Abstract
Summary: Skull base osteomyelitis (SBO) is typically bacterial in origin and caused by Pseudomonas, although the fungus Aspergillus has also rarely been implicated. SBO generally arises from ear infections and infrequently complicates sinonasal infection. Rhinocerebral Mucor infection is characteristically an acute, fulminant, and deadly infection also affecting the orbits and deep face and is associated with intracranial complications. Bony involvement is uncommon because of the angioinvasive nature of the fungus. More recently, chronic invasive Mucor sinusitis has been described. We report the unusual clinical and imaging features of a patient with biopsy-proven invasive mucormycosis arising from chronic isolated sphenoid sinus disease, who presented with extensive SBO and a paucity of deep facial, orbital, or intracranial involvement.
Skull base osteomyelitis (SBO) is uncommon and poses both a diagnostic and therapeutic challenge. Most cases occur from contiguous spread of ear infection. Typically, it follows partially treated necrotizing (formerly known as “malignant”) otitis externa in a diabetic patient, and is caused by the bacteria Pseudomonas aeruginosa (1–5). Fungal SBO caused by Aspergillus species occurs less frequently, afflicts immunosuppressed patients, and also arises from necrotizing otitis externa (6). SBO originates rarely from sinonasal infection, and in these cases is usually caused by Aspergillus, Pseudomonas, Salmonella, and Staphylococcus species (2, 5–7). A few reports of chronic invasive mucormycotic sinusitis causing skull base osteomyelitis have appeared in the otorhinolaryngologic literature (8, 9); however, skull base involvement is generally a late and uncommon finding in this situation. More frequently, mucormycosis results in sinonasal, orbital, and deep facial soft-tissue infiltration and intracranial complications such as cavernous sinus involvement, cerebral abscesses, and infarcts (10–15). The late occurrence of bony involvement is explained by the angioinvasive nature of the fungus and characteristically deep extension of infection through perivascular channels that precedes frank bony destruction (10, 12, 16). We report a patient with biopsy-proven invasive mucormycosis and relatively clinically silent sphenoid sinus disease, presenting clinically and radiographically as SBO, with relative paucity of typical soft-tissue findings.
Case Report
A 55-year-old man, under treatment for relapse of B-cell chronic lymphocytic leukemia, first noticed headaches in May 1999 that were attributed to chronic sinusitis based on results of CT and MR imaging of the head performed at an outside institution, which showed mucosal thickening in the sphenoid sinuses. He had no other significant sinonasal symptoms. One month later, he was admitted to our institution with low-grade fever. Investigation revealed infection of his central venous line with Pseudomonas. Blood cultures were positive for gram positive cocci and cytomegalovirus. The central line was withdrawn, and the fever, white cell count elevation, and blood cultures responded to wide-spectrum antibiotics, including vancomycin and nafcillin, prophylactic fluconazole, and low-dose amphotericin B administered over 6 weeks.
Three days after discharge, the patient was readmitted for new-onset diplopia and worsening headache. He remained afebrile, but neurologic examination revealed a right sixth cranial nerve palsy. A 5-mm-thick contrast-enhanced CT scan of the head showed mucosal thickening in the sphenoid sinus, bony erosion in the right sphenoid wall, and lytic foci in the clivus (Fig 1). The middle ear cavities and mastoids were clear, as was the remainder of his paranasal sinuses. In view of the patient's immunocompromised state, diagnostic considerations included neoplastic infiltration of the skull base and atypical fungal sinusitis. Sinus endoscopy showed well-aerated sinuses and absence of discharge, polyps, or masses. Right-sided sphenoidotomy revealed mild mucosal thickening but little inflammation. The patient underwent stripping of the sphenoid mucosa and biopsy of the anterior sphenoid wall, which appeared brittle and necrotic. No further necrotic bone could be removed safely from the lateral sphenoid wall possibly because of dehiscence of the carotid artery. The biopsy (Fig 2) showed acute and chronic inflammation of the deep submucosal soft tissues, with numerous fungal forms extensively invading the underlying necrotic bone. The overlying mucosa was largely unaffected. The fungi had broad, nonseptated hyphae with occasional 90° branching, showing the morphologic characteristics of mucormycosis. The organisms were easily visible with hematoxylin and eosin and periodic acid schiff staining, but stained weakly with Gomori's methenamine silver. The culture and smear of the cerebrospinal fluid were negative.
fig 1.
