Abstract
Background
The emergence and spread of antimicrobial resistance (AMR) in Neisseria gonorrhoeae, nationally and internationally, is a serious threat to the management and control of gonorrhoea. Limited and conflicting data regarding the epidemiological drivers of gonococcal AMR internationally have been published. We examined the antimicrobial susceptibility/resistance of gonococcal isolates (n = 15,803) collected across 27 European Union/European Economic Area (EU/EEA) countries in 2009–2016, in conjunction to epidemiological and clinical data of the corresponding patients, to elucidate associations between antimicrobial susceptibility/resistance and patients’ gender, sexual orientation and anatomical site of infection.
Methods
In total, 15,803 N. gonorrhoeae isolates from the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP), 2009–2016, were examined. Associations between gonococcal susceptibility/resistance and patients’ gender, sexual orientation and anatomical site of infection were investigated using univariate and multivariate logistic regression analysis. Statistical significance was determined by Pearson χ2-test or Fisher’s exact test with two-tailed p-values of < 0.05 indicating significance.
Results
The overall gonococcal resistance from 2009 to 2016 was 51.7% (range during the years: 46.5–63.5%), 7.1% (4.5–13.2%), 4.3% (1.8–8.7%), and 0.2% (0.0–0.5%) to ciprofloxacin, azithromycin, cefixime, and ceftriaxone, respectively. The level of resistance combined with decreased susceptibility to ceftriaxone was 10.2% (5.7–15.5%). Resistance to cefixime and ciprofloxacin, and resistance combined with decreased susceptibility to ceftriaxone were positively associated with urogenital infections and heterosexual males, males with sexual orientation not reported and females (except for ciprofloxacin), i.e. when compared to men-who-have-sex-with-men (MSM). Azithromycin resistance was positively associated with heterosexual males, but no association was significant regarding anatomical site of infection.
Conclusions
Overall, sexual orientation was the main variable associated with gonococcal AMR. Strongest positive associations were identified with heterosexual patients, particularly males, and not MSM. To provide evidence-based understanding and mitigate gonococcal AMR emergence and spread, associations between antimicrobial susceptibility/resistance and patients’ gender, sexual orientation and anatomical site of infection need to be further investigated in different geographic settings. In general, these insights will support identification of groups at increased risk and targeted public health actions such as intensified screening, 3-site testing using molecular diagnostics, sexual contact tracing, and surveillance of treatment failures.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-05931-0.
Keywords: Gonorrhoea, Ceftriaxone, Azithromycin, Antimicrobial resistance, Surveillance, European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP), Europe
Background
Globally, 87 million new gonorrhoea cases among adults each year were estimated in 2016 [1], which represent an increase with 12% compared to the 78 million estimated cases in 2012 [2]. In the European Union/European Economic Area (EU/EEA), the incidence of reported gonorrhoea cases increased from 7.8 (cases per 100,000 population) in 2008 to 26.4 in 2018 [3]. Effective antimicrobial treatment together with appropriate prevention, diagnostics, notification and treatment of sexual contacts, including test of cure (TOC), and a detailed understanding of the epidemiology, are the mainstays in controlling gonorrhoea. However, high levels of antimicrobial resistance (AMR) in Neisseria gonorrhoeae seriously threaten the management and control of the infection [3–7]. The susceptibility to the last remaining effective options for empiric first-line monotherapy, i.e. the extended-spectrum cephalosporin (ESC) ceftriaxone given in combination therapy together with azithromycin or as monotherapy [8–16], is also decreasing and since 2015 an international spread of at least one ceftriaxone-resistant gonococcal strain has been documented [4–8, 17–26]. Regular and quality-assured antimicrobial susceptibility surveillance for N. gonorrhoeae is imperative, to monitor current and emerging trends in AMR and to ensure effective patient management on an individual level as well as by timely revisions of treatment guidelines. In the EU/EEA, the antimicrobial susceptibility surveillance is conducted through the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP), which is a sentinel surveillance programme co-ordinated by the European Centre for Disease Prevention and Control (ECDC) since 2009 [6, 7, 27–29]. From 2009 to 2012, the 2009 European gonorrhoea management guideline was for urethral, cervical and rectal gonorrhoea recommending treatment as follows: ceftriaxone 250 mg intramuscular (IM) as a single dose or cefixime 400 mg oral as a single dose or spectinomycin 2 g IM as a single dose. For pharyngeal infection, ceftriaxone 250 mg IM as a single dose was recommended [30]. From 2012, the 2012 European gonorrhoea management guideline recommended dual therapy with ceftriaxone 500 mg plus azithromycin 2 g for both urogenital and extragenital gonorrhoea [31].
In several countries, emergence and spread of AMR N. gonorrhoeae strains have been associated with the gender and in particular sexual orientation of the gonorrhoea patients. For example, the spread of gonococcal AMR has been documented in high-frequency transmitting groups at increased risks such as men who have sex with men (MSM), after which the AMR has bridged over to the heterosexual population resulting in large and rapid spread across the country [32–35]. In Australia, significant differences in levels of resistance and decreased susceptibility were identified from gonococcal isolates derived from males versus females for all antimicrobials assessed [36]. Furthermore, it was recently suggested that genomic adaptation of gonococcal strains to the cervical environment is associated with increased antimicrobial susceptibility [37]. Regarding anatomical site of infection, pharyngeal gonorrhoea has been more common among MSM and gonococcal resistance to β-lactam antimicrobials, including the ESCs, may emerge in the pharynx through the acquisition of genetic AMR determinants from non-gonococcal commensal Neisseria species. For instance, N. cinerea and N. flavescens have been shown to harbour resistance-mediating penA sequences that can be transferred to gonococcal strains and cause resistance to penicillin, ceftriaxone and cefixime [33, 38–40]. Pharyngeal gonorrhoea can also support the spread of gonorrhoea and gonococcal AMR since these infections are mostly asymptomatic (> 90%), less frequently detected, and substantially more difficult to eradicate than infections at urogenital or anorectal sites. Accordingly, pharyngeal gonorrhoea can act as a reservoir for both infection and emergence and spread of AMR [33, 41]. Nevertheless, limited and also conflicting data regarding the level of gonococcal antimicrobial susceptibility/resistance in different anatomical sites of infection, e.g. pharyngeal and rectal sites compared to urogenital sites, have been published [41–46].
The aim of the present study was to investigate associations between the antimicrobial susceptibility/resistance of gonococcal isolates collected in 27 countries across EU/EEA from 2009 to 2016 and patients’ gender, sexual orientation and anatomical site of infection.
