Abstract
BACKGROUND AND PURPOSE: Although neuropsychological symptoms and signs are common in thyroid disease, their organic substrate is unknown. We performed brain MR imaging in patients with hyperthyroidism or hypothyroidism before and after treatment and correlated the results with hormonal markers.
METHODS. Eight patients with hyperthyroid disease and three with hypothyroid disease underwent imaging within 1–2 days of a thyroid hormone testing. Images were registered, and brain and ventricular sizes were measured by using a semiautomated contour and thresholding technique. Changes in brain and ventricular volume were correlated with serum levels of total thyroxine (T4), unbound triiodothyronine (free T3), and thyroid-stimulating hormone (TSH) before and after treatment.
RESULTS. With treatment, brain size decreased by 6,329–31,183 mm3 in the hyperthyroid group and increased by 2,599–48,825 mm3 in the hypothyroid group. Conversely, with treatment, ventricular size increased by 325–6,279 mm3 in the hyperthyroid group and decreased by 760–2,376 mm3 in the hypothyroid group. There was a highly significant correlation between reduction in brain size and reduction in T4, as well as between the increase in ventricular size and reduction in T4. There was a significant correlation between reduction in ventricular size and reduction in free T3. There were highly significant correlations between reduced levels of TSH and increase in brain size, as well as between increased levels of TSH and increase in ventricular size.
CONCLUSION. In thyroid disease, the size of the brain and ventricles significantly change after treatment, and these changes are correlated with T4, free T3, and TSH levels. The mechanism of these changes is uncertain, but it may involve osmolyte regulation, the sodium and water balance, and alterations in cerebral hemodynamics.
An excessive production or deficiency of thyroid hormones may result in symptoms and signs that can affect every organ in the body, including the brain (1). In patients with hyperthyroidism, feelings of nervousness, tension, and anxiety are common, whereas in patients with hypothyroidism, poor memory, mental slowing, and depression are frequently noted (2). Although the brain is clearly implicated in thyroid disease, no abnormalities have previously been demonstrated within the brain by using imaging techniques.
Our aims were to use MR imaging to determine whether brain changes could be observed in patients with hyperthyroidism and in those with hypothyroidism after treatment and to correlate any changes with serum markers of disease.
Methods
Eleven patients (six male, five female) with a mean age ± SD of 41 years ± 12 (range, 22–59 years) were prospectively examined. Approval of the study was obtained from our institution’s ethics committee, and all patients provided informed consent. At diagnosis, eight of the patients had hyperthyroidism and three had hypothyroidism. All patients with hyperthyroidism had serum antibodies against thyroid-stimulating hormone (TSH), consistent with Graves disease. At presentation, antithyroid therapy with thionamide drugs was initiated (seven patients received carbimazole, and one received propylthiouracil). Two of the three patients with hypothyroidism had primary disease, whereas hypothyroid develop in the third patient after 131I therapy for hyperthyroidism. In all three patients with hypothyroidism, thyroxine replacement therapy was started at presentation with a maintenance dose of 100 μg/dL.
MR Imaging
Three-dimensional, T1-weighted, radio-frequency, spoiled MR images (TR/TE/NEX, 21/6/2; flip angle, 35°; imaging matrix, 152 × 256 × 114; FOV, 25 cm; section thickness, 1.6 mm) were obtained by using a 1.-T machine (HPQ Plus; Marconi Medical Systems, Cleveland, OH). All 11 patients underwent imaging before treatment and 1 month after treatment. Further follow-up images were acquired at 3 months in eight patients, at 6 months in four patients, at 9 months in two patients, and at 14 months in one patient. All follow-up images were registered by using subvoxel image registration to ensure that they accurately matched the position on the pretreatment images (to approximately 0.01 mm) (3, 4). Registered-difference images were generated by subtracting the registered post-treatment image from pretreatment image.
Quantitation Method
In each patient, brain volume and ventricular volume (that of only the lateral and third ventricles) were measured from the anatomic images acquired before treatment and at the end point of treatment. A semiautomated contour and thresholding program was used (5, 6). Separate contours were drawn for the brain and ventricles. In each case, the contours were positioned so that they loosely enclosed the region of interest without impinging on it and so that they excluded any other structures of similar signal intensity. Threshold values were calculated for both the brain and CSF (5), and the number of voxels measured was converted into a volume by referencing them to the imaging matrix size and section thickness.