Axial CT image through the skull base. A 5-mm-section using a standard soft-tissue algorithm and bone windowing shows mucosal thickening in the sphenoid sinuses, focal bony destruction of the right lateral sphenoid sinus wall (white arrowheads), and subtle lytic foci in the clivus (black arrowheads).
fig 2. Photomicrograph of the sphenoid sinus wall reveals broad hollow-appearing hyphae with 90° branching (arrowheads at 90° to each other) characteristic of mucormycosis. A large necrotic bone fragment is seen centrally (methanamine silver stain, original magnification ×400)
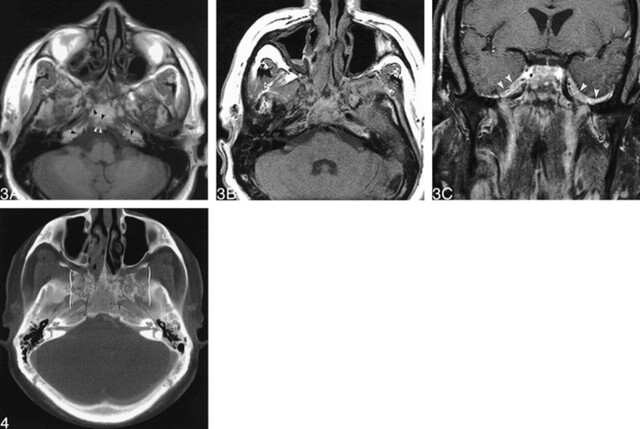
Review of the outside nonenhanced MR scan of the brain, performed 2 months earlier and not previously submitted for interpretation, showed patchy replacement of the normal fatty marrow of the clivus in addition to sphenoid sinus mucosal thickening (Fig 3A). An MR scan of the brain and paranasal sinuses performed after the sphenoidotomy and biopsy (Fig 3B and C) showed extensive, heterogeneous, and enhancing abnormal marrow signal in the central skull base, including the clivus, body, greater wings, and pterygoid plates of the sphenoid bone and both petrous apices. There was smooth dural enhancement of the floor and the anterior and medial walls of both middle cranial fossae. Postoperative changes were present in the sinuses. The normal fat planes of the deep face were preserved. No abnormalities were identified in the orbits. There was no evidence of cerebral abscesses or infarcts.
fig 3.
MR images through the skull base.
A, Axial T1-weighted (600/12/2 [TR/TE/excitations]) MR image, obtained from an outside institution and performed 10 weeks prior to CN VI palsy onset, reveals foci of signal hypointensity in the clivus and bilateral petrous apexes (arrowheads).
B, Axial T1-weighted (600/9/2) MR image, obtained at our institution 10 weeks after A, shows progressive heterogeneity of the marrow fat in the central skull base.
C, Contrast enhanced fat-suppressed coronal T1-weighted (600/9/2) MR image shows bilateral, thick, smooth enhancement of the dura of the medial wall and floor of the middle cranial fossae (arrowheads) and abnormal marrow enhancement in the clivus (asterisk).
fig 4. A 3-mm-thick CT image obtained using a high-resolution bone algorithm postoperatively more clearly shows the extensive erosive changes in the central skull base (brackets)
A 3-mm-thick contrast-enhanced CT scan of the paranasal sinuses performed with a high-resolution bone algorithm (unavailable on the earlier 8-mm-thick CT scan of the head obtained at an outside institution) depicted the striking and severe involvement of the skull base (Fig 4). This had a “ratty” moth-eaten appearance, with focal areas of cortical destruction.