Methods
European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP)
Euro-GASP has been previously described in detail [6, 7, 27–29, 47–51]. Briefly, participating EU/EEA countries submit a sample of consecutive N. gonorrhoeae isolates (ideally 100–200 isolates dependent on the number of gonorrhoea cases reported annually in the country) from their routine diagnostics for centralised antimicrobial susceptibility testing (AST) or, if countries have fulfilled set quality assurance criteria, they perform decentralised AST in their own countries. Countries include only one isolate per patient from those who were infected multiple times within a 4-week period or at several anatomical sites, to represent different gonorrhoea episodes. When multiple isolates per patient and gonorrhoea episode, priority is given to test extragenital isolates, particularly pharyngeal ones [48]. For decentralised AST, countries should have performed consistently well in the annual mandatory Euro-GASP external quality assessment (EQA) [50] and have had a good comparability between the laboratories own national or regional AST data and AST data generated by centralised testing [48]. During 2009–2016, the number of Euro-GASP countries increased from 17 to 23 and the number of decentralised Euro-GASP countries increased from 12 to 17. Centralised AST is currently conducted using minimum inhibitory concentration (MIC; mg/L) gradient strip tests for ceftriaxone, cefixime, and azithromycin, and MIC gradient strip test or agar dilution breakpoint method, which does not provide MIC and only susceptibility category (using agar plates with concentrations of 0.03 mg/L and 0.06 mg/L), for ciprofloxacin. For quality control of the AST in Euro-GASP [48], the 2016 WHO N. gonorrhoeae reference strains G, K, M, P and O (only when spectinomycin is tested) [51] are used. Furthermore, all centralised and decentralised Euro-GASP countries participate in the mandatory annual Euro-GASP EQA [50]. The MICs of each antimicrobial are interpreted into resistant (R); susceptible, increased exposure (I; formerly intermediate susceptibility); or susceptible (S) using current clinical breakpoints recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org/clinical_breakpoints/), i.e. ceftriaxone and cefixime (S ≤ 0.125 mg/L, R > 0.125 mg/L), and ciprofloxacin (S ≤ 0.032 mg/L, R > 0.064 mg/L). In 2019, EUCAST excluded their clinical breakpoints for azithromycin. However, because agar dilution breakpoint method was previously used by many Euro-GASP countries also for azithromycin and accordingly no MICs of azithromycin are available, in the present study, we had to use the previous clinical azithromycin breakpoints (S ≤ 0.25 mg/L; R > 0.5 mg/L). Due to the low number of isolates with ceftriaxone resistance, analysis was performed using isolates with resistance (MIC> 0.125 mg/L) combined with decreased susceptibility (DS; MIC> 0.032–0.125 mg/L) to ceftriaxone. In Euro-GASP, the AST and N. gonorrhoeae species identification should be repeated for all isolates that are resistant to ceftriaxone, have elevated resistance to cefixime (MIC> 0.25 mg/L), and all isolates showing high-level resistance to azithromycin (MIC≥256 mg/L). Those isolates are also recommended to be sent to the Euro-GASP Reference Laboratory Hub (Public Health England/Örebro University Hospital) for further verification and whole genome sequencing [48].
All countries participating in Euro-GASP report all results through The European Surveillance System (TESSy), a web-based reporting system managed by the ECDC, where in addition to antimicrobial susceptibility/resistance data also epidemiological and clinical data of the corresponding gonorrhoea patients are included. The collected epidemiological and clinical data include gender, age, country of origin, mode of transmission (referred to as sexual orientation below: females, heterosexual males and MSM), anatomical site of infection (urogenital, pharyngeal and anorectal), HIV status, previous gonorrhoea diagnosis, and probable country of infection [6, 7, 27–29, 47–50].
Statistical analysis
Associations between resistance/decreased susceptibility and patients’ gender, sexual orientation and anatomical site of infection were investigated using univariate and multivariate logistic regression analysis with MSM as the reference for sexual orientation and urogenital for site of infection. To control for the different years, year of isolate collection, with 2009 as the base, was included in the multivariate analysis. The odds ratios (OR) and 95% confidence intervals (CI) were calculated and Pearson’s χ2 test was used to measure if these odds ratios differed significantly from 1. Using a forward step-wise approach, the most significant and strongest associations from the univariate analysis were added to a multivariable logistic regression model sequentially. The statistical significance was determined by Pearson χ2-test or by Fisher’s exact test if cell numbers were less than 5, considering two-sided p-values of < 0.05 indicating significance. Statistical analysis was performed in Stata 15.1 (StataCorp, Texas, USA).
Results
Euro-GASP data from 2009 to 2016
From 2009 to 2016, 15,803 N. gonorrhoeae isolates from 27 countries were examined in Euro-GASP. Austria, Belgium, Denmark, France, Germany, Greece, Italy, Latvia, Malta, the Netherlands, Norway, Portugal, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom (17 countries) have participated in Euro-GASP since 2009, in 2010 four additional countries joined (Cyprus, Hungary, Ireland, and Romania), in 2013 Iceland, in 2014 Estonia and Poland, in 2015 Croatia, and finally in 2016 Czech Republic and Luxembourg.
The number of Euro-GASP isolates reported in 2009–2016, which significantly increased from 1366 isolates in 2009 to 2663 isolates in 2016, gender and sexual orientation of the corresponding patients, and anatomical site of infection have been summarised in Table 1.
Table 1.
Total number and proportion (%) of Neisseria gonorrhoeae isolates collected in Euro-GASP from 2009 to 2016 divided into gender, sexual orientation and anatomical site of infection
| 2009 No (%) | 2010 No (%) | 2011 No (%) | 2012 No (%) | 2013 No (%) | 2014 No (%) | 2015 No (%) | 2016 No (%) | Total No (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Total no of isolates | 1366 (8.6) | 1766 (11.2) | 1902 (12.0) | 1926 (12.2) | 1994 (12.6) | 2151 (13.6) | 2035 (12.9) | 2663 (16.9) | 15,803 (100) |
| Gender and sexual orientation | |||||||||
| Females | 219 (16.3) | 308 (17.6) | 321 (17.6) | 310 (16.3) | 302 (15.3) | 318 (14.9) | 363 (18.0) | 395 (14.9) | 2536 (16.2) |
| Males_Hetero | 314 (23.4) | 426 (24.4) | 423 (23.1) | 389 (20.4) | 376 (19.0) | 485 (22.7) | 419 (20.7) | 632 (23.8) | 3464 (22.2) |
| Males_MSM | 251 (18.7) | 395 (22.6) | 442 (24.2) | 408 (21.4) | 496 (25.1) | 594 (27.8) | 657 (32.5) | 696 (26.2) | 3939 (25.2) |
| Males_Other/UNK | 559 (41.6) | 620 (35.4) | 642 (35.1) | 798 (41.9) | 804 (40.6) | 743 (34.7) | 583 (28.8) | 931 (35.1) | 5680 (36.4) |
| Unknown | 23 | 17 | 74 | 21 | 16 | 11 | 13 | 9 | 184 |
| Site of infection | |||||||||
| Urogenital | 1163 (86.5) | 1426 (84.7) | 1466 (82.1) | 1536 (83.0) | 1531 (79.0) | 1549 (76.3) | 1496 (75.5) | 1946 (75.5) | 12,113 (79.7) |
| Anorectal | 138 (10.3) | 191 (11.4) | 216 (12.1) | 188 (10.2) | 255 (13.2) | 192 (9.5) | 276 (13.9) | 366 (14.2) | 1822 (12.0) |
| Pharyngeal | 34 (2.5) | 59 (3.5) | 79 (4.4) | 92 (5.0) | 122 (6.3) | 154 (7.6) | 178 (9.0) | 165 (6.4) | 883 (5.8) |
| Other | 10 (0.7) | 7 (0.4) | 24 (1.3) | 35 (1.9) | 30 (1.5) | 135 (6.7) | 31 (1.6) | 100 (3.9) | 372 (2.5) |
| Unknown | 21 | 83 | 117 | 75 | 56 | 121 | 54 | 86 | 613 |
No Number, Hetero Heterosexual, MSM Men who have sex with men, UNK Unknown sexual orientation
Briefly, the overall coverage of reporting gender, sexual orientation, and anatomical site of infection was 98.8% (range: 96.1–99.7%), 56.2% (range: 49.9–63.9%), and 96.1% (range: 93.8–98.5%), respectively. The isolates were mainly collected from males (n = 13,083, 83.8%; females n = 2536, 16.2%; gender not reported, n = 184). Of these patients, 3939 (25.2%), 3464 (22.2%), and 2536 (16.2%) reported sexual orientation as MSM, heterosexual males, and females, respectively. The isolates were cultured from urogenital samples (n = 12,113; 79.7%), anorectal samples (n = 1822; 12.0%), pharyngeal samples (n = 883; 5.8%), and samples from other anatomical sites (n = 372; 2.5%). The site of infection was not reported for 613 isolates (Table 1). Males who did not report as MSM and females were most likely to have urogenital infections, whereas MSM had the highest proportion of anorectal and pharyngeal infections (Table 2).
Table 2.
