Abstract
Summary: This study introduces a new, hybrid embolic device that in addition to offering all the important attributes of existing detachable platinum coils also shows an enhanced ability to fill aneurysm cavities. The device consists of a carrier platinum coil coupled to an expandable hydrogel material, which undergoes a ninefold increase in volume when placed into a physiological environment. Distinct from previous devices aimed at speeding the organization of thrombus, the new device has been designed to entirely fill the aneurysm cavity, with complete or near-complete exclusion of thrombus. Unlike thrombus, the hydrogel material is stable and unaffected by natural thrombolytic processes and thus may diminish observed rates of aneurysm recanalization. We report the angiographic and histologic findings of the new, hybrid device used to treat experimental aneurysms in rabbits.
Endovascular treatment of aneurysms was revolutionized by the introduction of the Guglielmi detachable coil (GDC; Boston Scientific, Natick, MA) during the early 1990s (1–4). The GDC offers several design features that allow placement into both ruptured and unruptured aneurysms with relatively low risk of aneurysm perforation or parent artery compromise. These features include trackability, softness, low profile design, radiopacity, low thrombogenicity, and retrievability. However, important limitations of the GDC have become manifest, including relatively low rates of complete aneurysm obliteration and high rates of aneurysm recanalization, especially in cases of large and giant aneurysms (5, 6). Even in densely packed aneurysms, the cavities are filled with only 20% to 30% of platinum, with the remaining 70% to 80% filled with thrombus (7). At least in large and giant aneurysms, the coil-induced thrombus remains unorganized for long periods of time. Natural thrombolytic processes acting on this unstable thrombus may, in part, explain the high rates of clinically observed aneurysm regrowth. Shortcomings of the GDC have led to numerous descriptions of alternative devices aimed at improving the long-term occlusion rates of aneurysms, primarily centered on surface modifications of platinum coils that might enhance the organization of thrombus within aneurysms (8–15).
Methods
Elastase-induced, saccular aneurysms were created in 16 New Zealand White rabbits. After aneurysm creation, at least 21 days elapsed before embolization. After embolization, the study animals were followed up for 2 weeks (n = 4), 1 month (n = 4), 3 months (n = 4), and 6 months (n = 4). Detailed procedures for aneurysm creation have been described elsewhere (16). Briefly, New Zealand White rabbits (3–4 kg) are anesthetized with ketamine and xylazine (60 and 6 mg/kg, respectively). Using a sterile technique, the right common carotid artery is exposed and ligated distally. A 5F sheath (Cordis Endovascular, Miami Lakes, FL) is advanced retrograde in the common carotid artery to a point approximately 3 cm cephalad to the common carotid artery origin. Through this indwelling sheath, a 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, CA) is advanced to the origin of the right common carotid artery at its junction with the subclavian artery. The balloon is inflated with iodinated contrast material just enough to achieve flow arrest in the common carotid artery. Porcine elastase (5.23 μ/mgP, 40.1 mgP/mL, approximately 200 U/mL; Worthington Biochemical Corporation, Lakewood, NJ) mixed with saline and iodinated contrast material is incubated in the dead space of the common carotid artery, above the inflated balloon. After incubation of the elastase solution, the balloon and sheath are removed, and the common carotid artery is ligated below the sheath entry site.
Coil Characteristics
Platinum Coils.—
MicroPlex Coiling System (MicroVention, Inc., Aliso Viejo, CA) 0.010- and 0.018-inch complex coils were deployed first in the aneurysm to establish a cagelike framework to stabilize the aneurysm. A variety of sizes (4–8 mm) and lengths (10–20 cm) of the complex coil were evaluated in this study.
Hybrid Hydrogel-Platinum Coil Device.—
The HydroCoil Embolization System (MicroVention, Inc.) is compatible with microcatheters of 0.017-inch inner diameter. These devices consist of a synthetic, polymeric hydrogel attached to a platinum coil (Fig 1). The hybrid hydrogel-platinum coil device was used as a filler device after placement of the complex platinum coils. The initial diameter of these devices is approximately 0.010 inch, and the expanded diameter is approximately 0.035 inch. Bench data suggested that the hydrogel material remained sufficiently constrained to allow deployment and retraction back into the microcatheter for at least 5 minutes. Thus, for the purposes of this study, the operator was limited to 5 minutes of repositioning time. In blood, the hydrogel swells to its maximum diameter approximately 20 minutes after submersion. Several sizes (2–6 mm) and lengths (5–15 cm) of the hybrid device were evaluated in this study.