The patient was treated with intravenous high-dose amphotericin B (15 mg/kg/day) and clinical improvement was noted. It was felt that further radical surgical debridement of the skull base could not be performed safely. He was discharged a month later with residual right–lateral gaze paresis. Repeat CT scanning of the paranasal sinuses 1 month after discharge showed stable bony findings. The patient remained clinically stable at the time of submission of this report.
Discussion
SBO is uncommon, and most cases arise from contiguous spread of ear infections (1–3). It typically occurs in a diabetic or immunosuppressed patient incompletely treated for necrotizing otitis externa. Pseudomonas is the usual bacterial pathogen; less frequently, Aspergillus afflicts immunosuppressed patients (6). SBO may originate rarely from sinonasal infection; microorganisms include Aspergillus, Pseudomonas, Salmonella, and Staphylococcus (2, 5, 7). More often, complicating infection of the paranasal sinuses associated with osteomyelitis tends to involve the cancellous bone of the cranial vault (such as the frontal bones) rather than the skull base (2, 4, 5). Mucormycotic SBO is rare, but has been described as a late finding (8, 9). Our patient had a highly unusual presentation of extensive SBO caused by isolated sphenoid sinus mucormycosis, with a paucity of sinonasal symptoms and erosions of the clivus demonstrable 10 weeks prior to the onset of his sixth cranial nerve palsy.
Classically, rhinocerebral mucormycosis is an acute, fulminant, and often lethal opportunistic infection (8–10, 12–14, 17). It is caused by one of the members of the mucoraceal family, including Absidia, Mucor, and Rhizopus (9–15, 17, 18). Histologic distinction of the Mucor fungi from the more common Aspergillus is based on the characteristically broad, “empty-appearing,” nonseptated hyphae of these fungi, which are readily visible after routine hematoxylin and eosin staining (8, 9, 15, 17). After inhalation into the nasal cavity and paranasal sinuses, the fungi infect the immunosuppressed or diabetic host by causing a necrotizing vasculitis of the nose and sinuses, and rapidly extend into the orbits, deep face, and cranial cavity (10–16). This results from perivascular, perineural (19), or direct soft-tissue invasion by the fungi, causing a suppurative arteritis, vascular thrombosis, and infarction of surrounding tissues. Patients often present acutely with headache, fever, facial swelling, sinusitis, and unilateral orbital apex syndrome (10–13, 15, 17, 20). Neurologic deficits and obtundation may occur secondary to intracerebral abscess formation and septic thrombosis of major intracranial vessels (10–13, 15).
In the appropriate clinical context, the imaging findings of rhinocerebral mucormycosis on CT and MR imaging are diagnostic (10–15). These include soft-tissue opacification of sinuses with hyperdense material, nodular mucosal thickening, and an absence of fluid levels (10, 15) in the maxillary, ethmoid, frontal, and sphenoid sinuses, in decreasing order of incidence (10). Sinus contents have a variety of MR signal characteristics, including T2 hyperintensity or marked hypointensity on all sequences, possibly secondary to the presence of iron and manganese in the fungal elements (11–13). Soft-tissue infiltration of the deep face and obliteration of the normal fat planes in the infratemporal fossa, pterygopalatine fossa, pterygomaxillary fissure, and periantral fat are often present (10, 11, 16). Typically, proptosis occurs because of enhancing soft-tissue masses crowding the orbital apex (15) and the cavernous sinuses (11, 12). Thickening and lateral displacement of the medial rectus muscle are characteristic of orbital invasion from disease in the ethmoid sinuses (15). Lack of enhancement of the superior ophthalmic vein or ophthalmic and internal carotid arteries may be seen and is related to vasculitis and thrombosis (14). Flow-sensitive gradient-echo MR sequences are useful in documenting arterial thrombosis (11, 12). MR angiography may also be used for this purpose. Intracranial findings include infarcts related to vascular thrombosis, mycotic emboli, and frontal lobe abscesses (10–13).