Total number and proportion (%) of Neisseria gonorrhoeae isolates collected in Euro-GASP from 2009 to 2016 by gender and sexual orientation divided into the anatomical site of infection
| Gender and sexual orientation | Urogenital No (%) | Anorectal No (%) | Pharyngeal No (%) | Other No (%) | UNK | Total |
|---|---|---|---|---|---|---|
| Females | 2154 (88.2) | 113 (4.6) | 134 (5.5) | 41 (1.7) | 94 | 2536 |
| Males_Hetero | 3343 (97.4) | 23 (0.7) | 26 (0.8) | 40 (1.2) | 32 | 3464 |
| Males_MSM | 1928 (50.0) | 1357 (35.2) | 542 (14.1) | 26 (0.7) | 86 | 3939 |
| Males_Other/UNK | 4621 (86.0) | 316 (5.9) | 175 (3.2) | 263 (4.9) | 305 | 5680 |
| Unknown | 67 | 13 | 6 | 2 | 96 | 184 |
| Total | 12,113 | 1822 | 883 | 372 | 613 | 15,803 |
No Number, Hetero Heterosexual, MSM Men who have sex with men, UNK Unknown sexual orientation
Antimicrobial resistance in Euro-GASP from 2009 to 2016 – association with gender and sexual orientation
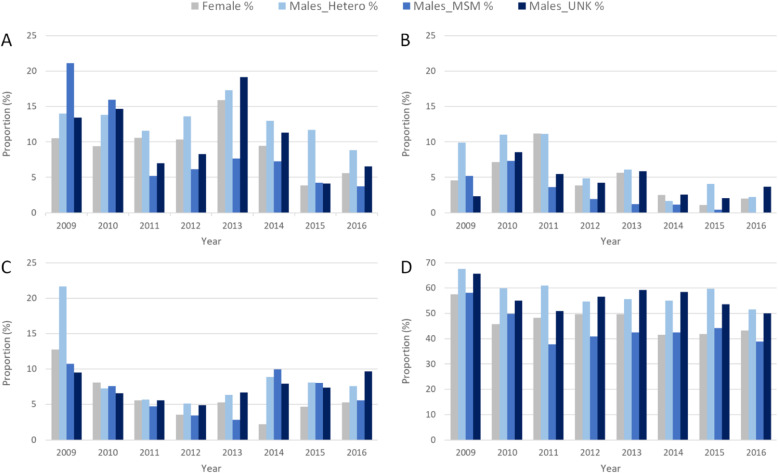
During 2009–2016, the overall resistance to ciprofloxacin, azithromycin, cefixime, and ceftriaxone was 51.7% (range during the years: 46.5–63.5%), 7.1% (4.5–13.2%), 4.3% (1.8–8.7%), and 0.2% (0.0–0.5%), respectively. The level of resistance combined with decreased susceptibility to ceftriaxone was 10.2% (5.7–15.5%). The proportion of isolates with resistance combined with decreased susceptibility to ceftriaxone, and resistance to cefixime, azithromycin and ciprofloxacin over time by gender and sexual orientation are shown in Fig. 1.
Fig. 1.
Proportion of Neisseria gonorrhoeae isolates with a resistance combined with decreased susceptibility to ceftriaxone, b resistance to cefixime, c resistance to azithromycin, and d resistance to ciprofloxacin, over time by gender and sexual orientation, 2009 to 2016
Despite that the overall level of resistance combined with decreased susceptibility to ceftriaxone was relatively high (10.2%), it decreased from 2009 to 2016 (Fig. 1a). Furthermore, the overall number of isolates with ceftriaxone resistance was low (n = 26, 0.2%) and none were collected from females. Rare isolates with ceftriaxone resistance initially emerged in 2011 (three isolates in Austria and seven in Germany) among five patients without gender or sexual orientation reported, four male patients with sexual orientation not reported, and one heterosexual male. Ceftriaxone resistance was subsequently identified in two MSM (Ireland and Slovenia) and one patient without gender or sexual orientation reported (Germany) in 2012, in six males with sexual orientation not reported (Spain) and one heterosexual male (Germany) in 2013, in three males with sexual orientation not reported (Germany, Greece, and Norway) and two heterosexual males (Greece) in 2014, and finally in one heterosexual male (Greece) in 2015 (no ceftriaxone resistance was found in 2016). The overall level of resistance combined with decreased susceptibility to ceftriaxone was 7.6% in MSM, 12.6% in heterosexual males, 10.6% in males with sexual orientation not reported, and 9.1% in females. Resistance combined with decreased susceptibility to ceftriaxone was significantly positively associated with isolates from heterosexual males, males with sexual orientation not reported, and females (p < 0.001, p < 0.001, and p = 0.025; ORs 1.76, 1.44, and 1.23, respectively). The level of gonococcal resistance to cefixime was 2.1, 6.0, 4.4, and 4.6% in MSM, heterosexual males, males with sexual orientation not reported, and females, respectively. Also cefixime resistance was significantly associated with isolates from heterosexual males, males with sexual orientation not reported, and females (all p < 0.001; ORs 3.0, 2.14, and 2.26). Regarding azithromycin, 6.5% of the isolates from MSM, 8.5% from heterosexual males, 7.3% from males with sexual orientation not reported, and 5.6% from females were resistant. Azithromycin resistance was significantly associated with heterosexual males (p < 0.001; OR 1.33). Finally, 43.2% of the isolates from MSM, 57.4% from heterosexual males, 56.0% from males with sexual orientation not reported, and 46.6% from females were resistant to ciprofloxacin. Ciprofloxacin resistance was significantly associated with heterosexual males, males with sexual orientation not reported, and females (p < 0.001, p < 0.001, and p = 0.007; ORs 1.78, 1.67, and 1.15). See supplementary tables S1, S2, S3 and S4 for all p-values, ORs and CIs.
Antimicrobial resistance in Euro-GASP from 2009 to 2016 – association with anatomical site of infection
The number of Euro-GASP isolates collected from different anatomical sites and the number and proportion of isolates with resistance combined with decreased susceptibility to ceftriaxone, and resistance to cefixime, azithromycin and ciprofloxacin, by anatomical site is summarised in Table 3.
Table 3.