Fig 1.
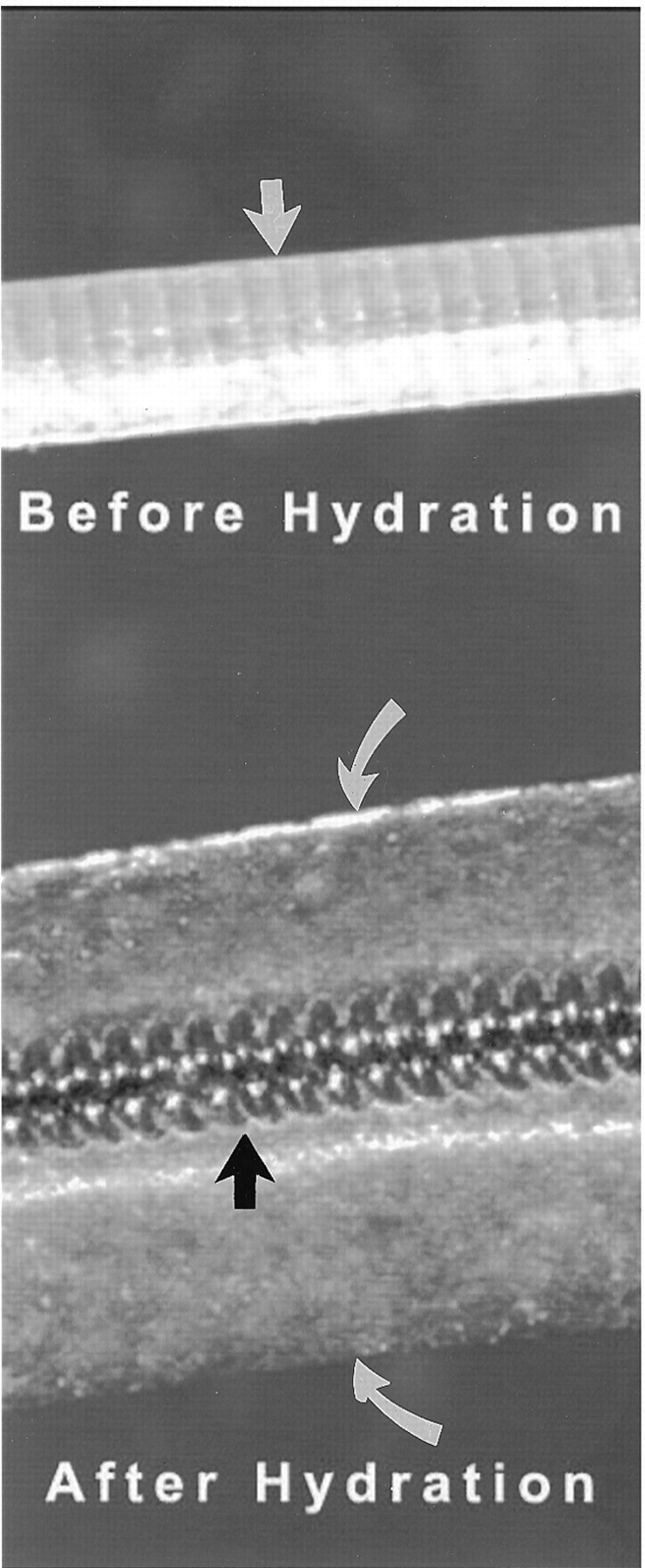
Hybrid hydrogel-platinum coil device. Top, prehydration image shows initial profile of the device. Highly compact hydrogel material is wrapped around a platinum coil. Indentations between winds of the underlying platinum coil can be seen through the compact hydrogel material (straight white arrow). The outer diameter of the coil is 0.008 inch; the thickness of the hydrogel is approximately 0.001 inch, such that the outer diameter of the device is 0.010 inch. Bottom, post-hydration image of the device shows marked expansion of the hydrogel material, which has become translucent. The outer edges of the hydrogel are denoted by the curved white arrows. The platinum coil is denoted by the black arrow. The radial thickness of the expanded hydrogel is approximately 0.013 inch, such that the total outer diameter of the hydrated device is 0.035 inch.
