Abstract
Microglia, the resident brain immune cells, play a critical role in normal brain development, and are impacted by the intrauterine environment, including maternal immune activation and inflammatory exposures. The COVID-19 pandemic presents a potential developmental immune challenge to the fetal brain, in the setting of maternal SARS-CoV-2 infection with its attendant potential for cytokine production and, in severe cases, cytokine storming. There is currently no biomarker or model for in utero microglial priming and function that might aid in identifying the neonates and children most vulnerable to neurodevelopmental morbidity, as microglia remain inaccessible in fetal life and after birth. This study aimed to generate patient-derived microglial-like cell models unique to each neonate from reprogrammed umbilical cord blood mononuclear cells, adapting and extending a novel methodology previously validated for adult peripheral blood mononuclear cells. We demonstrate that umbilical cord blood mononuclear cells can be used to create microglial-like cell models morphologically and functionally similar to microglia observed in vivo. We illustrate the application of this approach by generating microglia from cells exposed and unexposed to maternal SARS-CoV-2 infection. Our ability to create personalized neonatal models of fetal brain immune programming enables non-invasive insights into fetal brain development and potential childhood neurodevelopmental vulnerabilities for a range of maternal exposures, including COVID-19.
Subject terms: Molecular neuroscience, Stem cells
Introduction
Maternal immune activation can result from exposures ranging from metabolic conditions to stress and infection, with potential in utero consequences to the developing fetus1–6. In particular, epidemiologic studies strongly suggest that maternal viral and bacterial infections in pregnancy may be associated with adverse neurodevelopmental outcomes in offspring, particularly autism spectrum disorders, schizophrenia, and cerebral palsy, but potentially including mood and anxiety disorders as well1–3,7–9. For instance, individuals who were fetuses during the 1957 influenza pandemic had a significantly increased risk for being hospitalized for schizophrenia as an adult10. The magnitude of these effects varies, but their consistency is difficult to ignore. Although mechanisms underlying neurodevelopmental morbidity in offspring remain unclear, microglial priming toward a pro-inflammatory phenotype with consequent altered synaptic pruning has been suggested as a candidate mechanism11–17.
Microglia, brain-resident tissue macrophages, play a key role in normal neurodevelopment by modulating synaptic pruning, neurogenesis, phagocytosis of apoptotic cells, and regulation of synaptic plasticity18–21. Fetal yolk sac-derived macrophages are the progenitors for the permanent pool of brain microglia throughout an individual’s lifetime22–25. As such, inappropriate fetal microglial priming (“trained immunity”26) due to in utero immune activation may have lifelong neurodevelopmental consequences. The central role of mononuclear cells, including macrophages, in COVID-19 pathogenesis27 suggests that the potential risk to exposed fetal microglia requires investigation.
We have previously developed and validated adult patient-specific models of microglia-mediated pruning by reprogramming induced microglial cells from human peripheral blood mononuclear cells (PBMCs), and assaying them with isolated synapses (synaptosomes) derived from neural cultures differentiated from induced pluripotent stem cells (iPSCs)28,29. We demonstrated robust evidence of abnormalities in microglia and synaptosomes from individuals with schizophrenia, shown to be complement-dependent through a C3 receptor neutralizing antibody and rescued in a dose-responsive fashion with a small molecule probe28. Other groups have similarly applied in vitro synaptic pruning assays to provide insight into the pathogenesis of autism spectrum disorder and neurodegenerative disorders30–32.
In this study, we investigated whether our validated reprogramming methods for adult PBMCs could be successfully adapted and applied to umbilical cord blood-derived mononuclear cells (CB-MNCs) from both SARS-CoV-2 infected and uninfected pregnancies to create personalized models of fetal brain microglia. Such models could have a wide range of application in investigating effects of in utero exposure on neurodevelopment. To illustrate this application, we demonstrate successful induction of microglia-like cells (CB-iMGs) from CB-MNCs from both infected and uninfected pregnancies.
Materials/subjects and methods
Ethical statement
This study was approved by the Partners Institutional Review Board. Informed consent was obtained from all participants.