Neisseria gonorrhoeae isolates in Euro-GASP from 2009 to 2016 by anatomical site of infection, and resistance combined with decreased susceptibility to ceftriaxone, and resistance to cefixime, azithromycin, and ciprofloxacin
| Site of infection | Ceftriaxone | Cefixime | Azithromycin | Ciprofloxacin | |||||
|---|---|---|---|---|---|---|---|---|---|
| No of tested | DS No (%) | R No (%) | No of tested | R No (%) | No of tested | R No (%) | No of tested | R No (%) | |
| Urogenital | 12,113 | 1264 (10.4) | 17 (0.1) | 12,063 | 575 (4.8) | 12,062 | 872 (7.2) | 12,102 | 6508 (53.8) |
| Female | 2154 | 185 (8.6) | 0 (0.0) | 2154 | 96 (4.5) | 2153 | 117 (5.4) | 2153 | 993 (46.1) |
| Males_Hetero | 3343 | 415 (12.4) | 5 (0.1) | 3307 | 196 (5.9) | 3302 | 284 (8.6) | 3343 | 1921 (57.5) |
| Males_MSM | 1928 | 172 (8.9) | 0 (0.0) | 1917 | 57 (3.0) | 1921 | 134 (7.0) | 1926 | 900 (46.7) |
| Males_Other/UNK | 4621 | 485 (10.5) | 12 (0.3) | 4618 | 222 (4.8) | 4619 | 334 (7.2) | 4613 | 2653 (57.5) |
| Unknown | 67 | 7 | 0 | 67 | 4 | 67 | 3 | 67 | 41 |
| Anorectal | 1822 | 149 (8.2) | 1 (0.1) | 1822 | 35 (1.9) | 1822 | 118 (6.5) | 1822 | 774 (42.5) |
| Female | 113 | 15 (13.3) | 0 (0.0) | 113 | 9 (8.0) | 113 | 10 (8.9) | 113 | 61 (54.0) |
| Males_Hetero | 23 | 4 (17.4) | 0 (0.0) | 23 | 1 (4.4) | 23 | 3 (13.0) | 23 | 10 (43.5) |
| Males_MSM | 1357 | 99 (7.3) | 1 (0.1) | 1357 | 18 (1.3) | 1357 | 78 (5.8) | 1357 | 556 (41.0) |
| Males_Other/UNK | 316 | 29 (9.2) | 0 (0.0) | 316 | 6 (1.9) | 316 | 26 (8.2) | 316 | 140 (44.3) |
| Unknown | 13 | 2 | 0 | 13 | 1 | 13 | 1 | 13 | 7 |
| Pharyngeal | 883 | 72 (8.2) | 2 (0.2) | 883 | 22 (2.5) | 883 | 73 (8.3) | 883 | 365 (41.3) |
| Female | 134 | 20 (14.9) | 0 (0.0) | 134 | 10 (7.5) | 134 | 14 (10.5) | 134 | 66 (49.3) |
| Males_Hetero | 26 | 2 (7.7) | 0 (0.0) | 26 | 2 (7.7) | 26 | 0 (0.0) | 26 | 16 (61.5) |
| Males_MSM | 542 | 25 (4.6) | 1 (0.2) | 542 | 6 (1.1) | 542 | 43 (7.9) | 542 | 202 (37.3) |
| Males_Other/UNK | 175 | 24 (13.7) | 0 (0.0) | 175 | 1 (0.6) | 175 | 13 (7.4) | 175 | 78 (44.6) |
| Unknown | 6 | 1 | 1 | 6 | 3 | 6 | 3 | 6 | 3 |
| Other | 372 | 22 (5.9) | 0 (0.0) | 372 | 13 (3.5) | 372 | 22 (5.9) | 372 | 187 (50.3) |
| Female | 41 | 3 (7.3) | 0 (0.0) | 41 | 1 (2.4) | 41 | 1 (2.4) | 41 | 16 (39.0) |
| Males_Hetero | 40 | 5 (12.5) | 0 (0.0) | 40 | 4 (10.0) | 40 | 2 (5.0) | 40 | 26 (65.0) |
| Males_MSM | 26 | 0 (0.0) | 0 (0.0) | 26 | 0 (0.0) | 26 | 0 (0.0) | 26 | 11 (42.3) |
| Males_Other/UNK | 263 | 14 (5.3) | 0 (0.0) | 263 | 8 (3.0) | 263 | 19 (7.2) | 263 | 134 (51.0) |
| Unknown | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
| Unknown | 613 | 76 (12.4) | 6 (1.0) | 611 | 27 (4.4) | 613 | 33 (5.4) | 613 | 323 (52.7) |
| Female | 94 | 9 (9.6) | 0 (0.0) | 93 | 1 (1.1) | 94 | 1 (1.1) | 94 | 45 (47.9) |
| Males_Hetero | 32 | 7 (21.9) | 0 (0.0) | 32 | 3 (9.4) | 32 | 3 (9.4) | 32 | 17 (53.1) |
| Males_MSM | 86 | 1 (1.2) | 0 (0.0) | 86 | 1 (1.2) | 86 | 2 (2.3) | 86 | 31 (36.1) |
| Males_Other/UNK | 305 | 35 (11.5) | 1 (0.3) | 304 | 10 (3.3) | 305 | 23 (7.5) | 305 | 172 (56.4) |
| Unknown | 96 | 24 | 5 | 96 | 12 | 96 | 4 | 96 | 58 |
| Total | 15,803 | 1583 (10.0) | 26 (0.2) | 15,751 | 672 (4.3) | 15,752 | 1118 (7.1) | 15,792 | 8157 (51.7) |
No Number, R Resistance, DS Decreased susceptibility, UNK Unknown sexual orientation
The majority (79.3%) of AMR isolates (resistant to at least one antimicrobial) were cultured from urogenital samples, reflecting that most (76.7%) Euro-GASP isolates during 2009 to 2016 were obtained from urogenital sites.
Where site of infection was known for ceftriaxone-resistant isolates (20/26), all were from urogenital site (n = 17), with exception of two pharyngeal isolates (2012, Germany and Slovenia (MSM)) and one rectal isolate from Ireland (2012, MSM). Overall, resistance combined with decreased susceptibility to ceftriaxone was found in 10.6% of the isolates from urogenital site, 8.2% from anorectal, and 8.4% from pharyngeal. Resistance combined with decreased susceptibility to ceftriaxone was significantly associated with urogenital samples compared to anorectal and pharyngeal samples (p = 0.002, p = 0.039; ORs 0.76 and 0.77). The level of resistance to cefixime was 4.8, 1.9, and 2.5% in urogenital, anorectal, and pharyngeal isolates, respectively, and also significantly associated with urogenital samples compared to anorectal and pharyngeal samples (p < 0.001, p = 0.002; ORs 0.39 and 0.51). For azithromycin 7.2% of the isolates from urogenital, 6.5% from anorectal, and 8.3% from pharyngeal sites were resistant; with no significant association to any anatomical site of infection. Finally, 53.8% of the isolates from urogenital, 42.5% from anorectal, and 41.3% from pharyngeal sites were resistant to ciprofloxacin (Table 3). Ciprofloxacin resistance was significantly associated with isolates from urogenital site when compared to anorectal and pharyngeal samples (both p < 0.001; ORs 0.63 and 0.61, respectively). See supplementary tables S1, S2, S3 and S4 for all p-values, ORs and CIs.
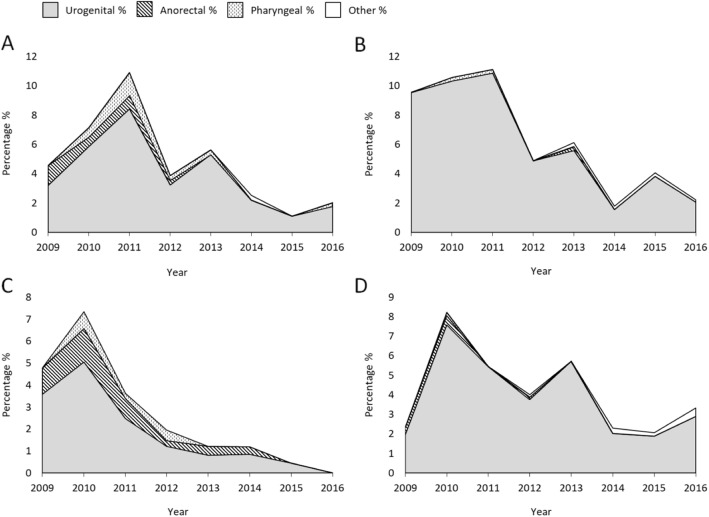
When combining gender, sexual orientation and anatomical site of infection over time, cefixime resistant isolates were mostly from the urogenital site. However, during the overall increase in 2010 and 2011 the proportion of anorectal and pharyngeal isolates increased by primarily anorectal isolates among MSM in 2010 and subsequently pharyngeal isolates among females in 2011 (Fig. 2).
Fig. 2.
Proportion of cefixime-resistant gonococcal isolates divided into gender, sexual orientation, and anatomical site of infection displayed over time. a Females b Heterosexual males c Men who have sex with men (MSM), and d Males with other sexual orientation or not reported
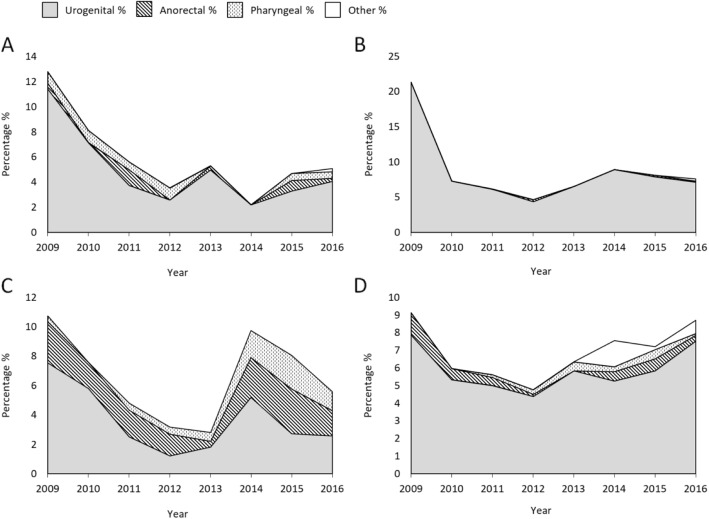
Also azithromycin-resistant isolates were most frequently from urogenital site, however, the proportion of anorectal and pharyngeal isolates increased in MSM in 2014, and a small increase of anorectal isolates was seen in females in 2015 (Fig. 3).