Device Detachment
The complex coils and hybrid devices were detached from the pusher tube by using a fluid-actuated detachment system. Manual pressure was applied to the proximal end of the pusher tube by using a 1-cc syringe filled with radiographic contrast material. The pressure was transmitted through the lumen of the pusher tube to its distal tip. The pressure actuated the detachment of the coil from the pusher tube, resulting in instantaneous detachment.
Embolization Procedures
Aneurysms were allowed to mature for at least 3 weeks after creation. For the embolization procedure, anesthesia was induced with an intramuscular injection of ketamine and xylazine and then anesthesia was maintained with IV administered pentobarbital. Using a sterile technique, surgical exposure of the right common femoral artery was performed. The artery was ligated distally by using 4-0 silk suture, and a 22-gauge angiocatheter was advanced retrograde into the artery. A 0.018-inch guidewire was passed through the angiocatheter, and a 5F vascular sheath was then placed. Heparin (100 U/kg) was IV administered. A 5F catheter (Envoy; Cordis Neurovascular Systems, Miami Lakes, FL) was advanced into the brachiocephalic artery. Using a coaxial technique, with continuous heparinized saline flush, an Excel (Target Therapeutics) 2-marker microcatheter was advanced into the aneurysm cavity. The size of the aneurysm cavity was assessed by using direct comparison with radiopaque sizing devices during digital subtraction angiography.
Aneurysms were embolized with a combination of detachable platinum coils and hybrid hydrogel-coil devices. MicroPlex complex coils were placed initially, and then subsequent packing was performed by using HydroCoil hybrid coil devices. In most cases, a single complex coil was used, but some oblong aneurysms required placement of two complex coils to ensure complete aneurysm filling. Aneurysms were packed as densely as possible (ie, until further device placement resulted in dislodgement of the microcatheter tip into the parent artery or until additional devices could no longer be readily advanced into the aneurysm cavity). Whenever possible, maximal packing of hybrid devices at the parent artery-aneurysm neck interface was achieved. After embolization, final control digital subtraction angiography was performed. We did not perform delayed angiography at the end of the procedures; therefore, the immediate postembolization angiograms were obtained only several minutes after the final device placement. The catheters and sheath were removed, the femoral artery ligated, and the skin closed with running sutures.
Angiographic Examination
Percent aneurysm occlusion was estimated by an experienced observer for all time points by using the following categories: 100% occluded; 95% occluded; 90% occluded; and <90% occluded. In addition, theoretical volumetric occlusion was estimated on the basis of volume of the aneurysm and volume of implanted devices. For these calculations, the aneurysm dome and length and a radiopaque calibration marker were measured with calipers. The volumes of the aneurysms were determined from the dome and length measurements of the aneurysms before embolization. The aneurysms were assumed to be cylindrical, and the volume was determined from the equation,
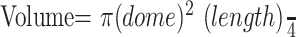 |
The volumes of the devices were determined by ex vivo measurement. Theoretical volumetric occlusion was determined by taking the sum of the volumes of all the devices implanted divided by aneurysm volume. Careful comparison was made between immediate postembolization angiograms and angiograms obtained before the animals were sacrificed to detect cases of increase in size of the aneurysm cavity over time, which would have been theoretically possible if the hydrogel expansion exceeded the capacity of the aneurysm cavity.
Follow-up Imaging
Study animals sacrificed at 2, 4, and 12 weeks underwent intraarterial digital subtraction angiography at the time they were sacrificed. Study animals that were followed for 24 weeks underwent IV digital subtraction angiography at 12 weeks and intraarterial digital subtraction angiography at the time they were sacrificed. All follow-up angiograms were compared with the immediate postembolization angiograms to assess for interval changes in coil configuration or aneurysm filling.
Tissue Harvest and Processing
On the day the animals were sacrificed, surgical access to the left common femoral artery was achieved in a manner similar to that used for access of the right common femoral artery, as described above. A 4F catheter was placed in the aortic arch, and digital subtraction angiography was performed. Immediately after angiography, the study animals were sacrificed by using a lethal injection of pentobarbital. The mediastinum was dissected, and the aortic arch and proximal great vessels, including the coil-embolized segment of artery, were exposed and dissected free from surrounding tissues. The tissue was immediately placed in 10% neutral buffered formalin. Tissue was embedded in methyl methacrylate, sectioned, and stained with hematoxylin and eosin. Sections were viewed by an experienced pathologist who paid particular attention to cell type and appearance of extracellular matrix within and around the coil mass.