Isolation and preparation of mononuclear cells from umbilical cord blood (CB-MNCs)
Umbilical cord blood was collected at the time of delivery into EDTA tubes for plasma and CB-MNC isolation. After spinning at 1000g for 10 min to separate plasma, samples were processed for CB-MNC isolation using a Ficoll density gradient33. Briefly, blood was transferred into a 50 mL conical tube and then diluted to 1:1 ratio with Hanks’ balanced salt solution without calcium or magnesium (HBSS minus). This diluted blood was then gently layered on top of Ficoll at 2:1 ratio (two volumes of blood diluted with HBSS minus to one volume Ficoll). The conical tube was then centrifuged at 1000g for 30 min at room temperature with brake inactivated to allow layering of cellular components. The cloudy ring below the plasma and above the Ficoll (i.e. the CB-MNC layer) was collected and placed in a new 15 mL conical tube, with HBSS minus added to bring the volume to 15 mL. This tube was then centrifuged at 330g for 10 min with high brake. The supernatant was removed and the CB-MNC pellet was washed with HBSS minus and resuspended in 10 mL HBSS minus for counting. Cells were counted on a hemocytometer in a 1:10 dilution of trypan blue. Cells were frozen in freezing medium consisting of RPMI 1640 Medium with 1% penicillin–streptomycin, l-glutamine, 1% sodium pyruvate, 1% non-essential amino acids, 20% fetal bovine serum (FBS), and 10% DMSO at 5–10 million cells/vial, placed in a chilled Mr. Frosty, then into −80 °C. The following day, CB-MNC cryovials were transferred to liquid nitrogen for long-term storage. Isolated cryopreserved adult peripheral blood mononuclear cells were obtained from a single healthy control donor by Vitrologic (https://vitrologic.com) cat# MNC-300.
Derivation of induced microglia-like cells from CB-MNCs by direct cytokine reprogramming
iMGs were derived using previously described methods28,29 while CB-iMGs were derived from CB-MNCs with modifications as noted. Briefly, cryopreserved PBMCs or CB-MNCs were rapidly thawed at 37 °C, diluted into media consisting of RPMI 1640 with 10% FBS and 1% penicillin/streptomycin. The cell suspension was centrifuged at 300g for 5 min at room temperature, with the brake off. After aspirating the supernatant, the cell pellet was resuspended in media, counted, and plated on Geltrex-coated 24-well plates at 1 × 106 cells per 0.5 mL per well. After cells were incubated at 37 °C for 24 h, the media was completely replaced with RPMI 1640 with GlutaMAX, 1% penicillin–streptomycin, 100 ng/mL of human recombinant IL-34 (Peprotech), and 10 ng/mL of GM-CSF (Peprotech). Media was replaced on day 13 after the initial cytokine reprogramming, and real-time live cell imaging or immunocytochemistry was performed on day 14.
iPSC generation and neural differentiation for synaptosome isolation
iPSCs were reprogrammed from fibroblasts and used to derive neural progenitor cells, which were differentiated into neural cultures, as previously described28,29. In brief, adult human fibroblasts were reprogrammed to iPSCs using non-integrating synthetic RNA pluripotency factors, expanded and cryopreserved by Cellular Reprogramming, Inc. (https://www.cellular-reprogramming.com). To initiate neural progenitor induction, iPSCs were cultured feeder-free in E8 medium (Gibco) on Geltrex-coated six-well plates and passaged using 50 mM EDTA and trituration with ROCK inhibitor (10 mM Thiazovivin; Stemgent). iPSCs were further purified using magnetic-activated cell sorting with Tra-1-60 microbeads (Miltenyi Biotec) on LS columns as described by vendor. Neural progenitor cells (NPCs) were derived from these iPSCs using neurobasal medium (Thermo Fisher Scientific) with 1× Neural Induction Supplement (Thermo Fisher Scientific), expanded using a neural expansion medium, and purified by double sorting using MACS against CD271 and CD133. NPCs were immunostained for markers, including Nestin, SOX1, SOX2, and Pax-6. Validated NPCs were seeded for neural differentiation on Geltrex-coated T1000 five-layer cell culture flasks (Millipore Sigma # PFHYS1008) and grown in neuronal differentiation medium (Neurobasal media (Gibco # 21103049) supplemented with 1× each (N2 supplement (Stemcell Technologies SCT # 7156), B27 supplement without Vitamin A (Gibco # 12587010), non-essential amino acids (NEAA Gibco # 11140050), penn/strep), 1 μM ascorbic acid, 10 ng/mL BDNF and GDNF (Peprotech), and 1 μg/mL mouse laminin (Sigma # L2020) for 8 weeks.
Synaptosome isolation by sucrose gradient
Synaptosome isolation by sucrose gradient was adapted for iPSC-derived differentiated neural cultures from previously described protocols34–36. First, media was aspirated from flasks and cells were washed or scraped with 1× gradient buffer (ice-cold 0.32 M sucrose, 600 mg/L Tris, 1 mM NaH3CO3, 1 mM EDTA, pH 7.4 with added HALT protease inhibitor—Thermo Fisher # 78442), homogenized using a dounce homogenizer and centrifuged at 700g for 10 min at 4 °C. The pellet was resuspended in 10 mL of 1× gradient buffer (transferred to a 30 mL and centrifuged at 15,000g for 15 min at 4 °C. The second pellet was resuspended in 1× gradient buffer and slowly added on top of a sucrose gradient with 1× gradient buffer containing 1.2 M (bottom) and 0.85 M (middle) sucrose layers. The gradient and cell mixture was centrifuged at 26,500 r.p.m. (~80,000g) for 2 h at 4 °C, with the brake set to “slow” so as not to disrupt the final bands. The mixtures were handled carefully and bands were inspected to confirm successful fractionation. The synaptosome band (in between 0.85 and 1.2 M sucrose) was removed with a 5-mL syringe and 19Gx1 ½″ needle and centrifuged at 20,000g for 20 min at 4 °C. The final pellet was resuspended in an appropriate volume of 1× gradient buffer with 1 mg/mL bovine serum albumin (BSA) with protease and phosphatase inhibitors, aliquoted and slowly frozen at −80 °C. Protein concentration was measured by BCA, and synaptosomes were further analyzed by transmission electron microscopy for heterogeneity of size and morphology, and enrichment of pre-synaptic (synapsin, SNAP-25) and post-synaptic (PSD-95) markers was determined by western blot analysis as previously described28,29.