Serum levels of total thyroxine (T4); unbound, or free, triiodothyronine (T3); and TSH were measured before and after treatment within 1 to 2 days of the MR imaging examinations. Changes in brain and ventricular size were correlated with changes in the levels of T4, free T3, and TSH.
Statistical Analysis
The Pearson correlation was used to correlate the change in the size of the brain and ventricles with the changes in the levels of T4, free T3, and TSH.
Results
Clinical and Biochemical Findings
The patients’ clinical assessments and the level of thyroid hormones before and after treatment and are shown in Table 1. In each case, the initial measured T4, free T3, and TSH levels were within the diagnostic range for either hyperthyroidism or hypothyroidism. In seven of the eight patients in the hyperthyroid group, the level of thyroid hormones returned to normal after treatment. In the one remaining patient, the hormone levels showed evidence of improvement after treatment, but did not reach the normal range. (For example, the T4 level was reduced from 307 to 228 nmol/L.) In all patients with hyperthyroidism, symptoms of anxiety, agitation, restlessness, and hyperactivity were reduced with treatment.
TABLE 1:
Clinical features and serum levels of T4, free T3, and TSH before and after treatment
| Patient | Initial Diagnosis* | Level Before Treatment |
Clinical Diagnosis After Treatment | Clinical Assessment After Treatment‖ | Level After Treatment |
||||
|---|---|---|---|---|---|---|---|---|---|
| T4 (nmol/L)† | T3 (pmol/L)‡ | TSH (mU/L)§ | T4 (nmol/L)† | T3 (pmol/L)‡ | TSH (mU/L)§ | ||||
| 1 | Hyperthyroidism | 284 | 32.2 | <0.1 | Euthyroidism | Symptoms remitted | 116 | 7.8 | <0.1 |
| 2 | Hyperthyroidism | 267 | 23+ | <0.1 | Euthyroidism | Symptoms remitted | 61 | 2.2+ | <0.1 |
| 3 | Hyperthyroidism | 263 | 13.2+ | <0.1 | Euthyroidism | Symptoms remitted | 112 | 4.6+ | <0.1 |
| 4 | Hyperthyroidism | 196 | 28.2 | <0.1 | Euthyroidism | Symptoms remitted | 73 | 7.2 | <0.1 |
| 5 | Hyperthyroidism | 142 | 11.1 | <0.1 | Euthyroidism | Symptoms remitted | 53 | 2.8+ | <0.1 |
| 6 | Hyperthyroidism | 287 | 39.7+ | <0.1 | Euthyroidism | Symptoms remitted | 121 | 6.1+ | 1.4 |
| 7 | Hyperthyroidism | >300 | 55.9 | <0.1 | Euthyroidism | Symptoms remitted | 66 | 6.2 | <0.1 |
| 8 | Hyperthyroidism | 307 | >45 | <0.1 | Toxicity | Symptoms remitted | 229 | 20.4 | <0.1 |
| 9 | Hypothyroidism | 2 | 2.9 | 43.3 | Euthyroidism | Symptoms remitted | 81 | 6.9 | 2.5 |
| 10 | Hypothyroidism | <15 | NA | 76.5 | Euthyroidism | Symptoms remitted | 94 | NA | 3.4 |
| 11 | Hypothyroidism | <15 | NA | >100 | Euthyroidism | Symptoms remitted | 113 | NA | 25.9 |
All patients with hyperthyroidism had neuropsychological symptoms of anxiety, agitation, restlessness, and hyperactivity. All patients with hypothyroidism had neuropsychological symptoms of tiredness, lethargy, mental slowing, and depression.
Normal range, 60–160 nmol/L.
Normal ranges, 5.4–9.3 pmol/L (assay 1) and 2.5–5.3 pmol/L (assay 2) (denoted with +).
Normal range, 0.4–4.0 mU/L.
In all patients, symptoms remitted at clinical assessment after treatment.
In the patients with hypothyroidism, thyroid hormone levels returned to the normal range except for a persistently elevated TSH level in one patient. Initially, all three patients reported fatigue, lethargy, mental slowing, and depression. After treatment, these symptoms improved.