Histologic Analysis
Qualitative evaluation was performed at all time points. The tissue or material within the aneurysm cavity was noted. Inflammatory change was assessed. The type and extent of tissue, including unorganized and organized thrombus and fibrotic tissue, were catalogued.
Results
Angiographic Examination
Aneurysms were successfully embolized in all 16 cases. The mean dome and height of the aneurysm cavities were 4.9 ± 0.9 mm and 8.8 ± 3.8 mm, respectively. The mean dome to neck ratio was 1.3 ± 0.3. A single complex coil was placed before inserting hybrid devices in 13 of 16 cases; two complex coils were used in each of the remaining three cases. A mean of 2.3 hybrid devices were placed per aneurysm (range, one to six hybrid devices). The mean percent occlusion based on angiographic findings immediately after embolization was 89 ± 10% (Figs 2–5). The percent occlusion was 100% in two (13%) of 16 aneurysms, 95% in six (38%), 90% in four (25%), and <90% in four (25%). Mean theoretical volumetric occlusion was 68 ± 28% (range, 33–124%). Minor parent artery compromise was observed in three cases, without evidence for compromise of flow in any case. There were no cases of inability to retrieve the hybrid device.
Fig 2.
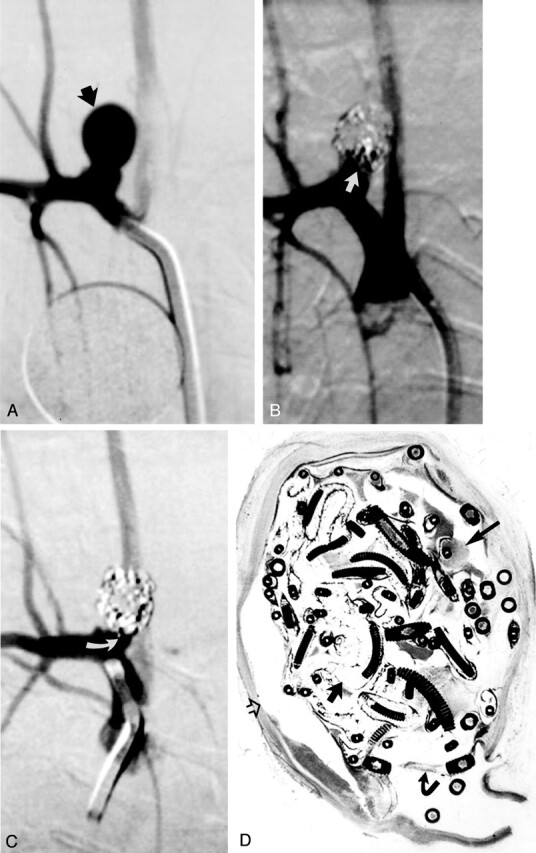
Study animal 4, 2-week implant.
A, Anteroposterior digital subtraction angiogram of the brachiocephalic artery shows a narrow-necked 5.4-mm-wide 7.8-mm-high aneurysm (black arrow).
B, Anteroposterior digital subtraction angiogram obtained immediately after embolization with a single complex coil and three hydrogel devices shows dense packing of the aneurysm dome, with persistent flow at the aneurysm neck (straight white arrow).
C, Anteroposterior digital subtraction angiogram obtained 2 weeks after embolization shows progressive occlusion of the aneurysm cavity, with the neck now occluded (curved white arrow).
D, Hematoxylin and eosin stain; original magnification, ×6.8. Coronal section obtained through the aneurysm cavity. The aneurysm cavity is filled with a mixture of unorganized thrombus (long straight arrow) and expanded hydrogel (short straight arrow). The hydrogel stains as faint, violet-colored, reticular material. There is no substantial inflammation. A thin fibrin membrane traverses the neck (curved arrow). Artifactual separation between the aneurysm wall and the coils and hydrogel occurred during processing (open arrow).
Fig 5.
