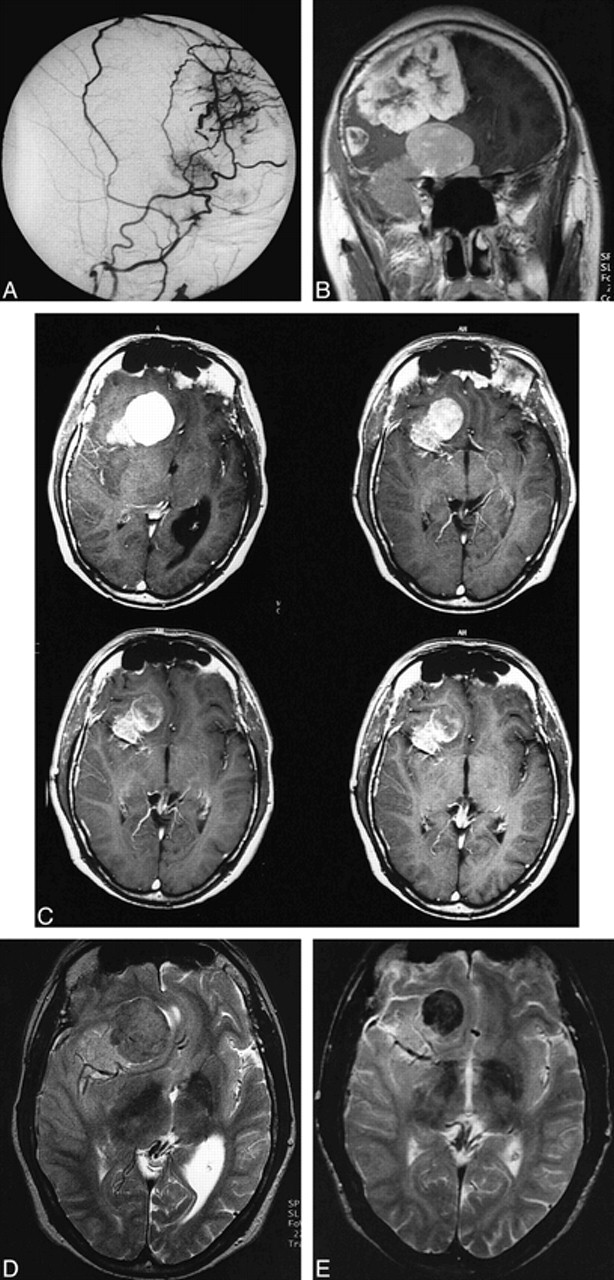
Patient 1.
A, Superselective angiogram of the right external carotid artery (lateral view) shows blood supply of frontal and frontobasal meningiomas by branches of the frontal and parietal branches of the right middle meningeal artery.
B, Coronal T1-weighted spin-echo MR image (532/17/2) 12 hours after embolization shows persistent uptake of contrast medium in both meningiomas despite complete devascularization of the external carotid artery feeders.
C, Contrast-enhanced axial T1-weighted images (490/17/2) 12 hours (top left), 8 months (top right), 14 months (bottom left), and 22 months (bottom right) after embolization show a reduction in size and contrast enhancement of the meningioma. After 22 months, there is only a slight peripheral uptake of contrast medium.
D and E, Axial T2-weighted spin-echo images (1957/80/2) before (D) and 22 months after (E) embolization. The intensity changed from an isointense signal to a marked hypointense signal, which was probably due to intratumoral thrombosis.
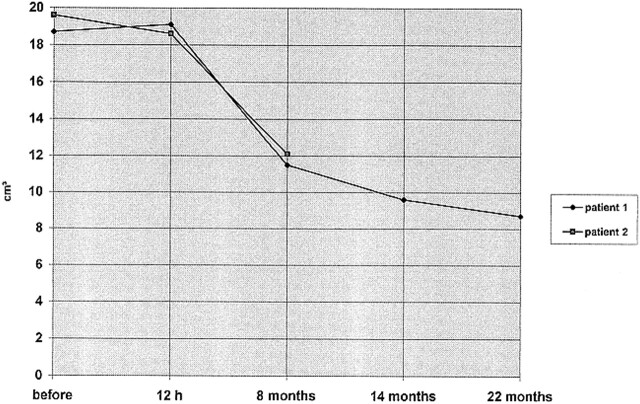
Postoperatively, the patient's mental status improved markedly. Because of the multiplicity of tumors, it was decided to observe the remaining meningiomas. Four weeks after embolization, MR spectroscopy showed a marked lipid peak in the frontobasal meningioma, which decreased on subsequent examinations. Figure 1C shows the follow-up MR images at 12 hours and at 8, 14, and 22 months. On T2-weighted images, signal intensity changed from isointense to markedly hypointense relative to adjacent brain tissue (Fig 1D and E). There was progressive shrinkage of the frontobasal meningioma and a decline of contrast uptake with only a slight residual peripheral enhancement after 22 months (Fig 2). At that time, MR spectroscopy no longer showed a distinct metabolic peak. The patient remains neurologically asymptomatic, with no progression of the remaining meningiomas.
fig 2.