Real-time live cell imaging of synaptosome phagocytosis by CB-iMGs
Real-time live cell imaging of CB-iMGs was performed as previously described28,29. Briefly, cells were imaged on the IncuCyte ZOOM live imaging system (Essen Biosciences) while incubated at 37 °C with 5% CO2. Synaptosomes were sonicated and labeled with pHrodo Red SE (Thermo Fisher Scientific) and added to CB-iMGs at 15 µg total protein per well in 24-well plates. Phase contrast and red fluorescence channel images were taken at a resolution of 0.61 µm per pixel every 45 min for a total of 315 min. Images were exported as 16-bit grayscale files and analyzed using CellProfiler37 to quantify cells and phagocytized particles. CellProfiler pipeline description are included in Supplementary Materials.
Immunocytochemistry and confocal microscopy
iMGs were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at 4 °C, washed twice with PBS, blocked for 1 h with 5% FBS and 0.3% Triton-X (Sigma Aldrich) in PBS, and then washed three times with 1% FBS in PBS. Cells were incubated with primary antibodies in 5% FBS and 0.1% Triton-X overnight at 4 °C (Anti-IBA1, 1:500; Abcam #ab5076; Anti-CX3CR1, 1:100, Abcam ab8021; Anti-PU.1, 1:11,000, Abcam #ab183327, and Anti-P2RY12, 1:100, Alomone Labs). Cells were then washed twice with 1% FBS in PBS and incubated in secondary antibodies (1:500) and Hoechst 33342 (1:5000) in 5% FBS and 0.1% Triton-X in PBS for 45 min at 4 °C, light-protected. Cells were washed twice and imaged using the IN Cell Analyzer 6000 (Cytiva). CellProfiler pipeline description used for quantification of marker immunopositive cell percentages are included in Supplementary Materials.
Quantitation of microglial marker gene expression by qRT-PCR
Total RNA was extracted using the RNeasy Plus Micro Kit (Qiagen # 74034) and treated with dsDNAse (Thermo Scientific # EN0771) for 10 min at 37 °C followed by heat inactivation for 5 min at 55 °C in the presence of 10 mM DTT. Forty-five nanograms of purified RNA was used for cDNA synthesis using the Superscript III First-Strand Synthesis System (Invitrogen # 18080051). Generated cDNA was diluted 1:3 and 3 μL were loaded into the quantitative PCR reaction containing TaqMan Fast Advanced Master Mix (Applied Biosystems # 4444556) and the TaqMan Gene Expression Assays (1×) for both the gene of interest and the housekeeping gene (Applied Biosystems; IBA1: Hs00610419_g1, PU.1: Hs02786711_m1, P2RY12: Hs00224470_m1, TMEM119: Hs01938722_u1, RPLP0: Hs00420895_gH). All reactions were run in technical triplicates in a 10 μL reaction volume in a 384-well plate with a Roche LightCycler 480 machine as follows: 50 °C for 2 min, 95 °C for 20 s, then 40 cycles of 95 °C for 1 s and 60 °C for 20 s. Ct values over 35 were set to the baseline Ct of 35 given inconsistent detection that occurs with low transcript levels. Relative quantification of gene expression was normalized to the endogenous housekeeping gene (RPLP0) then to the experimental negative control (PBMCs, CB-MNCs, respectively).