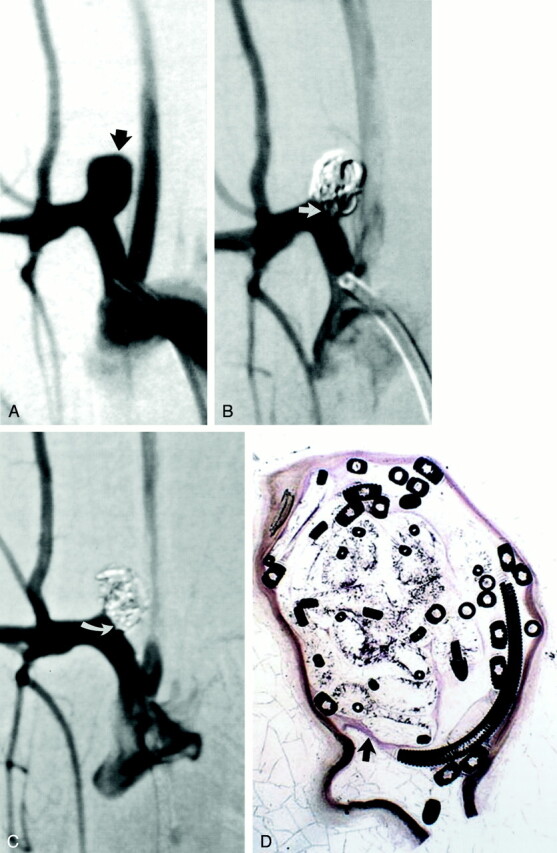
Study animal 16, 6-month implant.
A, Anteroposterior digital subtraction angiogram of the brachiocephalic artery shows a wide-necked 5.5-mm-wide 8.5-mm-high aneurysm (black arrow).
B, Anteroposterior digital subtraction angiogram obtained immediately after embolization with a single complex coil and a single hydrogel device shows dense packing of the aneurysm dome, with persistent flow at the aneurysm neck (straight white arrow).
C, Anteroposterior digital subtraction angiogram obtained 6 months after embolization shows progressive occlusion of the aneurysm cavity, with the neck now occluded (curved white arrow).
D, Hematoxylin and eosin stain; original magnification, ×6.8. Coronal section obtained through the aneurysm cavity. The sac is completely filled with hydrogel, which stains as faint, violet-colored, reticular material. A thin layer of organized tissue traverses the neck of the aneurysm (arrow).
The mean percent occlusion based on angiographic findings at follow-up was 93 ± 9% (Figs 2–5). Seven (44%) of 16 cases showed progressive aneurysm occlusion at follow-up as compared with that observed immediately after embolization. There were no cases of delayed, progressive compromise of the parent artery, even in cases in which dense packing of hybrid devices was achieved at the aneurysm neck–parent artery interface. In some cases, the border of the aneurysm cavity, as detected by the contour of the coil mass, was slightly more irregular than that in the baseline angiogram; however, there were no cases in which measurable increases in aneurysm volume were detected. At the time of treatment or at follow-up, distal emboli suggestive of hydrogel or thrombus dislodgement in the parent artery, either at the aneurysm neck or in the distal vasculature, were not observed.
Histologic Findings
Aneurysms embolized with hybrid devices, in general, showed marked swelling of the hydrogel material within the aneurysm cavities. In the 2-week group, the aneurysms were filled with poorly organized blood clot, desiccated hydrogel, and coils (Fig 2D). Most of the aneurysm lumen was filled with expanded hydrogel and a minority of thrombus. A thin fibrin rim traversed the neck of the aneurysms. In the 1-month group, three aneurysms were filled with well-organized tissue, hydrogel, and coils (Fig 3D). As seen at earlier time points, most of the aneurysm cavities were filled with expanded hydrogel, with small clefts of organized tissue remaining between areas of hydrogel. The fourth aneurysm in the 1-month group was filled with mostly organized thrombus, hydrogel, and coils. In the 3- and 6-month groups, histologic findings appeared similar to those seen at 1 month, with well-organized tissue, hydrogel, and coils filling the aneurysm (Figs 4D and 5D). As seen at earlier time points, most of the aneurysm cavities were filled with expanded hydrogel, with a small amount of organized clefts of tissue between hydrogel loops.
Fig 3.
