INTRODUCTION
In the year 1956, two researchers, Puck and Marcus, published a seminal paper detailing a new method of assessing the colony formation ability of mammalian cells (1). This type of culture analysis would later be deemed the clonogenic assay. The clonogenic assay is an in vitro cell survival assay that helps us characterize mammalian cell line features like the ability to grow in a colony. The simplicity and robustness of this method have allowed researchers to discover key differences between cellular behaviors found in tumors versus normal tissues, as well as how these behaviors can be altered when cells are exposed to potential drug therapies (2).
The characteristics that make the clonogenic assay a great tool in the research laboratory also make it a great technique to introduce in the undergraduate laboratory classroom. We have used this assay as a tool in the undergraduate biology laboratory, exposing students to a more specialized form of mammalian cell culture and helping them refine scientific research skills and knowledge. Moreover, the clonogenic assay’s relatedness and amenability to answering interesting research questions in cancer biology makes it an ideal hands-on method for undergraduates to gain familiarity with a research topic that has universal impact. Due to its prevalence, cancer biology is an effective way to engage students with life sciences research (3). While there are many didactic resources available to learn about cancer, direct access to undergraduate research experiences in the classroom lab regarding the topic is limited. In this paper, we describe how HeLa cells can be used as a mammalian model to teach undergraduate students to carry out clonogenic assays and implement techniques such as growing, harvesting, counting, diluting, fixing, staining, and imaging mammalian cells. These experiments also easily allow undergraduates to test the effects of different treatments on HeLa cell colony formation. Overall, this approach allows students to experience how changing the cellular environment can lead to differences in cancer cell behaviors. Furthermore, this activity allows students to acquire skills that can spark their interest in biomedical research.
PROCEDURE
Safety Considerations and Precautions
Prior to the first laboratory session, students were required to complete relevant online Collaborative Institutional Training Initiative (CITI) training, on-site blood-borne pathogen and laboratory safety guidelines training, a hepatitis B vaccine declination form, and also to sign a document confirming that they agreed to comply with laboratory regulations. These requirements follow standard Biosafety Level 2 (BSL-2) recommendations, which include use of a laminar flow hood or biosafety cabinet, protective eyewear, lab coats, closed-toe shoes, and gloves at all times, as well as proper disposal of BSL-2 materials (such as HeLa cells) and reagents (4). Students were also required to watch videos on laboratory safety (North Carolina Community Colleges BioNetwork Lab Safety Video Series; https://www.youtube.com/playlist?list=PL4qaj9envIYnBaQSPpcOMUqWiQUAgPoMq) and complete a quiz with a passing grade to demonstrate their understanding. More details about the safety considerations and precautions are provided in Appendix 1.
Materials and Methods
Instructor preparation
Detailed information on how instructors can best prepare to teach and implement using HeLa cells in the undergraduate lab can be found in our previous publication (5).
Implementation of experiments in the undergraduate laboratory
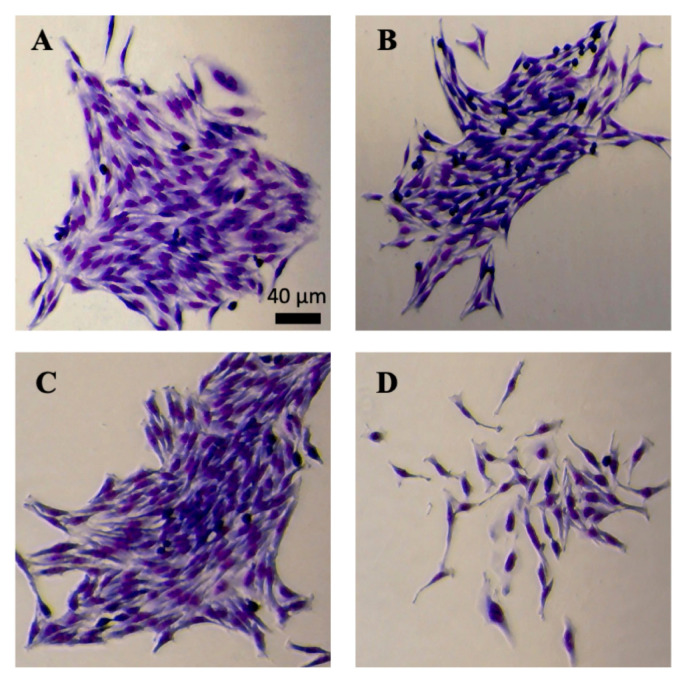
In preparation to perform clonogenic assays, students were required to complete safety training (Appendix 1), given a lab skills and aseptic technique review, and tissue culture introduction. Groups of two to three students discussed the video, watched the instructor passage HeLa cells onto new plates, and mirrored the technique independently (Appendix 2), ultimately generating their own HeLa cell starter plate to care for. Students grew a population of HeLa cells on this 100-mm plate to ≈50 to 80% confluency and used the cells harvested to set up their clonogenic assay (Appendix 3). In short, the spent medium from the plate was aspirated and the plate was washed with 3 mL of phosphate-buffered saline (PBS) twice. One milliliter of warm 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) solution was then added, and the plate was set to rest in the 37°C incubator for 5 minutes. After 5 minutes, 1 mL of warm medium was added to the plate to neutralize trypsin-EDTA, and the resulting cell suspension was pipetted approximately 10 to 15 times in order to break up cell clumps and make a homogeneous cell suspension. Students transferred 2 mL of this cell suspension to a 15-mL conical tube, and a 10-μL aliquot was taken from this to be used in a counting slide. Using a microscope, students calculated the average cell concentration per square centimeter with the counting slide. This average was then used to calculate the number of cells per microliter of the cell suspension. Using this calculated cell concentration, students adjusted the volume (or generated a new more dilute suspension) to 10 cells/μL. Finally, six-well plates were inoculated with 10 μLof the cell suspension, or 100 cells, per well for the clonogenic assay. Students then selected the conditions to test the clonogenic capacity of HeLa cells. One option included different concentrations of fetal bovine serum (FBS), either 5% FBS or 10% FBS. Another option included different anticancer agents, either fisetin or cycloheximide. When one of the anticancer agents was chosen, the effect of the agent was compared to the control treated with dimethyl sulfoxide (DMSO). Exemplary pictures of enlarged colonies after treating the cells with different FBS concentrations and fisetin are shown in Fig. 1A and B and 1C and D, respectively. As expected, when treated with the anticancer agent fisetin, the colony consisted of many fewer cells compared with the DMSO-treated control.