Fig. 3.
Proportion of azithromycin-resistant gonococcal isolates divided into gender, sexual orientation and anatomical site of infection displayed over time. a Females b Heterosexual males c Men who have sex with men (MSM), and d Males with other sexual orientation or not reported
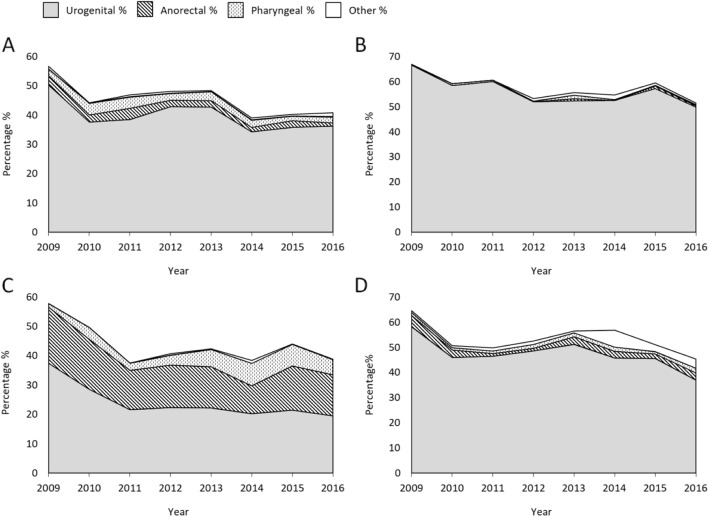
Ciprofloxacin resistance was relatively high and stable from 2009 to 2016 and mostly identified in urogenital isolates, but also in anorectal isolates from MSM (Fig. 4).
Fig. 4.
Proportion of ciprofloxacin-resistant gonococcal isolates divided into gender, sexual orientation and anatomical site of infection displayed over time. a Females b Heterosexual males c Men who have sex with men (MSM), and d Males with other sexual orientation or not reported
Finally, most of the statistical associations reported above remained valid in the multivariate analysis in respect to cefixime and ciprofloxacin resistance/susceptibility and gender, sexual orientation, and site of infection with the exception of the association between ciprofloxacin resistance and females (supplementary tables S1, S2, S3 and S4). Ceftriaxone resistance combined with decreased susceptibility remained associated with non-MSM males, as well as unknown site of infection. When controlling for year, there was no longer an association between cefixime susceptibility and pharyngeal infections. All other azithromycin, ceftriaxone and ciprofloxacin associations remained when controlling for year in a multivariate analysis.
Discussion
From 2009 to 2016, the overall resistance to ceftriaxone among the Euro-GASP isolates (n = 15,803) was only 0.2% (0.0–0.5% during the years), however, resistance combined with decreased susceptibility to ceftriaxone was 10.2% (5.7–15.5%). The overall resistance to cefixime, azithromycin, and ciprofloxacin was 4.3% (1.8–8.7%), 7.1% (4.5–13.2%), and 51.7% (46.5–63.5%), respectively. Resistance combined with decreased susceptibility to ceftriaxone, resistance to cefixime, and resistance to ciprofloxacin were all significantly associated with heterosexual males, males with sexual orientation not reported, and females (except for ciprofloxacin), i.e. when compared to MSM. Notably, none of the 26 ceftriaxone-resistant isolates in 2009–2016 was cultured from a female. Resistance to azithromycin was significantly associated with heterosexual males compared to MSM. Furthermore, resistance combined with decreased susceptibility to ceftriaxone, resistance to cefixime, and resistance to ciprofloxacin were all significantly associated with urogenital site of infection, while no significant association with site of infection was observed for azithromycin. Overall, it appeared that sexual orientation was the main variable associated with AMR, which resulted in some associations also with urogenital site of infection because these infections were more common among heterosexual patients, while anorectal and pharyngeal infections were more common among MSM. Nevertheless, the levels of AMR in all urogenital and extragenital sites were substantial and extragenital infections were relatively common also in females. Accordingly, diagnostic 3-site testing (urogenital, pharyngeal, and anorectal sites) for N. gonorrhoeae is of obvious importance, i.e. to limit the spread of infection as well as to detect gonococcal AMR. This 3-site testing should ideally use molecular diagnostics for the extragenital samples and be routine in MSM, considered in women particularly if sexual contacts of gonorrhoea patients, and be guided based on sexual history, risk, travel history and symptoms or signs in all other patients [8, 12, 52–54].
Previous studies from the Gonococcal Isolate Surveillance Project (GISP) in the USA have shown significantly higher geometric mean MICs of azithromycin in MSM than heterosexual males [55, 56]. However, in Euro-GASP we could not find the same association with azithromycin resistance or geometric mean (data not shown), and instead azithromycin resistance was significantly associated with heterosexual males. Nevertheless, it cannot be excluded that the low coverage on reporting on the epidemiological variable sexual orientation underestimates the proportion of MSM. This could potentially bias our associations, but this bias was considered limited because males with sexual orientation not reported were not significantly associated with azithromycin resistance. A feasible explanation to the higher prevalence of azithromycin resistance in males than females might be the frequent use of azithromycin 1 g to treat male non-gonococcal urethritis and Chlamydia trachomatis infections. Also in contrast to our findings, studies from the US GISP and the Gonococcal Resistance to Antimicrobials Surveillance Program (GRASP) in England and Wales have reported that ceftriaxone and cefixime resistance is strongly associated with MSM [56–58]. In Euro-GASP, this association with MSM was observed in earlier years, i.e. in 2009–2010 [59], however, in 2013 cefixime resistance and decreased susceptibility to ceftriaxone was more strongly associated with heterosexual patients [27]. In line with this, the initial wider spread of ciprofloxacin resistance documented in US GISP as well as in GRASP was predominantly among MSM, but then over time was bridged over to heterosexual males and females [34, 35, 60, 61]. A similar initial emergence and spread of ciprofloxacin resistance in many EU/EEA countries cannot be excluded.
In general, larger studies regarding differences in gonococcal antimicrobial susceptibility in different anatomical sites in both genders are extremely limited and nearly absent, which is especially because most larger gonococcal AMR surveillance programmes have predominantly or only collected urogenital samples in males (US GISP). However, it has been suggested that pharyngeal gonococcal infection is an asymptomatic reservoir for AMR and especially initial emergence of AMR [38–40]. Furthermore, it has also been hypothesized that gonococcal isolates with mtrR mutations, which today exist in the majority of circulating gonococcal isolates, may have enhanced survival in rectum and potentially can act as a reservoir for azithromycin resistance in especially MSM [62]. However, our present data did not support that isolates from the pharynx or anorectum were more resistant to any tested antimicrobial, as has been described in previous studies [43, 44, 46]. In contrast, overall resistance combined with decreased susceptibility to ceftriaxone, cefixime resistance, and ciprofloxacin resistance was significantly associated with urogenital site of infection.
The main limitation of the present study was that all analysis was performed on the entire Euro-GASP material, which includes samples of consecutive gonorrhoea patients and gonococcal isolates from many diverse countries [6, 7, 27–29, 47–50]. The gonorrhoea epidemiology, AMR levels, and level of reporting, especially in regard to sexual orientation, can differ in the countries participating in the Euro-GASP. However, country-by-country analysis was not possible to perform due to the lack of sufficient statistical power when analyzing the relatively small annual Euro-GASP samples from individual countries. We encourage similar studies to be performed on national level, analyzing all gonorrhoea cases and gonococcal isolates in the countries. Furthermore, the large proportion of urogenital isolates compared to rectal and pharyngeal isolates was an additional limitation.