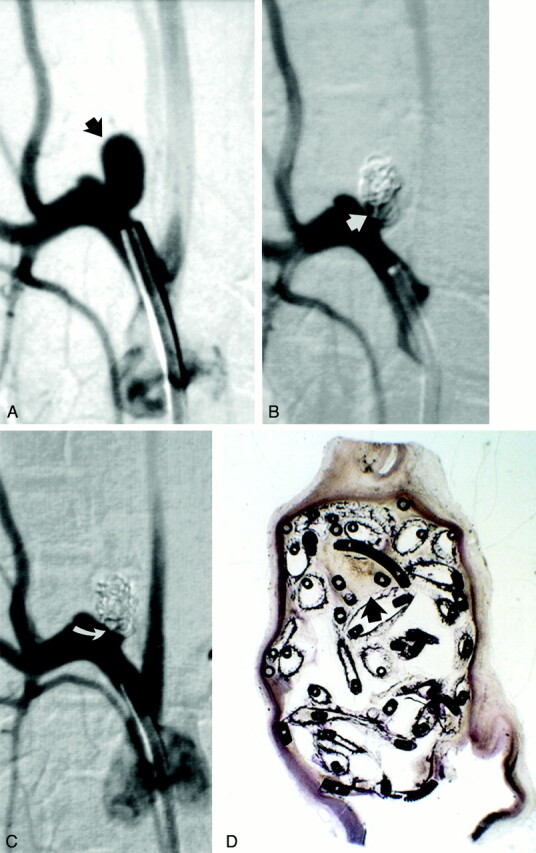
Study animal 6, 1-month implant.
A, Anteroposterior digital subtraction angiogram of the brachiocephalic artery shows a narrow-necked 4-mm-wide 7-mm-high aneurysm (black arrow).
B, Anteroposterior digital subtraction angiogram obtained immediately after embolization with a single complex coil and two hydrogel devices shows dense packing of the aneurysm dome, with persistent flow at the aneurysm neck (straight white arrow).
C, Anteroposterior digital subtraction angiogram obtained 1 month after embolization shows progressive occlusion of the aneurysm cavity, with the neck now occluded (curved white arrow).
D, Hematoxylin and eosin stain; original magnification, ×2.5. Coronal section obtained through the aneurysm cavity. The sac is nearly completely filled with hydrogel, which stains as faint, violet-colored, reticular material. There is a small amount of organizing thrombus near the dome (arrow).
Fig 4.
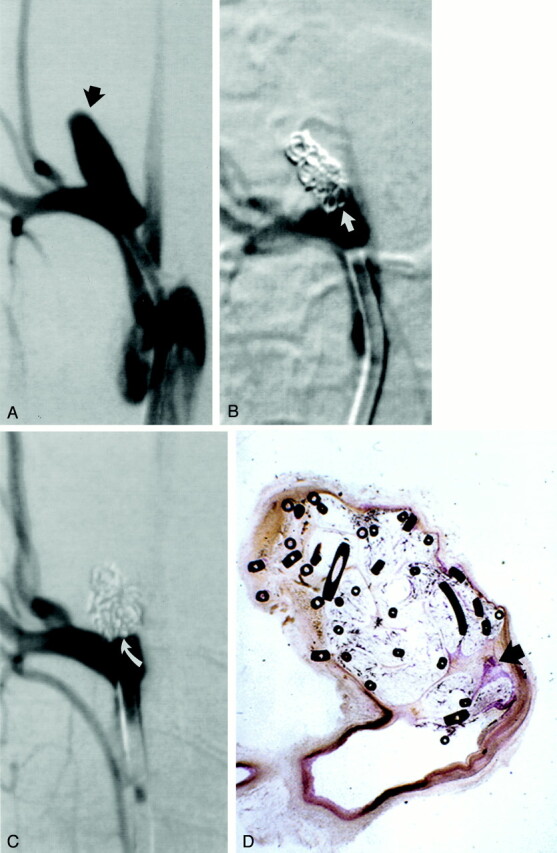
Study animal 10, 3-month implant.
A, Anteroposterior digital subtraction angiogram of the brachiocephalic artery shows a wide-necked 4-mm-wide 11-mm-high aneurysm (black arrow).
B, Anteroposterior digital subtraction angiogram obtained immediately after embolization with a single complex coil and a single hydrogel device shows dense packing of the aneurysm dome, with persistent flow at the aneurysm neck (straight white arrow).
C, Anteroposterior digital subtraction angiogram obtained 3 months after embolization shows progressive occlusion of the aneurysm cavity, with the neck now occluded (curved white arrow).
D, Hematoxylin and eosin stain; original magnification, ×6.3. Coronal section obtained through the aneurysm cavity. The sac is nearly completely filled with hydrogel, which stains as faint, violet-colored, reticular material. There is a small amount of organized tissue near the neck (arrow).