Qualitative Brain and Ventricular Changes on MR Images
In all patients, the size of the brain and ventricular system changed after treatment. These changes were evident only on the registered subtraction images. In the hyperthyroid group, brain size decreased and ventricular sized increased as the patients’ conditions reverted toward a euthyroid state (Fig 1). Conversely, in the hypothyroid group, the brain increased in size and the ventricles decreased in size as the patients’ conditions reverted to a euthyroid state.
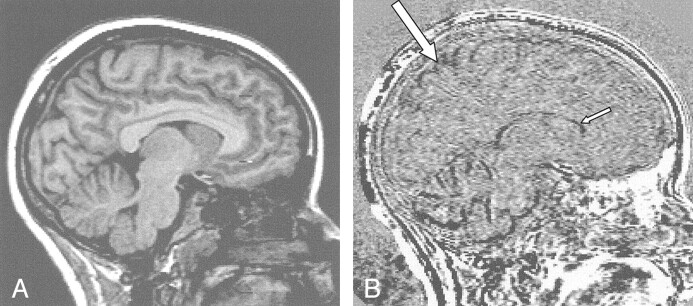
Fig 1.
Brain and ventricular changes before and after treatment in a patient with hyperthyroidism.
A, Sagittal T1-weighted image near the midline of the brain.
B, Registered subtraction image. The low-signal-intensity boundaries at the cortex (large arrow) indicate reduced brain size, and the low-signal-intensity seen more centrally (small arrow) indicates ventricular enlargement.
Brain and Ventricular Volume Measurements
The differences in brain and ventricular size before and after treatment are shown in Table 2. The correlations of volume change with changes in T4, free T3, and TSH levels before and after treatment are shown in Figures 2–4, respectively.
TABLE 2:
Brain and ventricular volumes before and after treatment
| Patient | Initial Diagnosis | Brain Size (mm3) |
Ventricular Size (mm3) |
||||
|---|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | Difference | Before Treatment | After Treatment | Difference | ||
| 1 | Hyperthyroidism | 1,406,076 | 1,394,841 | −11,235 | 10,933 | 12,973 | +2040 |
| 2 | Hyperthyroidism | 1,048,747 | 1,024,564 | −24,183 | 6276 | 6785 | +509 |
| 3 | Hyperthyroidism | 1,081,917 | 1,050,734 | −31,183 | 20,753 | 27,032 | +6279 |
| 4 | Hyperthyroidism | 1,139,104 | 1,129,720 | −9384 | 22,991 | 23,316 | +325 |
| 5 | Hyperthyroidism | 1,453,485 | 1,446,143 | −7342 | 12,941 | 12,691 | −250 |
| 6 | Hyperthyroidism | 1,117,777 | 1,107,486 | −10,291 | 9394 | 10,755 | +1361 |
| 7 | Hyperthyroidism | 1,261,845 | 1,231,219 | −30,626 | 24,503 | 26,729 | +2226 |
| 8 | Hyperthyroidism | 1,209,585 | 1,203,256 | −6329 | 35,018 | 35,371 | +353 |
| 9 | Hypothyroidism | 1,134,086 | 1,136,685 | +2599 | 9298 | 8220 | −1078 |
| 10 | Hypothyroidism | 1,322,421 | 1,371,246 | +48,825 | 15,159 | 12,783 | −2376 |
| 11 | Hypothyroidism | 1,171,701 | 1,179,529 | +7828 | 14,965 | 14,205 | −760 |
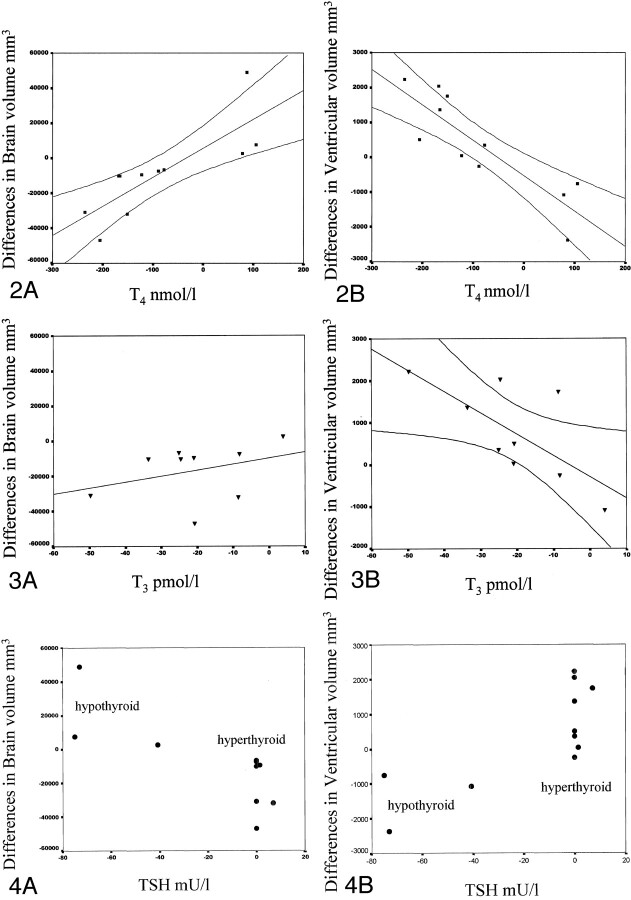
Fig 2.