Progressive shrinkage of tumor size in patients 1 and 2 (tumor volume in cm3)
Patient 2
A 79-year-old man presented with a history of headache, gait disturbance, and progressive psychiatric disorders. MR examination revealed a right-sided temporal meningioma with an extensive perifocal edema (Fig 3A). Owing to severe internal disease, it was decided not to operate on the meningioma. The patient improved clinically after intravenous application of glucocorticoids. Angiography, performed with the aim of embolizing the meningioma, showed a predominant external carotid artery blood supply by branches of the right middle meningeal artery. There was only a slight blood supply from the internal carotid artery. During two sessions, both feeders were extensively devascularized. Twelve hours after the second embolization, MR imaging showed areas of intratumoral necrosis and a slight reduction in tumor size (Fig 3B). The patient was discharged without any neurologic symptoms. Eight months later, however, he presented with a slight left-sided hemiparesis, headache, and gait disturbance.
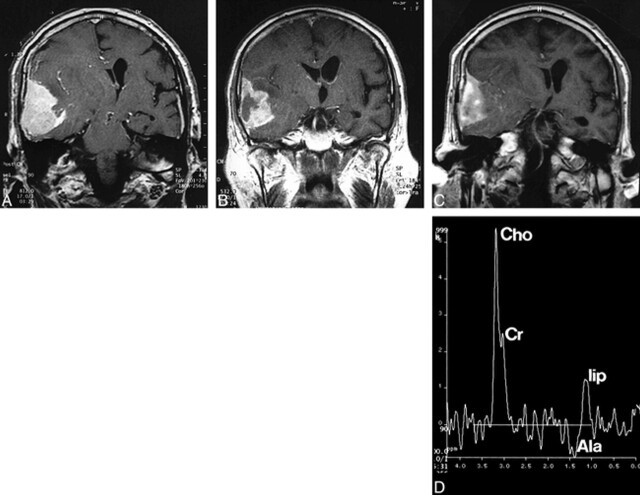
fig 3.
Patient 2.
A, Coronal contrast-enhanced T1-weighted spin-echo MR image (490/17/2) before embolization and before antiedematous treatment shows a large right temporal meningioma with extensive midline shift.
B, Twelve hours after embolization there is a central necrosis and a slight reduction in size. Moreover, the medial portions of the tumor show diminished uptake of contrast medium.
C, Eight months after embolization there is marked reduction in tumor size. The central necrosis has completely disappeared and the medial portions of the tumor show a slight uptake of contrast medium.
D, Spectrum of the meningioma (single-voxel PRESS sequence, 1500/135/128, voxel size, 20 mm3) shows persistence of the increased Cho/Cr ratio and an absence of NAA. There is an alanine (Ala) doublet at 1.45 ppm and lipid (lip) signals at 0.8 to 1.2 ppm, which were not present before embolization, indicating intratumoral necrosis.
Contrast-enhanced T1-weighted MR imaging performed immediately after embolization revealed further reduction in the size of the meningioma, especially in the nonenhancing areas (Fig 3C). Moreover, the medial portion of the tumor showed a decrease in enhancement as compared with the lateral portion. MR spectroscopy still showed a spectrum typical of a meningioma, with elevated Cho/Cr levels and an alanine peak at 1.45 ppm. There were, however, lipid signals at 0.8 to 1.3 ppm, which were not present before embolization (Fig 3D). T2-weighted images showed marked progression of the perifocal edema, which was attributed to the fact that the patient had stopped taking his oral glucocorticoids. The symptoms regressed after systemic administration of glucocorticoids, and the patient was neurologically asymptomatic at discharge.
