Abstract
BACKGROUND AND PURPOSE: Classification of variations of the superficial middle cerebral vein (SMCV) remains ambiguous. We propose a new classification system based on embryologic development for preoperative examination.
METHODS: Three-dimensional CT angiography was used to evaluate 500 SMCVs (in 250 patients). The outflow vessels from the SMCV were classified into seven types on the basis of embryologic development. The 3D CT angiograms in axial stereoscopic and oblique views and multiple intensity projection images were evaluated by the same neurosurgeon on two occasions. Inconsistent interpretations were regarded as equivocal.
RESULTS: Three-dimensional CT angiography clearly depicted the SMCV running along the lesser wing or the middle cranial fossa. However, the outflow vessel could not be confirmed as the sphenoparietal, cavernous, or emissary type in 39 (8%) of the sides. SMCVs running in the middle cranial fossa to join the transverse sinus or superior petrosal sinus were accurately identified. SMCVs were present in 456 sides: 62% entered the sphenoparietal sinus or the cavernous sinus and 12% joined the emissary vein. Nine vessels were the superior petrosal type, 10 the basal type, 12 the squamosal type, and 44 the undeveloped type.
CONCLUSION: Three-dimensional CT angiography can depict the vessels and their anatomic relationship to the bone structure, allowing identification of the SMCV variant in individual patients. Preoperative planning for skull base surgery requires such information to reduce the invasiveness of the procedure. With the use of our classification system, 3D CT angiography can provide exact and practical information concerning the SMCV.
The superficial middle cerebral vein (SMCV) usually runs downward and forward along the sylvian fissure and flows into the sphenoparietal sinus or directly into the cavernous sinus (1–3). However, there are several common variations. The terminology, assumptions, and classification of such variations differ widely, and definitions remain confusing.
Information concerning the location and outflow point of the SMCV and its anatomic relationship to the bone structure is important in the preoperative planning of skull base surgery. However, such information is not easy to obtain by many of the current methods of investigation. In this study, we used 3D CT angiography to analyze the characteristics of SMCVs and classified the variations into seven types on the basis of embryologic anatomy.
Methods
Two hundred fifty patients were included in the study, 132 men and 118 women, aged 28 to 85 years (mean age, 63 years). All patients were examined at our hospital between September 1996 and May 1999 for assessment of cerebrovascular disease, brain tumor, or abnormal findings identified at routine screening for cerebrovascular or other intracranial disease. No patient had edema or midline shift on CT scans or had undergone an operation.
Three-dimensional CT angiography was initiated 35 seconds after the start of intravenous administration of nonionizing contrast material, injected at a rate of 3 mL/s for 40 seconds, for a total volume of 120 mL. The x-ray tube potential and current were 120 kV and 175 mA, respectively. The sections were 2 mm thick, the table speed was 1.3 mm/s, and the scanning time was 1 second. Although a section thickness of 1 mm provides better quality images than a thickness of 2 mm, it is limited in the vertical direction owing to the table transfer. Therefore, we used a thickness of 2 mm and a table transfer range of 6 cm for optimum clarity. We used the shaded surface rendering method for 3D reconstruction with a threshold of 150 to 250 HU. Axial stereoscopic and oblique scans in the bilateral anterior or posterior directions were reconstructed. The 3D CT angiograms were evaluated by the same neurosurgeon on two separate occasions. In cases of inconsistent interpretations, the result was regarded as equivocal.
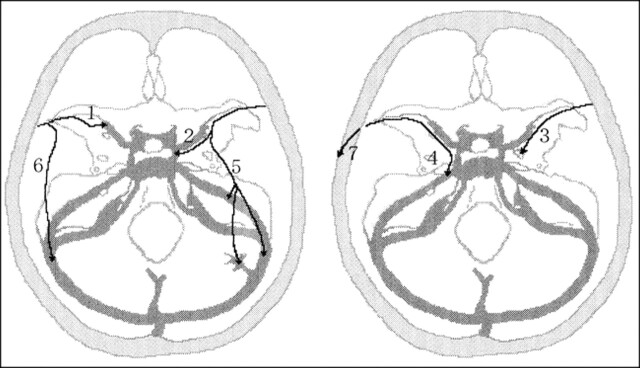
Variations among the SMCVs in the 500 sides of the 250 patients were classified according to alterations occurring during venous development in the embryo, as described on the following page (Fig 1).
fig 1.