Results
Generating and characterizing human microglia-like cells from umbilical cord blood-derived mononuclear cells (CB-iMGs)
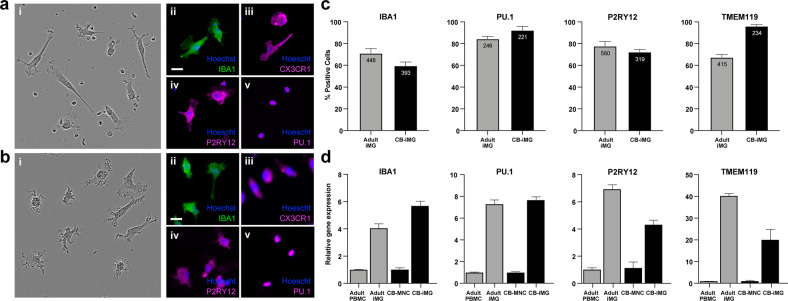
We adapted previously reported methods28,29 for generating iMGs from adult-derived PBMCs to reprogram umbilical CB-MNCs from neonates of SARS-CoV-2 negative (n = 4) and SARS-CoV-2 positive (n = 2) mothers, delivered between 7 June 2020 and 6 July 2020. After 2 weeks of cytokine exposure, analogous to PBMC-derived iMGs, CB-iMGs displayed typical ramified microglial morphology (Fig. 1a, i) and stained positive for canonical microglial markers IBA1, CX3CR1, PU.1, and P2RY12 (Fig. 1a, ii–v), demonstrating that iMGs can be generated from umbilical cord blood-derived CB-MNCs. These cells exhibit morphologic and features and markers of cell identity comparable to those we have demonstrated for adult blood-derived PBMCs (Fig. 1b, i–v). Quantitation of immunopositive cells for microglial markers (Fig. 1c) and upregulation of canonical microglial genes (Fig. 1d) indicate comparable efficiency of iMG transdifferentiation by PBMCs and CB-MNCs.
Fig. 1. Characterization of monocyte-derived induced microglia-like cells (iMGs) by direct cytokine reprogramming.
a Umbilical cord monocyte-derived CB-iMGs. b Adult PBMC-derived iMGs. (i) Morphology by phase contrast; immunostained images of iMG cells stained with nuclei (Hoechst) and indicated microglial markers (ii) IBA1, (iii) CX3CR1, (iv) P2RY12, and (v) PU.1. Scale bar: 30 μm. c Quantitation of positively immunostained cells as a percentage of total cells (nuclei) for indicated microglial markers. Bars indicate mean of indicated no. of cells measured, error bars indicating SEM. d Quantitation of fold increase in gene expression of indicated microglial markers normalized to input (PBMCs and CB-MNCs, respectively) after iMG reprogramming, error bars indicating SEM (n = 3 measurements).
CB-iMGs demonstrate capacity to engulf isolated synaptic material in an in vitro model of synaptic pruning
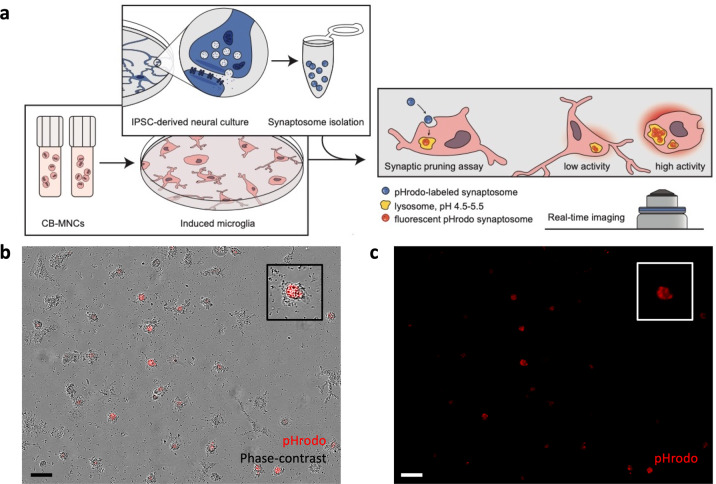
We next characterized CB-iMG function in a model of synaptic pruning using highly purified isolated nerve terminals (synaptosomes), allowing quantitation of synaptic engulfment in vitro with greater signal-to-noise than intact neural cultures28,29. Figure 2a illustrates the real-time imaging-based phagocytosis assay workflow with CB-iMG cultures upon addition of iPSC-derived neural culture purified synaptosomes labeled with pHrodo Red SE, a pH-sensitive dye that fluoresces upon localization to lysosomes post-engulfment. Engulfment of synaptosomes can be robustly quantified in real-time live imaging (Fig. 2b, c) as well as by endpoint confocal microscopy of fixed immunostained cells28,29.
Fig. 2. Characterizing synaptosome engulfment by CB-iMGs in an in vitro model of synaptic pruning.
a Overall schematic of pHrodo-labeled quantitative synaptosome phagocytosis assay by CB-iMGs. b Representative live real-time images in phase contrast/red fluorescence overlay mode showing cellular uptake and c red fluorescence channel alone of pHrodo (red)-labeled synaptosomes uptake at the end of the phagocytosis assay (315 min). Scale bar: 60 μm (boxes show magnified view of engulfing CB-iMG).