Correlation of changes in brain and ventricular size with changes in T4 levels (with 95% confidence limits).
A, Increases in brain size were strongly correlated with increases in T4 levels.
B, Reductions in ventricular size were strongly correlated with increases in T4 levels.
Fig 4.
Correlation of changes in brain and ventricular size with changes in TSH levels. The data formed two distinct groups. After treatment, TSH levels in the hyperthyroid patients either increased by a small amount or not at all. Although initially very high, TSH levels in the hypothyroid patients decreased to the normal range after treatment.
A, Low levels of TSH was correlated with reductions in brain size.
B, Low levels of TSH were correlated with increases in ventricular size.
In the eight patients with hyperthyroidism, brain size was reduced by 6329–31,183 mm3 after treatment, as the patients’ conditions reverted toward a euthyroid state (Table 2). A corresponding increase in ventricular size of 325–6279 mm3 was noted in seven of these patients. In one patient, the size of the ventricles was reduced by 250 mm3. In all three patients with hypothyroidism, brain size increased after treatment, as the patients’ conditions became euthyroid. Their brain sizes were 2599, 7828, and 48,825 mm3; the corresponding reduction in ventricular size were 1078, 760, and 2376 mm3, respectively (Table 2).
Change in Brain Size and Serum T4 Level
Figure 2 shows the change in brain size (Fig 2A) and ventricular size (Fig 2B), as correlated with the change in the level of T4 (with 95% confidence intervals). This analysis combined the changes occurring in the patients with hyperthyroidism and those occurring in patients hypothyroidism during treatment. In the brain, an increase in size was correlated with an increase in serum T4 levels (Fig 2A). The correlation was statistically significant (n = 11, r = +0.81, P = .003). In the ventricles (Fig 2B), an increase in size was correlated with a reduction of serum T4 levels. This correlation was also statistically significant (n = 11, r = −0.865, P = .001).
Change in Brain Size and Serum Free T3 Level
Figure 3 illustrates the change in brain size (Fig 3A) and ventricular size (Fig 3B) with changes in the serum levels of free T3. This analysis combined the changes in patients with hyperthyroidism and the changes in patients with hypothyroidism during treatment. In the brain, a trend similar to that of the serum T4 levels was observed, but it was not significant (n = 9, r = 0.333, P = .381). Increases in ventricular size and reductions in le free T3 levels were significantly correlated (n = 9, r = −7.0, P = .036).
Fig 3.
Correlation of changes in brain and ventricular size with changes in T3 levels (with 95% confidence intervals).
A, Brain size increased with increased in T3 levels, but the changes were not significant.
B, Reductions in ventricular size were strongly correlated with increases in T3 levels.
Change in Brain Size and Serum TSH Levels
Figure 4 shows the changes in brain (Fig 4A) and ventricular size (Fig 4B), as correlated with changes in TSH levels. The data fit into two distinct groups for both the brain and ventricles. The eight patients with hyperthyroidism formed one group, and the three patients with hypothyroidism formed another. In all patients in the hyperthyroid group, the serum TSH values at presentation were less than 0.1 mU/L. After treatment, the values remained suppressed at that level in all patients but one. In the hypothyroid group, the levels of TSH were elevated before treatment and markedly reduced after treatment as the patients’ conditions became euthyroid. The graphs (Fig 4) show a close relationship between the minimal change in TSH levels and a decrease in brain volume or an increase in ventricular size. Low levels of TSH were significantly correlated with an increase in brain size (n = 11, r = 0.788, P = .005) (Fig 4A), and elevated TSH levels were correlated with an increase in ventricular size (n = 11, r = 0.782, P = 0.004) (Fig 4B).