Only small amounts of blood or tissue were present, as compared with expanded hydrogel material, even in cases that showed progressive aneurysm occlusion between the time of embolization and the time the animals were sacrificed. This finding strongly suggests that the progressive occlusion resulted from hydrogel expansion during the early period after device implantation rather than thrombus propagation in the aneurysm cavities.
Inflammatory reaction at all time points was minimal and unremarkable. There was no evidence of egress of hydrogel or coils through the aneurysm walls. A thin layer of fibrous tissue traversed the neck of the aneurysm at 3 and 6 months.
Discussion
In this study, we showed feasibility of aneurysm embolization with a low profile, expandable hydrogel-platinum coil hybrid device. The device offers all the favorable attributes of currently available detachable platinum coils, including ready passage through microcatheters, radiopacity, atraumatic deployment, and retrievability. In addition to these favorable attributes, the hydrogel coil, with a length equivalent to that of a platinum coil, fills significantly more volume when fully expanded. It thus achieves markedly greater aneurysm filling than does a platinum coil. Histologic study at all time points after embolization showed only small amounts of unorganized thrombus or tissue within aneurysm cavities, with most of the aneurysm cavity filled with expanded hydrogel. Angiographic study showed high rates of progressive aneurysm occlusion with the hybrid device. In previous studies, we rarely, if ever, noted progressive aneurysm occlusion when using platinum coils alone (17). These findings suggest that the hydrogel coil may represent an important advance in the endovascular approach to aneurysm therapy.
Current limitations of coil embolization of aneurysms include incomplete initial occlusion rates (with <50% of aneurysms considered completely occluded immediately after therapy) and late aneurysm regrowth (6, 18). The mechanisms behind aneurysm regrowth remain unknown but likely relate to thrombolysis and recanalization of thrombus that are initially induced by the coils (15). The design of the hydrogel coil addresses both of these important shortcomings. First, whereas aneurysms densely packed with platinum coils are actually only 20% to 30% volumetrically occluded, experimental aneurysms treated with the hydrogel coil were volumetrically occluded by a mean of 68%. Second, the expanded hydrogel excludes thrombus from the aneurysm cavity, eradicating the tendency for aneurysm regrowth from thrombus resorption or recanalization.
Numerous coil modifications have previously been proposed to reduce the tendency for aneurysm recanalization. These modifications include the addition of biomaterials to carrier coils, including collagen coatings or filaments, basement membrane proteins, growth factors, cellular elements, or bioresorbable polymers (8–15). Many of these modifications have shown promise in preclinical models of aneurysms, but none has yet been applied clinically. The premise underlying these proposed modifications is that of inducing increased reaction to the coil implants by stimulating the inflammatory response or stimulating resident cellular elements and tissues. None of these previous coil-based modifications has addressed the physical constraints of coil therapy, with which coils are unable to achieve high degrees of aneurysm filling. The current hydrogel coil elegantly achieves the goal of aneurysm obliteration by using a device that behaves, for all practical purposes, as a coil. Long-term aneurysm occlusion with the hydrogel device is not dependent on stimulation of host reaction to the implant; instead, occlusion is achieved by filling of the aneurysm with the biomaterial.
We did not perform quantitative histopathologic examinations in this study. It may have been feasible to estimate the percent aneurysm lumen obliteration comprised by hydrogel. However, such estimates would have been difficult, considering that only two to three tissue sections were available for each aneurysm such that only a small portion of the lumen was represented. Furthermore, there is obligatory shrinkage of the hydrogel material as it undergoes tissue processing, so exact identification of hydrogel-containing lumen would have been difficult. It would also have been possible to calculate mean tissue thicknesses over the hydrogel at the aneurysm neck, but such measurements in isolation are considered by us to be of relatively little relevance. We consider the histopathologic features described in the text and shown in the figures to be relevant to researchers familiar with coil embolization of experimental and human aneurysms, because the tissue reaction and marked hydrogel expansion are well shown. Future studies aimed at quantitative assessment of percent aneurysm occlusion and thickness of tissue overlying the aneurysm neck should include control aneurysms embolized with platinum coils alone.
The hybrid device was designed to allow prolonged repositioning, even while achieving substantial, in vivo expansion. Theoretical concern exists regarding delayed hydration that might cause expansion of the coil-hydrogel mass into the parent artery or even over-expansion and rupture of the aneurysm itself. We endeavored to pack the aneurysm necks as tightly as possible, and in no case did we detect late, over-expansion of the hydrogel material. The expansion of the hydrogel material on any given coil is only 0.013 inch radially in any direction. Thus, expansion of hybrid coils at the neck would be imperceptible radiographically unless multiple coils displaced neighboring devices as an added effect.