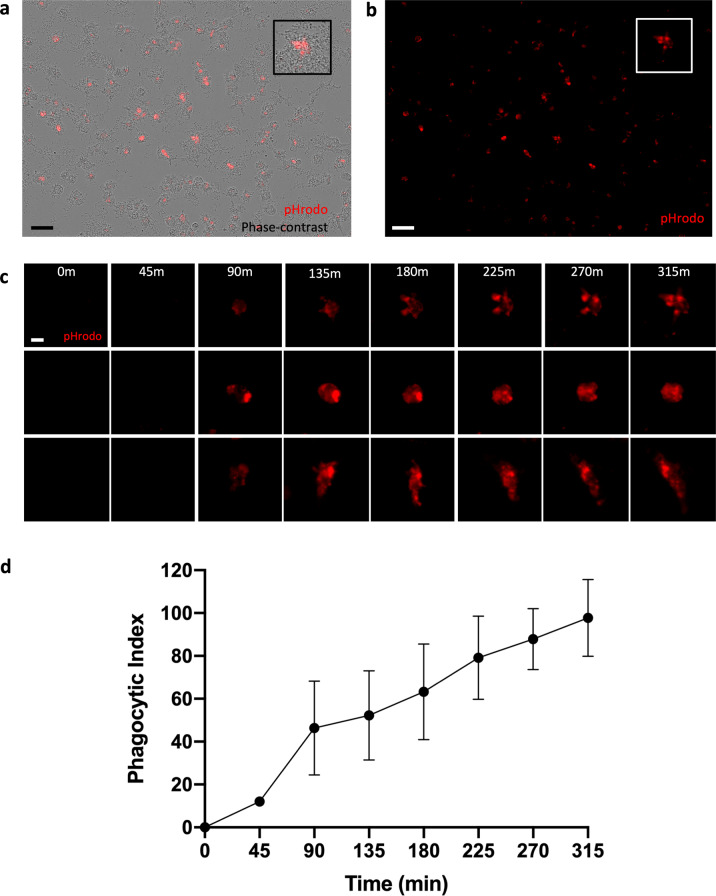
To characterize kinetics of phagocytosis over time, pHrodo-labeled synaptosomes were added to the CB-iMG cultures and engulfment was visualized every 45 min for approximately 5 h using real-time live fluorescence imaging (Fig. 3a). Phagocytotic index was determined at each time point by measuring pHrodo area per cell (Fig. 3b) using CellProfiler as described in “Methods” and Supplementary Information. CB-iMGs demonstrate robust phagocytosis of synaptosomes, with a time course of synaptosome engulfment rising over time qualitatively similar to that observed with adult PBMC-derived iMGs28,29.
Fig. 3. Quantifying synaptosome engulfment by CB-iMGs using live real-time imaging.
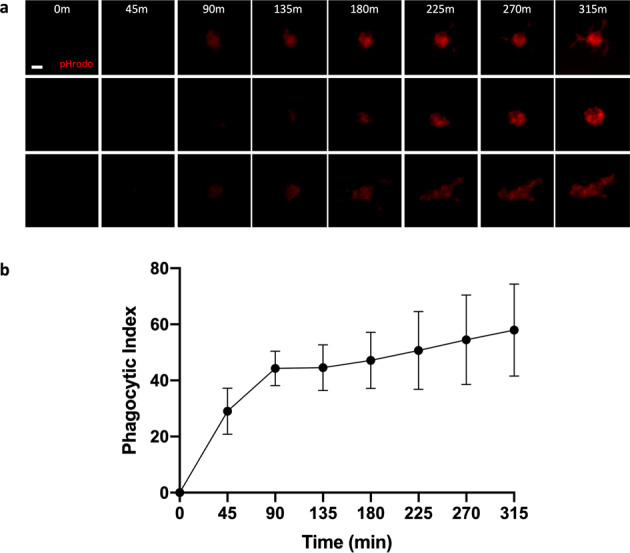
a Representative pHrodo (red)-labeled synaptosome engulfment in iMG cells during live real-time imaging used for quantification Scale bar: 20 μm. b Quantification of labeled synaptosome uptake by CB-iMGs cells during live imaging. The phagocytic index represents the mean pHrodo+ area per iMG cell over N = 8 fields per well × 3 wells per line and N = 4 separate healthy control CB-IMG line derivations. Error bars represent SEM.