Classification of the superficial sylvian venous drainage pathways. 1, Sphenoparietal type: the SMCV enters the sphenoparietal sinus and runs along the lesser wing of the sphenoid bone to enter the cavernous sinus. 2, Cavernous type: the SMCV directly enters the anterior end of the cavernous sinus. 3, Emissary type: the SMCV courses along the lesser wing, turns inferiorly to reach the floor of the middle cranial fossa, joins the sphenoidal emissary veins, and passes through the floor to reach the pterygoid plexus. 4, Superior petrosal type: the SMCV runs along the lesser wing and just before reaching the cavernous sinus, turns downward along the anterior inner wall of the middle cranial fossa, then runs along its floor medially to the foramen ovale to join the superior petrosal sinus. 5, Basal type: the SMCV runs along the lesser wing, turns downward along the anterior wall of the middle cranial fossa, then runs along its floor laterally to the foramen ovale over the petrous pyramid, presumably to join the transverse sinus through the lateral tentorial sinus or superior petrosal sinus. 6, Squamosal type: the SMCV fails to turn medially to join the sinus along the lesser wing, and instead turns directly backward along the inner aspect of the temporal squama and runs posteriorly to join the transverse sinus or lateral tentorial sinus. 7, Undeveloped type: the SMCV is absent, and the superficial sylvian drainage is through a large channel that extends forward, upward, upward and backward, or downward and backward into the superior sagittal sinus or transverse sinus
1. Sphenoparietal type: The SMCV enters the dura and runs along the lesser wing of the sphenoid bone (lesser wing) to enter the cavernous sinus. The dural portion of this channel along the lesser wing is frequently referred to as the sphenoparietal sinus.
2. Cavernous type: The SMCV enters the anterior end of the cavernous sinus directly.
3. Emissary type: The SMCV courses along the lesser wing, turns inferiorly to reach the floor of the middle cranial fossa, then joins the sphenoidal emissary veins and passes through the floor to reach the pterygoid plexus.
4. Superior petrosal type: The SMCV runs along the lesser wing, turns downward posteriorly along the anterior inner dural wall of the middle cranial fossa just before reaching the cavernous sinus, then runs along its floor medially to the foramen ovale and just lateral to the cavernous sinus and joins the superior petrosal sinus.
5. Basal type: The SMCV runs along the sylvian fissure, turns downward posteriorly along the anterior wall of the middle cranial fossa, then runs along its floor lateral to the foramen ovale to join the transverse sinus through the lateral tentorial sinus or superior petrosal sinus.
6. Squamosal type: The SMCV does not turn medially to join the sinus along the lesser wing on reaching the pterion, but turns directly backward along the inner aspect of the temporal squama and runs posteriorly to join the transverse sinus or the lateral tentorial sinus.
7. Undeveloped type: The SMCV is absent, and the venous drainage of the superficial sylvian area is through a large channel that extends forward, upward, upward and backward, or downward and backward into the superior sagittal sinus or the transverse sinus.
The restricted exploration area prevented inspection of the upward drainage system. Consequently, cases in which the SMCV could not be identified around the skull base area were included in the undeveloped type.
Results
Table 1 summarizes the distribution of each drainage type among our patients. Three-dimensional CT angiography clearly depicted the course of the SMCV running along the lesser wing or the middle cranial fossa. However, the outflow vessel could not be confirmed as the sphenoparietal, cavernous, or emissary types in 39 (8%) of the sides. The course and outflow point of the SMCV flowing into other cortical veins or running through the middle cranial fossa to join the transverse sinus or superior petrosal sinus could be accurately identified. The SMCV was present in 456 sides, and entered the cavernous sinus or sphenoparietal sinus in 62% of the sides (Fig 2A and B), joining the emissary veins in 12% (Fig 2C–F). The superior petrosal type was present in nine sides (Fig 2G), the basal type in 10 sides (Fig 2C–F, K, and L), the squamosal type in 12 sides (Fig 2M), and the undeveloped type in 44 sides (Fig 2N).