Discussion
We have shown a strong correlation between changes in brain and ventricular size and thyroid hormone levels after treatment. In hyperthyroidism, the brain decreased in size and the ventricles increased in size. In hypothyroidism, the brain increased in size, and the ventricles decreased in size.
Thyroid hormones are essential for the development and maintenance of cellular function and growth. Several functional changes occur with excessive or deficient levels of circulating thyroid hormones. In hyperthyroidism, the basal metabolic rate increases (7), and the oxygen consumption of organs such as the heart, liver, kidneys, and anterior pituitary gland is increased (8, 9). Other changes include increases in cardiac output with a reduction in peripheral resistance (10); increases in the glomerular filtration rate; (11–13), and increases in protein, carbohydrate, lipid, and vitamin metabolism (14). The reverse is seen in hypothyroidism (10, 13, 15, 16). With treatment, these metabolic changes are reversible.
Neurologic and psychological manifestations occur in both states. Patients with hyperthyroidism may experience anxiety, emotional lability, and poor concentration. More severe neuropsychological disorders may also occur; these can include delirium and mania (17). Patients with hypothyroidism can have depression, reversible dementia, and schizophrenia (myxedema madness) (18). Little is known about the etiology underlying the neuropsychological features of hyper- and hypothyroidism. Thyroid hormones are thought to affect neurotransmitter synthesis and the release of cytokines that affect brain function (19). In hyperthyroidism, oxidative metabolism is reduced, and this reduction in turn affects neuronal integrity. Cytokine release may result in the excessive release of neurotransmitters such as dopamine, norepinephrine, and glutamine, and cerebral confusion, which is often present in thyrotoxic patients, can result. The abnormalities in circulating thyroid hormone levels may also be associated with changes in cellular hydration, circulatory hemodynamics, the basal metabolic rate, and calcium homeostasis.
Much work has been performed to investigate how osmotic stress effects the regulation of brain-cell volume (20). With hyper- or hypo-osmotic stress, osmolyte levels are increased or decreased, respectively, in an attempt to regulate brain cellular hydration and the passage of water across the blood-brain barrier. Various molecules have been shown to be brain osmolytes. In patients with hepatic encephalopathy, a reduction in the brain myo-inositol signal intensity (as detected with proton (1H) magnetic MR spectroscopy) has been related to a reduction in the intracellular osmotic pressure. These findings are reversible once hepatic encephalopathy is successfully treated (21). These results have also been demonstrated in animal studies. Other cellular osmolytes, such as taurine and glycerophosphocholine, have been shown to be depleted in animals (22) and humans (23) during exposures to hypo-osmotic stress (24). These changes reverse once the stress factor is removed. In thyroid disease, the sodium and water homeostasis in brain cells may be disturbed. Some evidence suggests a reduction in brain-cell osmolyte levels in the hyperthyroid state; this reduction may be an adaptive response to increased amounts of cellular water and decreased cellular osmotic pressure. The reverse may be true in hypothyroidism. This possibility is interesting because patients with hyperthyroidism usually lose weight, but their brains appear to increase in size. In hypothyroidism, the reverse is evident. That is, weight gain occurs with a reduction in brain size.
In studies of hyperthyroid rats (25) and cats (26), a depletion of intracellular taurine has been seen secondary to a reduction in the sodium concentration. The reverse findings are seen in neonatal rats with induced hypothyroidism, in which an increase in taurine levels was detected (27). In humans, taurine concentrations in blood platelets are reduced in hyperthyroidism and increased in hypothyroidism. After treatment, the changes reverse (28). Furthermore, in patients with Graves disease, 1H MR spectroscopy of the frontal lobes shows that the choline-creatine (Cho/Cr) signal decreases when patients are thyrotoxic and increases after treatment when patients’ conditions change to euthyroidism (29). Findings suggest that a reduction in levels of glycerophosphocholine, another osmolyte, is associated with brain swelling. In hypothyroidism, the same explanation may also apply. The reverse findings were seen in a study of infants with hypothyroidism who underwent proton MR spectroscopy. The results showed that the choline signal increases in the hypothyroid state (30). Combined studies of both MR imaging and MR spectroscopy performed in patients before and after treatment may provide additional useful information.