FIGURE 1.
Micrographs of HeLa cell colonies grown under different conditions. Clonogenic assays were performed using six-well plates. Cells were grown in DMEM supplemented with 10% FBS (A), 5% FBS (B), 10% FBS and DMSO (C), or 10% FBS and 60 μM fisetin (D). All images were acquired after HeLa cell colonies were fixed, stained with eosin dye and then methylene dye, and rinsed with water. Scale bar, 40 μm.
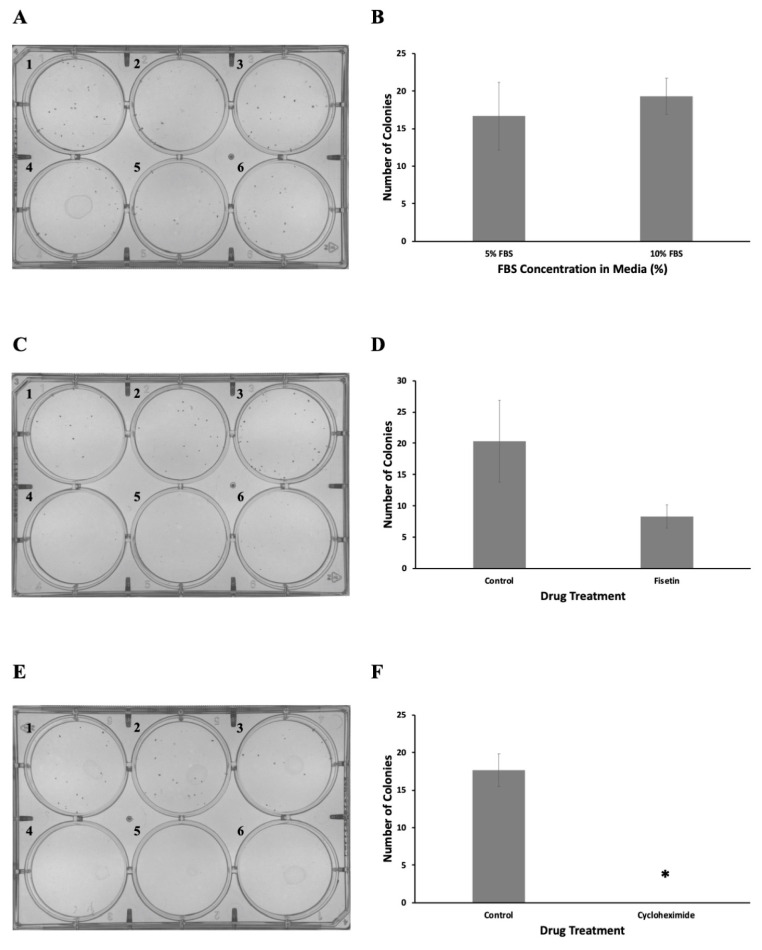
If the students selected the FBS, 3 mL of Dulbecco’s modified Eagle medium (DMEM) with 5% FBS was pipetted into each well in top row of the plate (wells A1 to A3). Then, 3 mL of 10% FBS containing DMEM was pipetted into the bottom row of the plate (A4 to A6). If the students selected one of the anticancer agents, 3 mL of DMSO-containing medium was added to the top rows of the plate (C1 to C3, E1 to E3), whereas the medium containing the anticancer agent (60 μM fisetin or 50 μg/mL cycloheximide) was added to the bottom rows of the plate (C4 to D6, E4 to E6). Finally, the plates were labeled and incubated at 37°C in a humidified atmosphere consisting 5% CO2 for 1 week. After 1 week, students removed the assay plates from the incubator, and the spent medium was carefully aspirated. Students then used the Richard-Allan Scientific three-step stain set (Thermo Fisher cat. no. 3300) to fix and stain cell colonies. Briefly, wells were filled with 0.5 mL of methanol fixative (fixative solution of the kit) and left to incubate at room temperature for 2 minutes. After removal of the fixative solution, 0.5 mL of eosin Y dye (solution A of the kit) was added to each well and incubated for 2 minutes at room temperature. Eosin dye was then removed and the plate was incubated with methylene blue–azure A solution (solution B of the kit) for 2 minutes, also at room temperature. Finally, all wells were aspirated and gently rinsed with deionized water. The resulting plates were analyzed under a microscope, and the numbers of colonies were counted and recorded. The plates with colonies are shown in Fig. 2A, C, and E. Graphs were generated with Microsoft Excel software. Briefly, treatments were carried out in triplicate and the numbers of colonies were counted. The average values were used to make graphs, and the error bars represent standard errors of the means (SEMs) (Fig. 2B, D, and F). As expected, we observed fewer colonies when cells were treated with the anticancer agents fisetin and cycloheximide. Statistical analysis of the data was performed using Excel software. The t test analyses were used to compare the means of the groups. We used unpaired two-sample t test as both samples consist of distinct test subjects. We performed a two-tailed test, as we wanted to test whether the treatment resulted in significantly more or fewer colonies than the control. P values of <0.01 were considered statistically significant.