Table 1:
Distribution of drainage veins from the superficial sylvian area by 3D CT angiography
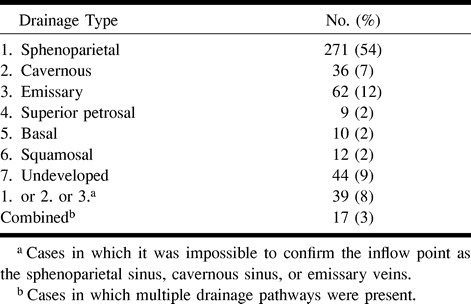
fig 2.

Variations of the superficial sylvian venous drainage pathway revealed by 3D CT angiography.
A, Axial view. The SMCV enters the sphenoparietal sinus (arrow).
B, Axial view. The SMCV directly enters the anterior end of the cavernous sinus (arrows).
C–F, Axial stereo and oblique views. The right SMCV courses along the lesser wing, turns inferiorly to reach the floor of the middle cranial fossa, and enters the foramen ovale (open arrows). This vein anastomoses with the cavernous sinus through the paracavernous sinus (arrowhead). The left SMCV turns downward along the anterior wall of the middle cranial fossa, then runs along its base laterally to the foramen ovale over the petrous pyramid, presumably to join the transverse sinus at the sigmoidal angle (closed arrows).
G–J, Axial stereo and oblique views. The SMCV turns medially into the sinus of the lesser wing, and before reaching the cavernous sinus turns downward along the anterior inner wall of the middle cranial fossa and runs posteriorly to join the superior petrosal sinus (arrows).
K and L, Axial stereo views. The SMCV turns downward along the anterior wall of the middle cranial fossa at the lesser wing and then runs along its floor laterally to the foramen ovale to drain into the transverse sinus (arrows).
M, Axial view. The SMCV turns directly backward along the inner aspect of the temporal squama and runs posteriorly to join the transverse sinus (arrows).
N, Axial view. The SMCV is absent, and the sylvian drainage area is taken over by a superficial temporal vein, which extends downward and backward into the lateral tentorial sinus (arrows).
O, Axial view. The remnant of the tentorial sinus originates from the cavernous sinus, runs beyond the petrous pyramid, and enters the lateral tentorial sinus. Note the anastomosis between the anterior end of the cavernous sinus and the basal vein of Rosenthal (arrowheads).
L, left; R, right; A, anterior; P, posterior.
Three-dimensional CT angiography could also depict the remnant of the tentorial sinus, originating from the cavernous sinus, running beyond the petrous pyramid, and entering the lateral tentorial sinus (Fig 2).
Discussion
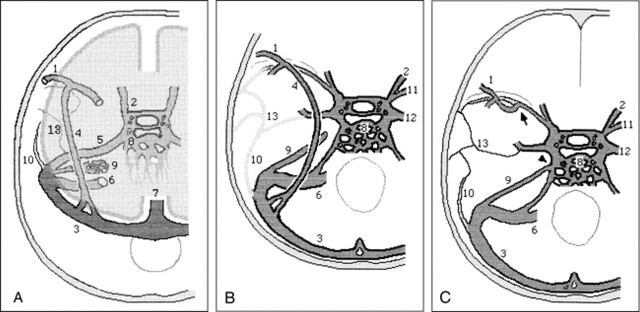
Adult venous patterns develop in the head region during the first three months of prenatal life, but the remnants of the embryonic tentorial sinus preserve a different drainage pattern for a little while after birth (4, 5). Two embryologic sinuses participate in the formation of the SMCV, the tentorial sinus, and the prootic sinus. The tentorial sinus can be identified by the 20-mm stage of embryonic development. Subsequent growth of the posterior cerebral hemisphere causes the tentorial sinus to elongate posteriorly, and the anterior portion is shifted medially (Fig 3A). The SMCV drains through the tentorial sinus into the transverse sinus rather than into the cavernous sinus during prenatal life and early infancy. The prootic sinus develops from the stem of the middle dural plexus by the 17- to 20-mm stage of development and is involved in the formation of the dura of the middle cranial fossa and the diploic vein (6, 7). The prootic sinus contributes to the cavernous sinus, the sphenoparietal sinus (3, 5), the meningeal sinus (eg, the emissary veins around the foramen ovale) (5, 8), and the anterior parietal temporal diploic veins in the 3-month-old embryo (Fig 3B). The superior orbital vein, which at first enters the prootic sinus, joins the cavernous sinus in the adult.
fig 3.