Skull base osteomyelitis and bony involvement from sinonasal mucormycosis is usually absent despite deep extension of disease and, when present, occurs last in the course of the disease (10, 12). This has been attributed to the angioinvasiveness of the fungi and their propensity to extend into the soft tissues of the orbit and deep face and into the brain by way of vessels penetrating through partitions in the skull base (10, 16). Therefore, we were surprised by the biopsy results from our patient not only because he was free of sinonasal symptoms other than headache, but because radiographic results indicated an infiltrating and destructive process of the skull base, with relative paucity of deep facial and orbital soft-tissue or intracranial findings. The presence of an isolated sixth nerve palsy in the absence of other (third, fourth and fifth) cranial neuropathies was consistent with a predominantly skull base process.
Our patient was immunosuppressed by the chemotherapeutic regimen for his leukemia, making him susceptible to fungal infection. Isolated sphenoid sinusitis in the immunocompromised patient is notoriously occult save for the nonspecific symptoms of headache (21). The low-dose antifungal agents administered as part of the treatment regimen for his fever and positive blood cultures may have prevented him from succumbing to a more acute and fulminant form of rhinocerebral mucormycosis, but at risk for SBO. There has been increasing evidence supporting a more chronic indolent form of invasive mucormycosis (8, 9, 17, 20), occasionally complicated by SBO (8, 9, 17). Indeed, rhinocerebral mucormycosis with SBO may be under-recognized if a high-resolution bone algorithm CT study is not employed (3). MR imaging, however, especially the nonenhanced T1-weighted sequence, is quite sensitive to minor pathologic abnormalities in the skull base. The characteristic finding is lost T1 signal hyperintensity of skull base marrow fat, which was observed in our case.
Management of chronic rhinocerebral mucormycosis is not well established (8, 9, 17, 20). Wide surgical debridement where feasible, prolonged high-dose systemic amphotericin B, control of underlying comorbid factors, and hyperbaric oxygen are used. Survival rates range from 21% to 70%, with higher mortality among patients without diabetes (22). For patients with underlying hematologic malignancies, resolution of chemotherapy-induced neutropenia is correlated with recovery (18). Nevertheless, prognosis for our patient is guarded, and close surveillance with high-resolution CT and MR imaging is warranted (2, 3). The role of gallium-67 imaging in monitoring response to therapy is well recognized in SBO of bacterial and fungal origin (1–3, 6, 7), although its use in SBO from mucormycosis has not been reported.
In this era of aggressive use of multipotent prophylactic antibiotics, chronic/subacute forms of invasive mucormycosis such as SBO may rise in incidence, especially in the immunosuppressed. A high index of suspicion needs to be maintained, and an awareness of the atypical clinical and radiographic features of this potentially fatal invasive fungal infection is important.
Footnotes
Address reprint requests to Lawrence E. Ginsberg, MD, Diagnostic Radiology, Box 57, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.