CB-IMGs derived from CB-MNCs exposed to maternal SARS-CoV-2 infection
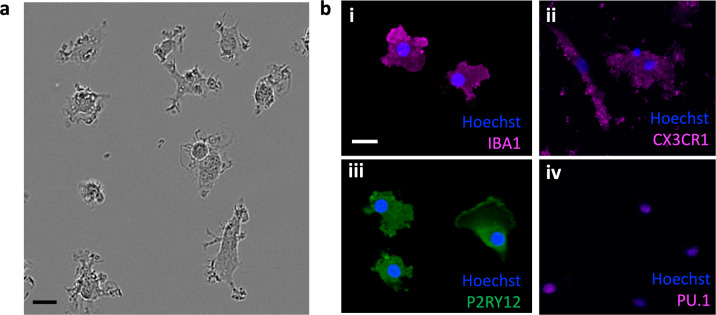
To illustrate the application of these models to study maternal exposures, we generated CB-iMGs from umbilical cord blood of neonates from mothers who tested positive for SARS-CoV-2 (n = 2). As observed above for CB-iMGs derived from SARS-CoV-2 unexposed pregnancies, these cells also display ramified and amoeboid morphology by phase contrast imaging (Fig. 4a), express microglial-specific markers IBA1, CX3CR1, PU.1, and P2RY12 by immunostaining (Fig. 4b, i–iv), and engulf pHrodo-labeled synaptosomes in our synaptic pruning assay as visualized by real-time live imaging (Fig. 5a–c) and quantification (Fig. 5d). These results demonstrate the capacity to create phenotypically characteristic and functionally active CB-iMGs in a quantitative model of synaptic pruning to provide for further investigations into potential effects of maternal exposures such as SARS-CoV-2.
Fig. 4. Phenotypic characterization of CB-iMGs derived from CB-MNCs exposed to maternal SARS-CoV-2 infection.
a Phase contrast ramified morphology of patient-derived CB-iMGs. Scale bar: 30 μm. b Immunostained images of exposed CB-iMG cells with nuclei (Hoechst) and indicated microglial markers (i) IBA1, (ii) CX3CR1, (iii) P2RY12, and (iv) PU.1. Scale bar: 25 μm.
Fig. 5. Functional characterization of synaptosome engulfment by CB-iMGs derived from CB-MNCs exposed to maternal SARS-CoV-2 infection.
a Representative live real-time images in phase contrast/red fluorescence overlay mode showing cellular uptake and b red fluorescence channel alone of pHrodo (red)-labeled synaptosomes uptake at the end of the phagocytosis assay (315 min). Scale bar: 60 μm (boxes show the magnified view of engulfing CB-iMG). c Representative pHrodo (red)-labeled synaptosome engulfment in exposed CB-iMGs during live real-time imaging used for quantification. Scale bar: 20 μm. d Quantification of labeled synaptosome uptake by exposed CB-iMGs cells during live imaging. The phagocytic index represents the mean pHrodo+ area per iMG cell over N = 8 fields per well × 3 wells per line and N = 2 separate SARS-CoV-2-exposed CB-IMG line derivations. Error bars represent SEM.
Discussion
Our results demonstrate the successful creation of neonatal patient-specific models of microglia-mediated synaptic pruning via cellular reprogramming of neonatal cord blood mononuclear cells. These models should facilitate novel insights into fetal brain development in the setting of maternal exposures, including but not limited to SARS-CoV-2 infection. Both SARS-CoV-2-exposed and -unexposed umbilical cord blood-derived microglia-like cells express canonical microglial markers IBA1, CX3CR1, PU.1, and P2RY12, and demonstrate a range of morphologies with varying degrees of ramification, potentially reflecting a range of activation states that can be perturbed in experimental systems. Importantly, the induced microglia phagocytose synaptosomes demonstrating that they can recapitulate this key function of microglia in the developing brain. This work suggests the potential for umbilical blood mononuclear cells to serve as a non-invasive, personalized biomarker of fetal brain microglial priming. To our knowledge, microglia have not previously been modeled from umbilical cord blood or used to predict neurodevelopmental vulnerability at a time when there is a window for intervention. These models provide the potential for quantifiable endpoints that can be used to assess microglial programming in the setting of various maternal exposures, and later can be used to test the efficacy of potential therapies to ameliorate in utero priming of microglia toward a pro-inflammatory phenotype38.
Previous studies have demonstrated the impact of various maternal exposures, including maternal stress, metabolic disorders, air pollution, and infections, on fetal brain development2–4,7. Such exposures may lead to offspring neurodevelopmental morbidity via maternal immune activation39–46, which results in aberrant microglial programming in the developing brain12–15,18. In turn, maternal immune activation models have pointed to aberrant differentiation of fetal microglia and dysregulation of cytokine networks as key mechanisms underlying abnormal fetal brain development, with microglia primed toward a pro-inflammatory phenotype and altered synaptic pruning implicated in offspring morbidity11–15. Given the extent of synapse formation and pruning that occurs in fetal and neonatal life, developmental microglial function represents a critical target for investigation.
We have previously demonstrated feasibility for the concept of using other monocyte populations to model microglial behavior using another monocyte type, fetal placental macrophages or Hofbauer cells, as a potential biologic surrogate for fetal microglial function in pre-clinical models of maternal obesity16, and have shown that maternal obesity-associated inflammation primes both fetal brain microglia and resident placental macrophages toward a highly correlated pro-inflammatory phenotype16. Personalized assays using more readily available cord blood mononuclear cells, as presented here, can be used to assess both baseline microglial function, and behavior in response to “second hit” inflammatory stimuli, which will be helpful in informing risk assessments for an individual fetus.