Abnormalities in circulating thyroid hormone levels are known to affect sodium and water handling. In hyperthyroidism, the glomerular filtration rate (GFR) increases (10, 13), as does the amount of excreted sodium and creatinine (31, 32). Changes in plasma vasopressin levels and sensitivity have also been found. In hypothyroidism, renal blood flow and the GFR are reduced, and excretion of a water load is reduced. Excessive or deficient levels of circulating thyroid hormones may also directly affect brain cellular hydration and sodium content. This possibility was reflected in changes in brain volume seen in this study.
Other possible causes for the changes in brain size in thyroid disease include alterations in the cerebral hemodynamics. In hyperthyroidism, cardiac output is increased and peripheral resistance is reduced. These changes have been shown to be reversible with treatment (33, 34). In rats, a reduction in peripheral resistance is associated with an increase in cerebral blood flow, which may lead to or contribute to the development of cerebral edema (35, 36). These same phenomena may also occur in humans. Although the theory is still controversial, our findings support the cell hydration and dehydration theory.
Conclusion
We have shown that brain and ventricle sizes change with treatment of hyperthyroid and hypothyroid states in humans and that these alterations are correlated with changes in the levels of circulating thyroid hormones. The reasons for the changes in brain size are uncertain, but they may involve osmolyte regulation, the sodium and water balance, and alterations in cerebral hemodynamics.
Acknowledgments
We wish to thank Marconi Medical Systems for their continued support.
References
- 1.Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia: Lippincott;1990. :331–370
- 2.Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia: Lippincott;1990. :1479–1488
- 3.Hajnal JV, Saeed N, Soar E, et al. A registration and interpolation procedure for subvoxel matching of serially acquired MR images. J Comput Assist Tomogr 1995;19:677–691 [DOI] [PubMed] [Google Scholar]
- 4.Saeed N. Magnetic Resonance image segmentation using pattern recognition, and applied to registration and quantitation. MR Biomed. 1998;11:157–167 [DOI] [PubMed] [Google Scholar]
- 5.Saeed N, Puri BK, Oatridge A, et al. Two methods for semi-automated quantification of changes in ventricular volume and their use in schizophrenia. Magn Reson Imaging 1998;16:1237–1247 [DOI] [PubMed] [Google Scholar]
- 6.Oatridge A. The Use of Subvoxel Image Registration and Subtraction of Serial Magnetic Resonance Imaging for Detecting Small Changes to the Brain. Leeds: Metropolitan University;1999
- 7.Iossa S, Liverini G, Barletta A. Relationship between the resting metabolic rate and hepatic metabolism in rats: effect of hyperthyroidism and fasting for 24 hours. J Endocrinol 1992;135:45–51 [DOI] [PubMed] [Google Scholar]
- 8.Singh G, Sharma AC, Thompson EB, et al. Renal endothelin mechanism in altered thyroid states. Life Sci 1994;54:1901–1908 [DOI] [PubMed] [Google Scholar]
- 9.Gomberg-Maitland M, Frishman WH. Thyroid hormone and cardiovascular disease. Am Heart J 1998;135:187–196 [DOI] [PubMed] [Google Scholar]
- 10.Weissel M. Hyperthyroidism and heart (review). Wien Klin Wochenschr 2001;113:157–161 [PubMed] [Google Scholar]
- 11.Montenegro J, Gonzalez O, Saracho R, et al. Changes in renal function in primary hypothyroidism. Am J Kidney Dis 1996;27:195–198 [DOI] [PubMed] [Google Scholar]
- 12.Kryvych NV, Pishak VP. Water-salt balance in patients with diffuse toxic goiter and hypothyroidism. Lis Sprava 1997;4:110–114 [PubMed] [Google Scholar]
- 13.Capasso G, De Tommaso G, Pica A, et al. Effects of thyroid hormones on the heart and kidney functions. Miner Electrolyte Metab 1996;25:56–64 [DOI] [PubMed] [Google Scholar]