Conclusions
In the light of the recent treatment failures, mainly of pharyngeal gonorrhoea, and the potential onward transmission of ceftriaxone-resistant gonococcal isolates, antimicrobial susceptibility surveillance as well as verification and reporting of treatment failures should be enhanced, and actions to develop alternative gonorrhoea therapeutic antimicrobials and treatment strategies are essential [63, 64]. In the present study, sexual orientation was the main variable associated with gonococcal AMR. Unexpectedly, the strongest associations with AMR were identified with heterosexual patients, particularly males, and not MSM. To provide evidence-based understanding and mitigate gonococcal AMR emergence and spread, associations between antimicrobial susceptibility/resistance and patients’ gender, sexual orientation and anatomical site of infection, as well as individual-level behavioural risk factors, need to be further investigated in different geographic settings. In general, these insights will support identification of risk groups and targeted public health actions such as intensified screening, 3-site testing using molecular diagnostics to more effectively identify also pharyngeal and anorectal gonorrhoea, sexual contact tracing, and enhanced surveillance of culture-based AMR and treatment failures.
Supplementary Information
Additional file 1: Table S1. Univariate association of ceftriaxone resistance (R) combined with decreased susceptibility (DS) or susceptibility and patient characteristics, Euro-GASP, 2009–2016. Table S2. Univariate association of cefixime resistance/susceptibility and patient characteristics, Euro-GASP, 2009–2016. Table S3. Univariate association of azithromycin resistance/susceptibility and patient characteristics, Euro-GASP, 2009–2016. Table S4. Univariate association of ciprofloxacin resistance/susceptibility and patient characteristics, Euro-GASP, 2009–2016.
Acknowledgements
We would like to thank the members of the European STI network for their active participation in Euro-GASP: Austria: Claudia Eder, Sonja Pleininger, Alexander Indra, Steliana Huhlescu; Belgium: Irith De Baetselier, Wim Vanden Berghe; Croatia: Blaženka Hunjak, Tatjana Nemeth Blažić; Cyprus: Panayiota Maikanti-Charalambous, Despo Pieridou; Czech Republic: Hana Zákoucká, Helena Žemličková; Denmark: Steen Hoffmann, Lasse Jessen Schwartz; Estonia: Rita Peetso, Jevgenia Epstein, Jelena Viktorova; France: Ndeindo Ndeikoundam, Beatrice Bercot, Cécile Bébéar, Florence Lot; Germany: Susanne Buder, Klaus Jansen; Greece: Vivi Miriagou, Georgios Rigakos, Vasilios Raftopoulos; Hungary: Eszter Balla, Mária Dudás; Iceland: Lena Rós Ásmundsdóttir, Guðrún Sigmundsdóttir, Guðrún Svanborg Hauksdóttir, Thorolfur Gudnason; Ireland: Aoife Colgan, Brendan Crowley, Sinéad Saab; Italy: Paola Stefanelli, Anna Carannante, Patrizia Parodi; Latvia: Gatis Pakarna, Raina Nikiforova, Antra Bormane, Elina Dimina; Luxembourg: Monique Perrin, Tamir Abdelrahman, Joël Mossong, Jean-Claude Schmit, Friedrich Mühlschlegel; Malta: Christopher Barbara, Francesca Mifsud; the Netherlands: Alje Van Dam, Birgit Van Benthem, Maartje Visser, Ineke Linde; Norway: Hilde Kløvstad, Dominique Caugant; Poland: Beata Młynarczyk-Bonikowska; Portugal: Jacinta Azevedo, Maria-José Borrego, Marina Lurdes Ramos Nascimento; Slovak Republic: Peter Pavlik; Slovenia: Irena Klavs, Andreja Murnik, Samo Jeverica, Sandra Kosmac, Tanja Kustec; Spain: Julio Vázquez Moreno, Asuncion Diaz, Raquel Abad; Sweden: Inga Velicko, Magnus Unemo; United Kingdom: Gwenda Hughes, Jill Shepherd, Lynsey Patterson.
Abbreviations
- AMR
Antimicrobial resistance
- CI
Confidence interval
- DS
Decreased susceptibility
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- ESC
Extended-spectrum cephalosporin
- EU
European Union
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- Euro-GASP
European Gonococcal Antimicrobial Surveillance Programme
- GISP
Gonococcal Isolate Surveillance Project
- GRASP
Gonococcal Resistance to Antimicrobials Surveillance Programme
- Hetero
Heterosexual
- I
Susceptibility, increased exposure
- MIC
Minimum inhibitory concentration
- MSM
Men who have sex with men
- No
Number
- R
Resistance
- S
Susceptibility
- TESSy
The European Surveillance System
- UNK
Unknown
Authors’ contributions
S.J., M.C., M.D., G.S. and M.U. initiated and designed the study. S.J., M.C. and M.U. analysed and interpreted the combined Euro-GASP data, and wrote a first draft of the paper. All authors read, commented and approved the final manuscript.
Funding
The study was funded by the ECDC (Framework Contract No. ECDC/2017/004). Open Access funding provided by Örebro University.
Availability of data and materials
The data that support the findings of this study are available from the ECDC, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data may however be available from the authors upon reasonable request and with permission of the ECDC.
Declarations
Ethics approval and consent to participate
All examined gonococcal isolates were cultured and preserved as part of the routine diagnostics (standard care), and isolates or data were submitted to the Euro-GASP surveillance study with no patient identification information. Separate ethical approval was therefore not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Susanne Jacobsson and Michelle J. Cole are joint first authors.
Contributor Information
Magnus Unemo, Email: magnus_unemo@yahoo.com.
on behalf of The Euro-GASP Network:
Claudia Eder, Sonja Pleininger, Alexander Indra, Steliana Huhlescu, Irith De Baetselier, Wim Vanden Berghe, Blaženka Hunjak, Tatjana Nemeth Blažić, Panayiota Maikanti-Charalambous, Despo Pieridou, Hana Zákoucká, Helena Žemličková, Steen Hoffmann, Lasse Jessen Schwartz, Rita Peetso, Jevgenia Epstein, Jelena Viktorova, Ndeindo Ndeikoundam, Beatrice Bercot, Cécile Bébéar, Florence Lot, Susanne Buder, Klaus Jansen, Vivi Miriagou, Georgios Rigakos, Vasilios Raftopoulos, Eszter Balla, Mária Dudás, Lena Rós Ásmundsdóttir, Guðrún Sigmundsdóttir, Guðrún Svanborg Hauksdóttir, Thorolfur Gudnason, Aoife Colgan, Brendan Crowley, Sinéad Saab, Paola Stefanelli, Anna Carannante, Patrizia Parodi, Gatis Pakarna, Raina Nikiforova, Antra Bormane, Elina Dimina, Monique Perrin, Tamir Abdelrahman, Joël Mossong, Jean-Claude Schmit, Friedrich Mühlschlegel, Christopher Barbara, Francesca Mifsud, Alje Van Dam, Birgit Van Benthem, Maartje Visser, Ineke Linde, Hilde Kløvstad, Dominique Caugant, Beata Młynarczyk-Bonikowska, Jacinta Azevedo, Maria-José Borrego, Marina Lurdes Ramos Nascimento, Peter Pavlik, Irena Klavs, Andreja Murnik, Samo Jeverica, Sandra Kosmac, Tanja Kustec, Julio Vázquez Moreno, Asuncion Diaz, Raquel Abad, Inga Velicko, Magnus Unemo, Gwenda Hughes, Jill Shepherd, and Lynsey Patterson
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–62P. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control Surveillance Atlas of Infectious Diseases. Surveillance atlas of infectious diseases: ECDC. https://atlas.ecdc.europa.eu/public/index.aspx
- 4.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 2017;14:e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health. 2019;16:412–425. doi: 10.1071/SH19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole MJ, Spiteri G, Jacobsson S, Woodford N, Tripodo F, Amato-Gauci AJ, et al. Overall low extended-spectrum cephalosporin resistance but high azithromycin resistance in Neisseria gonorrhoeae in 24 European countries, 2015. BMC Infect Dis. 2017;17:617. doi: 10.1186/s12879-017-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day MJ, Spiteri G, Jacobsson S, Woodford N, Amato-Gauci AJ, Cole MJ, et al. Stably high azithromycin resistance and decreasing ceftriaxone susceptibility in Neisseria gonorrhoeae in 25 European countries, 2016. BMC Infect Dis. 2018;18:609. doi: 10.1186/s12879-018-3528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unemo M, Ross JDC, Serwin AB, Gomberg M, Cusini M, Jensen JS. European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2020;2020:956462420949126. doi: 10.1177/0956462420949126. [DOI] [PubMed] [Google Scholar]
- 9.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for Gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1911–6. [DOI] [PMC free article] [PubMed]
- 10.Romanowski B, Robinson J, Wong T. Gonococcal infections chapter. Canadian guidelines on sexually transmitted infections. Wong T, Latham-Carmanico C, editors. Ottawa: Public Health Agency of Canada; 2013. http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf. Accessed 5 Feb 2021.