The COVID-19 pandemic, with its associated maternal immune activation and pro-inflammatory cytokine-mediated physiology47,48, may pose risk to the developing fetal brain. While current data suggest that vertical transmission of SARS-CoV-2 is relatively rare49–51, the profound immune activation observed in a subset of infected individuals suggests that, even if the virus itself does not cross placenta, the developing fetal brain may be impacted by maternal inflammation and altered cytokine expression during key developmental windows52–54. In this work, we suggest a model system that may be applied to investigate risk associated with the COVID-19 pandemic. Extending our work with adult peripheral blood mononuclear cells to cord blood-derived microglial models will allow for rapid, scalable models to investigate risk, yielding non-invasive, personalized assays of the impact of SARS-CoV-2 on fetal brain microglial priming and synaptic pruning function. This approach can complement more traditional approaches that will require large longitudinal cohort studies and may require years or even decades (in the case of schizophrenia, for example) to fully capture risk. The ability to detect priming of fetal brain microglia toward a pro-inflammatory phenotype extends beyond SARS-CoV-2 to include numerous other maternal infections in pregnancy, as well as the myriad maternal exposures that have been suggested to impact fetal microglial development.
In sum, we demonstrate the potential for umbilical cord blood mononuclear cells to serve as a non-invasive, personalized model of fetal brain microglial priming. These models provide the potential for quantifiable endpoints that can be used to assess microglial programming in the setting of various maternal exposures. We determined that CB-iMGs can recapitulate microglial characteristics and function in vitro, providing key insight into cells from the neonatal brain that are otherwise inaccessible at birth and throughout childhood. We illustrated their application to investigate effects of maternal infection, including SARS-CoV-2, on the developing brain28. Beyond characterizing any consequent abnormalities, the scalability of this approach may enable investigation of targeted therapeutic strategies to rescue such dysfunction.
Supplementary information
Supplemental Material: Description of CellProfiler image analysis
Acknowledgements
We are grateful to Dana Cvrk, CNM; Muriel Schwinn, NP; Lydia Shook, MD; Adeline Boatin, MD, MPH; Robin Azevedo, RN; Laurel Gardner, RN; Suzanne Stanton, RN; Natalie Croul, BA; Nicola Young, BA; and Samantha Devane, BS for their assistance with recruitment and sample collection; to all members of the MGH Obstetric-Pediatric COVID-19 Biorepository Processing Team for their assistance with sample processing and storage, to Lael Yonker, MD and Alessio Fasano, MD for their partnership on the Obstetric-Pediatric biorepository; to Jon Li, MD and Xu Yu, MD for critical infrastructural and regulatory support; and to Anjali Kaimal, MD,MAS, and Jeffrey Ecker, MD, for assistance with study infrastructure and departmental support. Most importantly, we thank the participants for being part of the study. This work was supported by R01-MH120227 (to R.H.P.) and R01HD100022 and 3R01HD100022-02S2 (to A.G.E.).
Conflict of interest
R.H.P. has received personal fees from Burrage Capital, RID Ventures, Genomind, Takeda, Psy Therapeutics, and Outermost Therapeutics, unrelated to the work described. He holds equity in Psy Therapeutics and Outermost Therapeutics. The other authors have declared no competing financial interests in relation to the work described.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Steven D. Sheridan, Jessica M. Thanos
Contributor Information
Roy H. Perlis, Email: rperlis@mgh.harvard.edu
Andrea G. Edlow, Email: aedlow@mgh.harvard.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01287-w.