FIGURE 2.
Representative student results for the clonogenic assays. Clonogenic assays were performed in six-well plates with HeLa cells. (A) Cells were grown in media supplemented with 5% FBS (plates 1 to 3) or 10% FBS (plates 4 to 6). On average, 16.7 and 19.3 colonies were formed under 5% and 10% FBS conditions, respectively. (B) The data are presented as means ± SEM. P > 0.01 versus control. (C) Cells were grown in 10% FBS containing DMEM with DMSO (control; plates 1 to 3) or 60 μM fisetin (plates 4 to 6). On average, 20.3 and 8.3 colonies were formed under control and fisetin treatment, respectively. (D) The data are presented as means ± SEM. P > 0.01 versus control. (E) Cells were grown in 10% FBS containing DMEM with DMSO (control, plates 1 to 3) or 50 μg/mL cycloheximide (plates 4 to 6). On average, 17.7 colonies formed in the control group. No colonies formed when treated with cycloheximide. (F) The data are presented as means ± SEM. *, P < 0.01 versus control. All assays were performed in triplicate.
Intended audience
This laboratory exercise is intended for undergraduate students in college or university. At High Point University (HPU), this exercise was implemented in an upper-level cancer biology class to explore concepts related to tumor growth and characteristics of human cancer cells in culture.
Alternative applications
The simplicity and robustness of the experiments described above also make it appropriate for implementation in lower-level biology courses, as long as the class size and cell culture facilities can accommodate the students enrolled in the courses of interest.
CONCLUSION
This laboratory activity is an effective way for undergraduate students to explore mammalian cell colony formation, reinforcing student understanding of cell biology and cancer biology. Furthermore, students have opportunities to formulate hypotheses, draw inquiry-based conclusions, and utilize the scientific process.
SUPPLEMENTAL MATERIALS
Footnotes
Supplemental materials available at http://asmscience.org/jmbe
REFERENCES
- 1.Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]; Puck TT, Marcus PI. 1956. Action of x-rays on mammalian cells. J Exp Med 103653–666. 10.1084/jem.103.5.653.
- 2.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]; Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. 2006. Clonogenic assay of cells in vitro. Nat Protoc 12315–2319. 10.1038/nprot.2006.339.
- 3.Hawkins AJ, Stark LA. More than metaphor: Online resources for teaching cancer biology. CBE Life Sci Educ. 2017;16:fe5. doi: 10.1187/cbe.17-05-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hawkins AJ, Stark LA. 2017. More than metaphor Online resources for teaching cancer biology. CBE Life Sci Educ 16fe5. 10.1187/cbe.17-05-0087.
- 4.Emmert EAB. Biosafety guidelines for handling microorganisms in the teaching laboratory: development and rationale. J Microbiol Biol Educ. 2013;14:78–83. doi: 10.1128/jmbe.v14i1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]; Emmert EAB. 2013. Biosafety guidelines for handling microorganisms in the teaching laboratory development and rationale. J Microbiol Biol Educ 1478–83. 10.1128/jmbe.v14i1.531.
- 5.Bowey-Dellinger K, Dixon L, Ackerman K, Vigueira C, Suh YK, Lyda T, Sapp K, Grider M, Crater D, Russell T, Elias M, Coffield VM, Segarra VA. Introducing mammalian cell culture and cell viability techniques in the undergraduate biology laboratory. J Microbiol Biol Educ. 2017;18(2) doi: 10.1128/jmbe.v18i2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bowey-Dellinger K, Dixon L, Ackerman K, Vigueira C, Suh YK, Lyda T, Sapp K, Grider M, Crater D, Russell T, Elias M, Coffield VM, Segarra VA. 2017. Introducing mammalian cell culture and cell viability techniques in the undergraduate biology laboratory. J Microbiol Biol Educ 18(2). 10.1128/jmbe.v18i2.1264.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.