Developmental stages of the basal cranial veins (after Padget).
A, 60-mm stage of embryologic development. The transverse sinus has swung backward on the sigmoid sinus and receives the elongated tentorial sinus. A lateral tributary of the prootic sinus, which primarily receives the middle meningeal sinus, is continuous with the definitive petrosquamosal sinus. The tentorial sinus becomes plexiform caudally as it is shifted toward the sigmoid sinus. The ophthalmic veins primarily drain laterally through the prootic sinus, and secondarily drain medially through the cavernous sinus and inferior petrosal sinus.
B, Typical infant stage. The SMCV still drains through the tentorial sinus, which has a variable position. The superior petrosal sinus has not yet joined the cavernous sinus.
C, Typical adult stage. Note the secondary anastomoses between the cavernous sinus and sphenoparietal sinus, derived from the prootic sinus (arrow), and between the cavernous sinus and superior petrosal sinus (arrowhead). The typical lateral wing of the cavernous sinus, just below the mandibular nerve root, is a remnant of the prootic sinus and extends to the foramen ovale and emissary veins. The petrosquamosal sinus and remnant of the prootic sinus draining the dura and bone become diploic in the adult, when the middle meningeal sinuses drain through the foramen ovale and sigmoid sinus.
1, SMCV; 2, superior orbital vein; 3, transverse sinus; 4, embryonic tentorial sinus; 5, prootic sinus; 6, sigmoid sinus; 7, superior sagittal sinus; 8, cavernous sinus and inferior petrosal sinus; 9, superior petrosal sinus; 10, petrosquamosal sinus; 11, sphenoparietal sinus; 12, emissary venous drainage; 13, middle meningeal sinus.
Two secondary intradural anastomoses involving the cavernous sinus have generally developed before the adult stage: one is between the tentorial sinus and the anterior end of the cavernous sinus (Fig 3C, arrow), whereas the caudal part of the tentorial sinus, which drains into the transverse sinus, has dwindled, and the other is between the cavernous sinus and the superior petrosal sinus (Fig 3C, triangle) (5). The former anastomosis is located between the sinus originating from the tentorial sinus and the sinus from the prootic sinus.
A secondary anastomosis between the cavernous sinus and the tentorial sinus is less likely to occur when the tentorial sinus is located more laterally (5). This depends on the position of the tentorial sinus in the middle cranial fossa (ie, a more medial location is more likely to form anastomoses with the cavernous sinus). Therefore, the adult SMCV variation is based on the relative development of the anastomoses between the pathway from the SMCV through the tentorial to the transverse sinus and the pathway from the cavernous sinus to the pterygoid plexus to the superior or inferior petrosal sinus.
The lateral wall of the cavernous sinus is usually composed of two layers of dura mater (9–13). In one study, a venous channel was present between these layers in 24% of specimens (14). This channel connects with the SMCV and runs posteriorly through the middle cranial fossa toward the pterygoid plexus, the transverse sinus, or the superior petrosal sinus. The vascular channel and the two layers of the lateral dural wall do not occur concomitantly. This channel pattern corresponds to the description of the paracavernous sinus under the term tentorial sinus (15). Presumably, this channel protrudes laterally because of an incomplete anastomosis between the cavernous sinus and the tentorial sinus. This structure of two layers is formed from the complete anastomosis of these two sinuses.
Our classification system includes seven variations of the SMCV. The cavernous type is the anastomosis between the cavernous sinus and tentorial sinus. The emissary type is that which connects the tentorial sinus with the emissary vein stemming from the prootic sinus, attributed to incomplete medial transfer and partial persistence. The superior petrosal type is the persistent remnant of the tentorial sinus and a possible anastomosis with the superior petrosal sinus. The basal type is that in which the tentorial sinus does not reach and anastomose with the prootic sinus, and therefore has developed compensatory vessels to drain the SMCV. The squamosal type is that in which the tentorial sinus is only slightly displaced medially or laterally by the developing hemisphere toward the temporal squama. Furthermore, there may be continuance between the petrosquamosal sinus and the tentorial sinus. The undeveloped type is the form of compensatory surrounding veins, since the tentorial sinus has dwindled without any anastomosis with the prootic sinus.