- 11.Australasian Sexual Health Alliance (ASHA). Gonorrhoea. Australian STI management guidelines for use in primary care: ASHA; 2016. http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management. Accessed 5 Feb 2021
- 12.Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS. 2020;31:4–15. doi: 10.1177/0956462419886775. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Society for Sexually Transmitted Infections Gonococcal infection. Sexually transmitted infections, diagnosis and treatment guidelines 2011. Jpn J Sex Transm Dis. 2011;22(Suppl. 1):529. [Google Scholar]
- 14.Boiko I, Golparian D, Krynytska I, Bezkorovaina H, Frankenberg A, Onuchyna M, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates and treatment of gonorrhoea patients in Ternopil and Dnipropetrovsk regions of Ukraine, 2013-2018. APMIS. 2019;127:503–509. doi: 10.1111/apm.12948. [DOI] [PubMed] [Google Scholar]
- 15.Golparian D, Bazzo ML, Golfetto L, Gaspar PC, Schörner MA, Benzaken AS, et al. Genomic epidemiology of Neisseria gonorrhoeae elucidating the gonococcal antimicrobial resistance and lineages/sublineages across Brazil, 2015-2016. J Antimicrob Chemother. 2020;75:3163–3172. doi: 10.1093/jac/dkaa318. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: WHO; 2016. [PubMed] [Google Scholar]
- 17.Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill. 2018;23(27):1800323. doi: 10.2807/1560-7917.ES.2018.23.27.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiley DM, Jennison A, Pearson J, Lahra MM. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis. 2018;18:717–718. doi: 10.1016/S1473-3099(18)30340-2. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother. 2016;60:4339–4341. doi: 10.1128/AAC.00504-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poncin T, Fouere S, Braille A, Camelena F, Agsous M, Bebear C, et al. Multidrug-resistant Neisseria gonorrhoeae failing treatment with ceftriaxone and doxycycline in France, November 2017. Euro Surveill. 2018;23(21). 10.2807/1560-7917.ES.2018.23.21.1800264. [DOI] [PMC free article] [PubMed]
- 21.Terkelsen D, Tolstrup J, Johnsen CH, Lund O, Larsen HK, Worning P, et al. Multidrug-resistant Neisseria gonorrhoeae infection with ceftriaxone resistance and intermediate resistance to azithromycin, Denmark, 2017. Euro Surveill. 2017;22(42). 10.2807/1560-7917.ES.2017.22.42.17-00659. [DOI] [PMC free article] [PubMed]
- 22.Golparian D, Rose L, Lynam A, Mohamed A, Bercot B, Ohnishi M, et al. Multidrug-resistant Neisseria gonorrhoeae isolate, belonging to the internationally spreading Japanese FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, Ireland, August 2018. Euro Surveill. 2018;23(47):1800617. doi: 10.2807/1560-7917.ES.2018.23.47.1800617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefebvre B, Martin I, Demczuk W, Deshaies L, Michaud S, Labbé AC, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Canada, 2017. Emerg Infect Dis. 2018;24:381–383. doi: 10.3201/eid2402.171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahra MM, Martin I, Demczuk W, Jennison AV, Lee KI, Nakayama SI, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis. 2018;24(4):735–740. doi: 10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eyre DW, Town K, Street T, Barker L, Sanderson N, Cole MJ, et al. Detection in the United Kingdom of the Neisseria gonorrhoeae FC428 clone, with ceftriaxone resistance and intermediate resistance to azithromycin, October to December 2018. Euro Surveill. 2019;24(10). 10.2807/1560-7917.ES.2019.24.10.1900147. [DOI] [PMC free article] [PubMed]
- 26.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, Unemo M. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med. 2016;374:2504–2506. doi: 10.1056/NEJMc1512757. [DOI] [PubMed] [Google Scholar]
- 27.Harris SR, Cole MJ, Spiteri G, Sánchez-Busó L, Golparian D, Jacobsson S, et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis. 2018;18:758–768. doi: 10.1016/S1473-3099(18)30225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole MJ, Unemo M, Hoffmann S, Chisholm SA, Ison CA, van de Laar MJ. The European gonococcal antimicrobial surveillance programme, 2009. Euro Surveill. 2011;16(42):19995. [PubMed] [Google Scholar]
- 29.Cole MJ, Spiteri G, Jacobsson S, Pitt R, Grigorjev V, Unemo M, Euro-GASP Network Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in Europe? Results from the 2013 European surveillance. BMC Infect Dis. 2015;15:321. doi: 10.1186/s12879-015-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bignell C, IUSTI/WHO 2009 European (IUSTI/WHO) guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2009;20:453–457. doi: 10.1258/ijsa.2009.009160. [DOI] [PubMed] [Google Scholar]
- 31.Bignell C, Unemo M, European STI Guidelines Editorial Board 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein E, Kirkcaldy RD, Reshef D, Berman S, Weinstock H, Sabeti P, et al. Factors related to increasing prevalence of resistance to ciprofloxacin and other antimicrobial drugs in Neisseria gonorrhoeae, United States. Emerg Infect Dis. 2012;18:1290–1297. doi: 10.3201/eid1808.111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unemo M, Del Rio C, Shafer WM. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr. 2016;4(3). 10.1128/microbiolspec.EI10-0009-2015. [DOI] [PMC free article] [PubMed]
- 35.Fenton KA, Ison C, Johnson AP, Rudd E, Soltani M, Martin I, et al. Ciprofloxacin resistance in Neisseria gonorrhoeae in England and Wales in 2002. Lancet. 2003;361:1867–1869. doi: 10.1016/S0140-6736(03)13489-7. [DOI] [PubMed] [Google Scholar]
- 36.George CRR, Kundu RL, Whiley DM, Lahra MM. Are sex norms the norm in gonococcal surveillance? Lancet Microbe. 2020;1(4):e143–e144. doi: 10.1016/S2666-5247(20)30087-2. [DOI] [PubMed] [Google Scholar]
- 37.Ma KC, Mortimer TD, Hicks AL, Wheeler NE, Sánchez-Busó L, Golparian D, et al. Adaptation to the cervical environment is associated with increased antibiotic susceptibility in Neisseria gonorrhoeae. Nat Commun. 2020;11(1):4126. doi: 10.1038/s41467-020-17980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igawa G, Yamagishi Y, Lee KI, Dorin M, Shimuta K, Suematsu H, et al. Neisseria cinerea with high ceftriaxone MIC is a source of ceftriaxone and cefixime resistance-mediating penA sequences in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2018;62(3):e02069–e02017. doi: 10.1128/AAC.02069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol. 1992;34:115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 40.Lewis DA. Will targeting oropharyngeal gonorrhoea delay the further emergence of drug-resistant Neisseria gonorrhoeae strains? Sex Transm Infect. 2015;91:234–237. doi: 10.1136/sextrans-2014-051731. [DOI] [PubMed] [Google Scholar]
- 41.Abraha M, Egli-Gany D, Low N. Epidemiological, behavioural, and clinical factors associated with antimicrobial-resistant gonorrhoea: a review. F1000Res. 2018;7:400. doi: 10.12688/f1000research.13600.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole MJ, Spiteri G, Town K, Unemo M, Hoffmann S, Chisholm SA, et al. Risk factors for antimicrobial-resistant Neisseria gonorrhoeae in Europe. Sex Transm Dis. 2014;41:723–729. doi: 10.1097/OLQ.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 43.Hottes TS, Lester RT, Hoang LM, McKay R, Imperial M, Gilbert M, et al. Cephalosporin and azithromycin susceptibility in Neisseria gonorrhoeae isolates by site of infection, British Columbia, 2006 to 2011. Sex Transm Dis. 2013;40:46–51. doi: 10.1097/OLQ.0b013e31827bd64c. [DOI] [PubMed] [Google Scholar]