References
- 1.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146:R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Haddad BJS, et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry. 2019;76:594–602. doi: 10.1001/jamapsychiatry.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Haddad BJS, et al. The fetal origins of mental illness. Am. J. Obstet. Gynecol. 2019;221:549–562. doi: 10.1016/j.ajog.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018;299(Pt A):241–251. doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RM, Messaoudi I. The impact of maternal obesity during pregnancy on offspring immunity. Mol. Cell Endocrinol. 2015;418(Pt 2):134–142. doi: 10.1016/j.mce.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordeiro CN, Tsimis M, Burd I. Infections and brain development. Obstet. Gynecol. Surv. 2015;70:644–655. doi: 10.1097/OGX.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yockey LJ, Lucas C, Iwasaki A. Contributions of maternal and fetal antiviral immunity in congenital disease. Science. 2020;368:608–612. doi: 10.1126/science.aaz1960. [DOI] [PubMed] [Google Scholar]
- 9.Zerbo O, et al. Maternal infection during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2015;45:4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mednick SA. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1988;45:189. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 11.Nunez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp. Neurol. 2003;181:270–280. doi: 10.1016/S0014-4886(03)00051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai Y, et al. Apoptosis and microglial activation in influenza encephalopathy. Acta Neuropathol. 2003;105:233–239. doi: 10.1007/s00401-002-0605-x. [DOI] [PubMed] [Google Scholar]
- 13.Smolders, S., Notter, T., Smolders, S. M. T., Rigo, J. M. & Brone, B. Controversies and prospects about microglia in maternal immune activationmodels for neurodevelopmental disorders. Brain Behav. Immun.73, 51–65 (2018). [DOI] [PubMed]
- 14.Fernandez de Cossio L, Guzman A, van der Veldt S, Luheshi GN. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017;63:88–98. doi: 10.1016/j.bbi.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q, et al. Maternal immune activation-induced PPARgamma-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring. Neurobiol. Dis. 2019;125:1–13. doi: 10.1016/j.nbd.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Edlow AG, et al. Placental macrophages, a window into fetal microglial function in maternal obesity. Int. J. Dev. Neurosci. 2019;77:60–68. doi: 10.1016/j.ijdevneu.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21:306–321. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria PM, Stevens B. Microglia function during brain development, new insights from animal models. Brain Res. 2015;1617:7–17. doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 20.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ginhoux F, Prinz M. Origin of microglia: current concepts and past controversies. Cold Spring Harb. Perspect. Biol. 2015;7:a020537. doi: 10.1101/cshperspect.a020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia. 2013;61:112–120. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- 26.Haley MJ, Brough D, Quintin J, Allan SM. Microglial priming as trained immunity in the brain. Neuroscience. 2019;405:47–54. doi: 10.1016/j.neuroscience.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Merad, M. & Martin, J. C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol.20, 355–362 (2020). [DOI] [PMC free article] [PubMed]
- 28.Sellgren CM, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019;22:374–385. doi: 10.1038/s41593-018-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellgren CM, et al. Patient-specific models of microglia-mediated engulfment of synapses and neural progenitors. Mol. Psychiatry. 2017;22:170–177. doi: 10.1038/mp.2016.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum, M. L. et al. CUB and Sushi Multiple Domains 1 (CSMD1) opposes the complement cascade in neural tissues. Preprint at bioRxiv, 10.1101/2020.09.11.291427 (2020).
- 31.Lui H, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarn, N. et al. Cytoplasmic-predominant Pten increases microglial activation and synaptic pruning in a murine model with autism-like phenotype. Mol. Psychiatry10.1038/s41380-020-0681-0 (2020). [DOI] [PMC free article] [PubMed]
- 33.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. 1976;Suppl 5:9–15. doi: 10.1111/j.1365-3083.1976.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 34.Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 35.Kamat PK, Kalani A, Tyagi N. Method and validation of synaptosomal preparation for isolation of synaptic membrane proteins from rat brain. MethodsX. 2014;1:102–107. doi: 10.1016/j.mex.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tenreiro P, et al. Comparison of simple sucrose and percoll based methodologies for synaptosome enrichment. Anal. Biochem. 2017;517:1–8. doi: 10.1016/j.ab.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 37.McQuin C, et al. CellProfiler 3.0: next-generation image processing for biology. PLoS Biol. 2018;16:e2005970. doi: 10.1371/journal.pbio.2005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammadi, A., Esmaeilzadeh, E., Li, Y., Bosch, R. J. & Li, J. Z. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. EBioMedicine59, 102903 (2020). [DOI] [PMC free article] [PubMed]
- 39.Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog. Neurobiol. 2019;175:1–19. doi: 10.1016/j.pneurobio.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Careaga M, Murai T, Bauman MD. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol. Psychiatry. 2017;81:391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddad FL, Patel SV, Schmid S. Maternal immune activation by Poly I:C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci. Biobehav. Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav. Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown AS, Meyer U. Maternal immune activation and neuropsychiatric illness: a translational research perspective. Am. J. Psychiatry. 2018;175:1073–1083. doi: 10.1176/appi.ajp.2018.17121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conway F, Brown AS. Maternal immune activation and related factors in the risk of offspring psychiatric disorders. Front. Psychiatry. 2019;10:430. doi: 10.3389/fpsyt.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Missault S, et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav. Immun. 2014;42:138–146. doi: 10.1016/j.bbi.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotlyar, A. M. et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 224, 35–53.e3 (2021). [DOI] [PMC free article] [PubMed]
- 50.Flaherman, V. J. et al. Infant outcomes following maternal infection with SARS-CoV-2: first report from the PRIORITY Study. Clin. Infect. Dis. ciaa1411, 10.1093/cid/ciaa1411 (2020). [DOI] [PMC free article] [PubMed]
- 51.Vivanti AJ, et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr. Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res. 2001;47:27–36. doi: 10.1016/S0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material: Description of CellProfiler image analysis