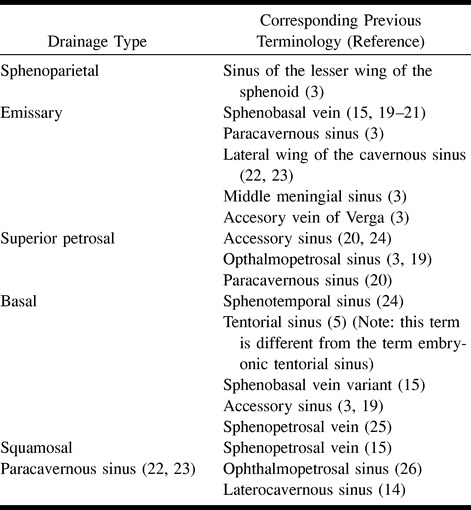
Previous classifications of the SMCV have been confusing and contradictory, without any basis in embryology. In some cases, several terms have been used to describe the same variant, and other times the same term has been used to describe different variations. Table 2 lists the terms of our classification system and the corresponding previous terminology. The confusion seems to have derived from a poor knowledge of embryology, a limited number of cadaveric specimens, inferior (low spatial resolution) angiographic images, and a poor comprehension of recent literature.
Table 2:
Comparison of classification systems for variants of the superficial middle cerebral vein
Three-dimensional CT angiography allows a variety of possible manipulations of the images from only one examination, high spatial resolution, and simultaneous visualization and evaluation of vessels and bone. However, in some cases, the sphenoparietal, cavernous, and emissary types of drainage were difficult to distinguish (16–18). This problem may be solved by examining images obtained in multiple directions and by limiting the images to the cavernous area by making reconstructions from thin-slice volume data. The superior petrosal, basal, and squamosal types of drainage were clearly differentiated. In addition, 3D CT angiography delineated the course and locality of veins that were previously obscure. In particular, we found that the emissary type flows out through the foramen ovale and the basal type runs laterally to the foramen ovale from anterior to posterior.
For neurosurgeons, information about the location of the SMCV along the floor of the middle cranial fossa, the outflow vein, and its junction is important for planning less invasive surgical approaches via the pterional, anterior temporal, subtemporal, or other routes. Hacker's classification (15) based on angiographic studies or examination of cadavers cannot provide accurate information about individual differences. In contrast, our classification, based on embryologic development and 3D CT angiographic evaluation, offers precise and practical information. Preoperative 3D CT angiography, by displaying multiple axial and oblique views, is likely to be useful for showing the vessels and their anatomic relationship to the bone structure as well as variations of the cranial venous system.
Conclusion
The classification of SMCV variants into seven types based on the changes seen during embryologic development combined with 3D CT angiographic evaluation can help identify and categorize the SMCV and its anatomic relationship to the middle cranial fossa bone structure in individual patients.
fig 2.
Continued
Footnotes
Address reprint requests to Yasuhiro Suzuki, MD, PhD.