- 44.McAuliffe GN, Smith M, Brokenshire M, Forster R, Reid M, Roberts SA. Keeping track of antimicrobial resistance for Neisseria gonorrhoeae in Auckland, New Zealand: past, present and future considerations. N Z Med J. 2018;131:71–77. [PubMed] [Google Scholar]
- 45.Stefanelli P, Vescio MF, Landini MP, Dal Conte I, Matteelli A, Cristaudo A, et al. Time trend analysis (2009-2016) of antimicrobial susceptibility in Neisseria gonorrhoeae isolated in Italy following the introduction of the combined antimicrobial therapy. PLoS One. 2017;12(12):e0189484. doi: 10.1371/journal.pone.0189484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole MJ, Tan W, Fifer H, Brittain C, Duley L, Hepburn T, et al. Gentamicin, azithromycin and ceftriaxone in the treatment of gonorrhoea: the relationship between antibiotic MIC and clinical outcome. J Antimicrob Chemother. 2020;75:449–457. doi: 10.1093/jac/dkz436. [DOI] [PubMed] [Google Scholar]
- 47.Spiteri G, Cole M, Unemo M, Hoffmann S, Ison C, van de Laar M. The European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP)--a sentinel approach in the European Union (EU)/European Economic Area (EEA) Sex Transm Infect. 2013;89(Suppl 4):iv16–iv18. doi: 10.1136/sextrans-2013-051117. [DOI] [PubMed] [Google Scholar]
- 48.European Centre for Disease Prevention and Control . Gonococcal antimicrobial surveillance reporting protocol 2020 - Euro-GASP - surveillance data for 2019 and 2020. Stockholm: European Centre for Disease Prevention and Control; 2020. [Google Scholar]
- 49.Cole MJ, Quinten C, Jacobsson S, Day M, Amato-Gauci AJ, Woodford N, et al. The European gonococcal antimicrobial surveillance programme (Euro-GASP) appropriately reflects the antimicrobial resistance situation for Neisseria gonorrhoeae in the European Union/European Economic Area. BMC Infect Dis. 2019;19:1040. doi: 10.1186/s12879-019-4631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole MJ, Quaye N, Jacobsson S, Day M, Fagan E, Ison C, et al. Ten years of external quality assessment (EQA) of Neisseria gonorrhoeae antimicrobial susceptibility testing in Europe elucidate high reliability of data. BMC Infect Dis. 2019;19(1):281. doi: 10.1186/s12879-019-3900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unemo M, Golparian D, Sánchez-Busó L, Grad Y, Jacobsson S, Ohnishi M, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71:3096–3108. doi: 10.1093/jac/dkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow EPF, Chen MY, Williamson DA, Bradshaw CS, Vodstrcil LA, Trumpour S, et al. Oropharyngeal and genital gonorrhoea infections among women and heterosexual men reporting sexual contact with partners with gonorrhoea: implication for oropharyngeal testing of heterosexual gonorrhoea contacts. Sex Transm Dis. 2019;46:743–747. doi: 10.1097/OLQ.0000000000001068. [DOI] [PubMed] [Google Scholar]
- 53.Comninos NB, Garton L, Guy R, Callander D, Fairley CK, Grulich AE, et al. Increases in pharyngeal Neisseria gonorrhoeae positivity in men who have sex with men, 2011–2015: observational study. Sex Transm Infect. 2019. 10.1136/sextrans-2019-054107 [Online ahead of print]. [DOI] [PubMed]
- 54.Hiransuthikul A, Sungsing T, Jantarapakde J, Trachunthong D, Mills S, Vannakit R, et al. Correlations of chlamydia and gonorrhoea among pharyngeal, rectal and urethral sites among Thai men who have sex with men: multicentre community-led test and treat cohort in Thailand. BMJ Open. 2019;9(6):e028162. doi: 10.1136/bmjopen-2018-028162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirkcaldy RD, Soge O, Papp JR, Hook EW, 3rd, del Rio C, Kubin G, Weinstock HS. Analysis of Neisseria gonorrhoeae azithromycin susceptibility in the United States by the Gonococcal Isolate Surveillance Project, 2005 to 2013. Antimicrob Agents Chemother. 2015;59:998–1003. doi: 10.1128/AAC.04337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirkcaldy RD, Hook EW, 3rd, Soge OO, del Rio C, Kubin G, Zenilman JM, Papp JR. Trends in Neisseria gonorrhoeae susceptibility to cephalosporins in the United States, 2006-2014. JAMA. 2015;314:1869–1871. doi: 10.1001/jama.2015.10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ison CA, Town K, Obi C, Chisholm S, Hughes G, Livermore DM, et al. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007-2011. Lancet Infect Dis. 2013;13:762–768. doi: 10.1016/S1473-3099(13)70143-9. [DOI] [PubMed] [Google Scholar]
- 58.Town K, Obi C, Quaye N, Chisholm S, Hughes G, GRASP Collaborative Group Drifting towards ceftriaxone treatment failure in gonorrhoea: risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales. Sex Transm Infect. 2017;93:39–45. doi: 10.1136/sextrans-2016-052583. [DOI] [PubMed] [Google Scholar]
- 59.Chisholm SA, Unemo M, Quaye N, Johansson E, Cole MJ, Ison CA, et al. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrug-resistant clone. Euro Surveill. 2013;18(3):20358. [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention (CDC) Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men--United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:335–338. [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention (CDC) Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332–336. [PubMed] [Google Scholar]
- 62.Shafer WM, Balthazar JT, Hagman KE, Morse SA. Missense mutations that alter the DNA-binding domain of MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology. 1995;141:907–911. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- 63.Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(8):e235–e279. doi: 10.1016/S1473-3099(17)30310-9. [DOI] [PubMed] [Google Scholar]
- 64.Seña AC, Bachmann L, Johnston C, Wi T, Workowski K, Hook EW, 3rd, et al. Optimising treatments for sexually transmitted infections: surveillance, pharmacokinetics and pharmacodynamics, therapeutic strategies, and molecular resistance prediction. Lancet Infect Dis. 2020;20:e181–ee91. doi: 10.1016/S1473-3099(20)30171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Univariate association of ceftriaxone resistance (R) combined with decreased susceptibility (DS) or susceptibility and patient characteristics, Euro-GASP, 2009–2016. Table S2. Univariate association of cefixime resistance/susceptibility and patient characteristics, Euro-GASP, 2009–2016. Table S3. Univariate association of azithromycin resistance/susceptibility and patient characteristics, Euro-GASP, 2009–2016. Table S4. Univariate association of ciprofloxacin resistance/susceptibility and patient characteristics, Euro-GASP, 2009–2016.
Data Availability Statement
The data that support the findings of this study are available from the ECDC, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data may however be available from the authors upon reasonable request and with permission of the ECDC.