Discussion
Surgery with complete removal of the tumor, including the site of dural attachment, is indisputably the treatment of choice for meningiomas (1). In some patients, however, surgery may be withheld because of age, clinical condition, multiplicity of meningiomas, or a problematic location. In these cases, there is no general agreement on treatment. Besides a solely observant attitude, various chemotherapeutic regimes (2–4) and radiotherapy protocols (5, 6) have been suggested as palliative measures. Preoperative embolization of meningiomas is commonly used to reduce intraoperative blood loss. Some investigators have also speculated that there may be a lower rate of recurrence in embolized meningiomas owing to necrosis at the site of dural attachment caused by the embolization (7). We know of only two other case reports on embolization of meningiomas without subsequent surgery (8, 9). In both those cases, shrinkage of the tumor was associated with clinical improvement. Only one report has described long-term CT follow-up, and in this patient there was a regrowth of the tumor (9).
Both our patients had a marked reduction in tumor size after embolization. In case 1, complete angiographic devascularization was achieved. The persistent contrast enhancement seen in this patient immediately after embolization subsided on the follow-up examinations. This may have been caused by a secondary intratumoral thrombosis after embolization. In case 2, complete devascularization of the tumor was not possible owing to a partial internal carotid artery blood supply. On postembolization MR studies, a central area with complete absence of contrast enhancement was present, as well as areas of reduced enhancement at the periphery of the tumor. On follow-up examinations, the central area had nearly completely vanished, and the medial portion of the tumor was reduced in size. In this patient, immediate necrosis may have occurred in the center of the tumor as a result of complete disruption of the blood supply and a peripheral area of secondary thrombosis with gradual resorption of this tissue. Nonetheless, this case clearly represents the limits of embolization, especially in patients with incomplete devascularization: despite shrinkage of the tumor on MR images, MR spectroscopy showed areas of elevated Cho/Cr levels and an alanine peak at 1.45 ppm, which is the typical spectral pattern of meningiomas (10). Moreover, the patient was still dependent on antiedematous drugs, which is also indicative of vital tumor tissue. In patient 1, there was progressive shrinkage of the meningioma on follow-up studies at 22 months without any evidence of regrowth.
Conclusion
Further long-term studies will be necessary to determine whether a definite cure can be accomplished in cases of complete devascularization of meningiomas or whether there will always be residual vital tumor tissue with regrowth on long-term follow-up studies.
Footnotes
Address reprint requests to Dr. M. Bendszus, Department of Neuroradiology, University of Würzburg, Josef-Schneider-Str. 11, 97080 Würzburg, Germany.
References
- 1.Black P. Meningiomas. Neurosurgery 1993;32:643-6657 [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain MC. Adjuvant combined modality therapy for malignant meningiomas. J Neurosurg 1996;84:733-736 [DOI] [PubMed] [Google Scholar]
- 3.Kyritsis AP. Chemotherapy for meningiomas. J Neurooncol 1996;29:269-272 [DOI] [PubMed] [Google Scholar]
- 4.Schrell UMH, Rittig MG, Anders M, et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas, II: decrease of size in meningiomas in patients treated with hydroxyurea. J Neurosurg 1997;86:840-844 [DOI] [PubMed] [Google Scholar]
- 5.Hudgins WR, Barker JL, Schwartz DE, Nichols TD. Gamma knife treatment of 100 consecutive meningiomas. Stereotact Funct Neurosurg 1996;66: (Suppl 1): 121-128 [DOI] [PubMed] [Google Scholar]
- 6.Hakim R, Alexander E, Loeffler JS, et al. Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery 1998;42:446-453 [DOI] [PubMed] [Google Scholar]
- 7.Berenstein A, Kricheff II. Microembolization technique of vascular occlusion: radiologic, pathologic and clinical correlation. AJNR Am J Neuroradiol 1981;2:261-267 [PMC free article] [PubMed] [Google Scholar]
- 8.Koike T, Saski O, Tanaka R, Arai H. Long-term results in a case of meningiomas treated by embolization alone: case report. Neurol Med Chir (Tokyo) 1990;30:173-177 [DOI] [PubMed] [Google Scholar]
- 9.Wakhloo AK, Juengling FD, Van Velthoven V, Schumacher M, Hennig J, Schwechheimer K. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol 1993;14:571-582 [PMC free article] [PubMed] [Google Scholar]
- 10.Bruhn H, Frahm J, Gyngell M, Merboldt KD, Harnicke W, Sauter R. Noninvasive differentiation of tumors with use of localized H—1 MR spectroscopy in vivo: initial experience in patients with tumors. Radiology 1988;172:541-548 [DOI] [PubMed] [Google Scholar]