References
- 1.DiChiro G. Angiographic patterns of cerebral convexity veins and superficial dural sinuses. AJR Am J Roentgenol 1962;87:308-321 [PubMed] [Google Scholar]
- 2.Jones FW, ed Buchanan's Manual of Anatomy.. London: Bailliere, Tindall and Cox; 1950:262
- 3.Wolf BS, Huang YP, Newton CM. The superficial sylvian venous drainage system. AJR Am J Roentgenol 1963;89:398-410 [PubMed] [Google Scholar]
- 4.Knosp E, Muller G, Perneczky A. Anatomical remarks on the fetal cavernous sinus and the veins of the middle cranial fossa. In: Dolenc VV, ed. The Cavernous Sinus: A Multidisciplinary Approach to Vascular and Tumorous Lesions. New York: Springer 1987 104-116
- 5.Padget DH. The cranial venous system in man in reference to development, adult configuration, and relation to the arteries. Am J Anat 1956;98:307-355 [DOI] [PubMed] [Google Scholar]
- 6.Zuckerkandle E. Beitrag zur Anatomie des Schlafenbeins. Monatsschr Ohrenheilk 1873;9:101-108 [Google Scholar]
- 7.Bailey P. Intracranial Tumors.. Springfield, Ill: Charles C Thomas; 1933
- 8.Padget DH. Development of cranial venous system in man, from view point of comparative anatomy (Carnegie Institution of Washington publication 611). Contrib Embryol 1957;36:79-140 [Google Scholar]
- 9.Bisaria KK. The superficial sylvian vein in humans: with special reference to its termination. Anat Rec 1958;212:319-325 [DOI] [PubMed] [Google Scholar]
- 10.Bonneville JF, Cattin F, Racle A, et al. Dynamic CT of the laterosellar extradural venous spaces. AJNR Am J Neuroradiol 1989;10:535-542 [PMC free article] [PubMed] [Google Scholar]
- 11.Mercier R, Patouillard P, Vanneuville G, Aussilhou A. Contribution a l'etude du sinus caverneux par l'utilisation simultanee de plusieurs modes d'investigation. C R Assoc Anat 1970;149:877-890 [PubMed] [Google Scholar]
- 12.Pauret G. Traite d'Anatomie Humaine.. Paris: Masson; 1958;745–752, tome III, fasc 2
- 13.Umansky F, Nathan H. The lateral wall of the cavernous sinus with special reference to the nerves related to it. J Neurosurg 1982;56:228-234 [DOI] [PubMed] [Google Scholar]
- 14.San Millan Ruiz D, Gailloud P, de Miquel Miquel MA, et al. Laterocavernous sinus. Acta Rec 1999;254:7-12 [DOI] [PubMed] [Google Scholar]
- 15.Hacker H. Abflusswege der Sylvischen Venengruppe. Radiologe 1968;8:383-387 [PubMed] [Google Scholar]
- 16.Aoki S, Sasaki Y, Machida T, Ohkubo T, Minami M, Sasaki Y. Cerebral aneurysms: detection and delineation using 3-D-CT angiography. AJNR Am J Neuroradiol 1992;13:1115-1120 [PMC free article] [PubMed] [Google Scholar]
- 17.Harbaugh RE, Schlusselberg DS, Jeffery R, Hayden S, Cromwell LD, Pluta D. Three-dimensional computerized tomography angiography in the diagnosis of cerebrovascular disease. J Neurosurg 1992;76:408-414 [DOI] [PubMed] [Google Scholar]
- 18.Takahana Y, Uno E, Wakamatsu K, Okada Y, Kaneko T, Tsuchiya Y. Three-dimensional computerized tomography angiography in a persistent primitive hypoglossal artery: a case report [in Japanese]. Jpn J Neurosurg 1998;7:125-128 [Google Scholar]
- 19.Ito J. Supratentorial venous system. In: Maki Y, Kuru H, eds. Neuroradiology I. Tokyo: Asakura Shoten 1986 443-484
- 20.Lang J. Tentorial sinus and transverse sinus. In: Lang L, ed. Clinical Anatomy of the Head. Berlin: Springer;1983;324–325
- 21.Oka K, Photon AL Jr, Barry M, Rodriguez R. Microsurgical anatomy of the superficial veins of the cerebrum. Neurosurgery 1985;17:711-748 [DOI] [PubMed] [Google Scholar]
- 22.Padget DH. The development of the cranial arteries in the human embryo (Carnegie Institution of Washington publication 575). Contrib Embryol 1948;32:205-261 [Google Scholar]
- 23.Padget DH. Designation of the embryonic intersegmental arteries in reference to the vertebral arteries and subclavian stem. Anat Rec 1954;119:349-356 [DOI] [PubMed] [Google Scholar]
- 24.Knott JF. On the cerebral sinus and their variations. J Anat Physiol (London) 1881;16:27-42 [PMC free article] [PubMed] [Google Scholar]
- 25.Yamakami I, Hirai S, Yamaura A, Ono J. Venous system playing a key role in transpetrosal approach [in Japanese]. No Shinkei Geka 1998;26:699-707 [PubMed] [Google Scholar]
- 26.Hyrtl J. Der sinus ophthalmo-petrosus. Wien Med Wochenschr 1862;19:291-292 [Google Scholar]