References
- 1.Chandler JR, Grobman L, Quencer R, et al. Osteomyelitis of the base of the skull. Laryngoscope 1986;96:245-251 [DOI] [PubMed] [Google Scholar]
- 2.Grobman LR, Ganz W, Casiano R, Goldberg S. Atypical osteomyelitis of the skull base. Laryngoscope 1989;99:671-676 [DOI] [PubMed] [Google Scholar]
- 3.Murray ME, Britton J. Osteomyelitis of the skull base: the role of high resolution CT in diagnosis. Clin Radiology 1994;49:408-411 [DOI] [PubMed] [Google Scholar]
- 4.Senegor M, Lewis HP. Samonella osteomyelitis of the skull base. Surg Neurol 1991;36:37-39 [DOI] [PubMed] [Google Scholar]
- 5.Hoistad DL, Duvall AJ. Sinusitis with contiguous abscess involvement of the clivus and petrous apices. Ann Otol Rhinol Laryngol 1999;108:463-466 [DOI] [PubMed] [Google Scholar]
- 6.Kountakis SE, Kemper JV, Chang CY, DiMaio DJ, Stiernberg CM. Osteomyelitis of the base of the skull secondary to Aspergillus. Am J Otolaryngol 1997;18:19-22 [DOI] [PubMed] [Google Scholar]
- 7.Parker KM, Nicholson JK, Cezayirli RC, Biggs PJ. Aspergillosis of the sphenoid sinus: presentation as a pituitary mass and postoperative gallium-67 imaging. Surg Neurol 1996;45:354-358 [DOI] [PubMed] [Google Scholar]
- 8.Bahna MS, Ward PH, Konrad HR. Nasopharyngeal mucormycotic osteitis: a new syndrome characterized by initial presentation of multiple cranial nerve palsies. Otolaryngol Head Neck Surg 1980;88:146-153 [DOI] [PubMed] [Google Scholar]
- 9.Finn DG, Farmer JC. Chronic mucormycosis. Laryngoscope 1982;92:761-766 [DOI] [PubMed] [Google Scholar]
- 10.Gamba JL, Woodruff WW, Djang WT, Yeats AE. Craniofacial mucormycosis: assessment with CT. Radiology 1986;160:207-212 [DOI] [PubMed] [Google Scholar]
- 11.Press GA, Weindling SM, Hesselink JR, et al. Rhinocerebral mucormycosis: MR manifestations. JCAT 1988;12:744-749 [DOI] [PubMed] [Google Scholar]
- 12.Yousem DM, Galetta SL, Gusnard DA, Goldberg HI. MR findings in rhinocerebral mucormycosis. JCAT 1988;13:878-882 [DOI] [PubMed] [Google Scholar]
- 13.Terk MR, Underwood DJ, Zee C, Colletti PM. MR imaging in rhinocerebral and intracranial mucormycosis with CT and pathological correlation. MRI 1992;10:81-87 [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick C, Tress B, King J. Computed tomography of rhinocerebral mucormycosis. Neuroradiology 198;26:71-73 [DOI] [PubMed] [Google Scholar]
- 15.Centeno RS, Bentson JR, Mancuso AA. CT scanning in rhinocerebral mucormycosis and aspergillosis. Radiology 1981;140:383-389 [DOI] [PubMed] [Google Scholar]
- 16.Silverman CS, Mancuso AA. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: CT and MR findings. AJNR Am J Neuroradiol 1998;19:321-325 [PMC free article] [PubMed] [Google Scholar]
- 17.Harrill WC, Stewart MG, Lee AG, Cernoch P. Chronic rhinocerebral mucormycosis. Laryngoscope 1996;106:1292-1297 [DOI] [PubMed] [Google Scholar]
- 18.Pagano L, Ricci P, Tonso A, et al. Mucormycosis in patients with hematological malignancies: a retrospective clinical study of 37 cases. Br J Haematology 1997;99:331-336 [DOI] [PubMed] [Google Scholar]
- 19.McLean FM, Ginsberg LE, Stanton CA. Perineural spread of rhinocerebral mucormycosis. AJNR Am J Neuroradiol 1996;17:114-116 [PMC free article] [PubMed] [Google Scholar]
- 20.DeShazo RD, O'Brien M, Chapin K, et al. A new classification and diagnsotic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg 1997;123:1181-1188 [DOI] [PubMed] [Google Scholar]
- 21.Holt GR, Standefer JA, Brown WE, Gates GA. Infectious diseases of the sphenoid sinus. Laryngoscope 1984;94:330-335 [DOI] [PubMed] [Google Scholar]
- 22.Blitzer A, Lawson W, Meyers BR, Biller HF. Patient survival factors in paranasal sinus mucormycosis. Laryngoscope 1980;90:635-648 [DOI] [PubMed] [Google Scholar]