Several cases showed theoretical volumetric occlusion rates >100%. However, in no case were increases in aneurysm size observed radiographically after embolization. Furthermore, there were no cases of delayed parent artery compromise from over-expansion of the hydrogel material. The theoretical occlusion rate was subject to errors inherent in estimating aneurysm volume, because irregular aneurysm shapes rendered exact volumetric calculations difficult.
In this study, we attempted to simulate the clinical environment, where we anticipate packing aneurysms as densely as possible with any embolic material. We packed devices by using techniques identical to those used for standard platinum coils, without waiting for hydration either between coils or after the final coil was placed. We did not perform serial angiography on the day of embolization to detect at what time point the progressive aneurysm occlusion occurred. Although potentially interesting, we do not consider the exact timing of expansion to be clinically relevant, if standard, dense-packing practices are used.
The scoring scheme for aneurysm occlusion rate used in this study was subjective and has not been validated. However, semiquantitative metrics for aneurysm occlusion rates are widely used and we have no reason to think that our method was less valid than previously unvalidated methods. We think that the angiographic and histopathologic data contained herein substantiate the efficacy of the hybrid device, even without use of a validated percent occlusion technique.
Conclusion
This new hydrogel-platinum coil hybrid device is easily tracked, deployed, repositioned, and detached through standard microcatheters without the use of nonaqueous solvents. High rates of delayed, progressive aneurysm occlusion were observed.
Acknowledgments
We acknowledge assistance in animal care and surgery by Cynthia Dodson and assistance in embolization procedures by Soma Sinha Roy, MD.
Footnotes
Supported by Microvention, Inc., Aliso Viejo, CA.
References
- 1.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004 [DOI] [PubMed] [Google Scholar]
- 2.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 3.Tateshima S, Murayama Y, Gobin YP, Duckwiler GR, Guglielmi G, Viñuela F. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery 2000;47:1332–1339 [PubMed] [Google Scholar]
- 4.Cognard C, Weill A, Castaings L, Rey A, Moret J. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 1998;206:499–510 [DOI] [PubMed] [Google Scholar]
- 5.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793–803 [DOI] [PubMed] [Google Scholar]
- 6.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery 1998;43:1016–1025 [DOI] [PubMed] [Google Scholar]
- 7.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–293 [DOI] [PubMed] [Google Scholar]
- 8.Dawson RC, Krisht AF, Barrow DL, Joseph GJ, Shengelaia GG, Bonner G. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133–139 [DOI] [PubMed] [Google Scholar]
- 9.de Gast AN, Altes TA, Marx WF, Do HM, Helm GA, Kallmes DF. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 2001;49:690–694 [DOI] [PubMed] [Google Scholar]
- 10.Abrahams JM, Forman MS, Grady MS, Diamond SL. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol 2001;22:1410–1417 [PMC free article] [PubMed] [Google Scholar]
- 11.Kallmes DF, Williams AD, Cloft HJ, Lopes MB, Hankins GR, Helm GA. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998;207:519–523 [DOI] [PubMed] [Google Scholar]
- 12.Marx WE, Cloft HJ, Helm GA, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001;22:323–333 [PMC free article] [PubMed] [Google Scholar]
- 13.Murayama Y, Viñuela F, Suzuki Y, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms: part II. an experimental study in a swine aneurysm model. AJNR Am J Neuroradiol 1999;20:1992–1999 [PMC free article] [PubMed] [Google Scholar]
- 14.Murayama Y, Viñuela F, Tateshima S, Song JK, Gonzalez NR, Wallace MP. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg 2001;94:454–463 [DOI] [PubMed] [Google Scholar]
- 15.Bavinzski G, Richling B, Binder BR, et al. Histopathological findings in experimental aneurysms embolized with conventional and thrombogenic/antithrombolytic Guglielmi coils. Minim Invasive Neurosurg 1999;42:167–174 [DOI] [PubMed] [Google Scholar]
- 16.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award: creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices: American Roentgen Ray Society. AJR Am J Roentgenol 2000;174:349–354 [DOI] [PubMed] [Google Scholar]
- 17.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217–222 [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Viñuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg 2000;93:561–568 [DOI] [PubMed] [Google Scholar]