- 14.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Intl J Obes Relat Metab Disord 2000;2(suppl):109–112 [DOI] [PubMed] [Google Scholar]
- 15.Bengel FM, Nekolla SG, Ibrahim T, Weniger C, Ziegler SI, Schwaiger M. Effect of thyroid hormones on cardiac function, geometry, and oxidative metabolism assessed non-invasively by positron emission tomography and magnetic resonance imaging. J Clin Endocrinol Metab 2000;85:1822–1827 [DOI] [PubMed] [Google Scholar]
- 16.Villabonna C, Sahun M, Roca M, et al. Blood volumes and renal function in overt and subclinical primary hypothyroidism. Am J Med Sci 1999;318:277–280 [DOI] [PubMed] [Google Scholar]
- 17.van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol 1998;11:138–145 [DOI] [PubMed] [Google Scholar]
- 18.Hierholzer K, Finke R. Myxedema. Kidney Int Suppl 1997;59:82–89 [PubMed] [Google Scholar]
- 19.Rasmussen AK. Cytokine actions on the thyroid gland. Dan Med Bull 2000;47:94–114 [PubMed] [Google Scholar]
- 20.Haussinger D, Kircheis G, Fischer R, et al. Hepatic encephaolopathy in chronic liver disease: a clinical manifestation of astrocytic swelling and low-grade cerebral edema. J Hepatol 2000;32:1035–1038 [DOI] [PubMed] [Google Scholar]
- 21.Haussinger D. Role of cellular hydration in the regulation of cell function. Biochem J 1996;313:697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estevez AY, O’Regan MH, Song D, et al. Hyposmotically induced amino acid release from the rat cerebral cortex: role of phospholipases and protein kinases. Brain Res 1999;844:1–9 [DOI] [PubMed] [Google Scholar]
- 23.Burg MB Molecular basis of osmotic regulation. Am J Physiol 1995;268:983–996 [DOI] [PubMed] [Google Scholar]
- 24.Law RO. The role of taurine in the regulation of brain cell volume in chronically hyponatraemic rats. Neurochem Int 1998;33:467–472 [DOI] [PubMed] [Google Scholar]
- 25.Nozaki M. Changes in free amino acids in the central nervous system of hypo- and hyperthyroid rats. Rinsho Shinkeigaku 1989;29:713–719 [PubMed] [Google Scholar]
- 26.Fox PR, Trautwein EA, Hayes KC, et al. Comparison of taurine, alpha-tocopherol, retinol, selenium, and total triglycerides and cholesterol. Am J Vet Res 1993;54:563–569 [PubMed] [Google Scholar]
- 27.Sugie H, Tsurui S, Ishikawa A, et al. Effects of neonatal hypothyroidism on brain development: analysis of brain metabolites using high resolution phosphorus and proton magnetic resonance (NMR) spectroscopy. No To Hattatsu 1990;22:166–172 [PubMed] [Google Scholar]
- 28.Baskin SI, Klekotka SJ, Kendrick ZV, et al. Correlation of taurine levels with thyroid function. J Endocrinol Invest 1979;2:245–249 [DOI] [PubMed] [Google Scholar]
- 29.Bhatora VS, Tripathi RP, Sankar R, et al. Frontal lobe proton magnetic-resonance spectroscopy in Graves’ disease: a pilot study. Psychoneuroendocrinology 1998;23:605–612 [DOI] [PubMed] [Google Scholar]
- 30.Gupta RK, Bhatia V, Poptani H, et al. Brain metabolite changes on in vivo proton magnetic resonance spectroscopy in children with congenital hypothyroidism. J Pediatr 1995;126:389–392 [DOI] [PubMed] [Google Scholar]
- 31.Shirota T. Studies on the renal handling of urea nitrogen, creatine, water and electrolytes in hyperthyroid patients with Graves’ disease. Nippon Naibunpi Gakkaiz Asshi 1991;67:611–621 [DOI] [PubMed] [Google Scholar]
- 32.Shitora T, Shinoda T, Yamada T, et al. Alteration of renal function in hyperthyroidism: increased tubular secretion of creatine and decreased distal tubule delivery of chloride. Metabolism 1992;41 ;402–405 [DOI] [PubMed] [Google Scholar]
- 33.Toft P, Botker HE. Hyperthyroidism and heart disease: is thyrotoxic cardiomyopathy a disease entity? Ugeskr Laeger 1993;155:1354–1357 [PubMed] [Google Scholar]
- 34.Araki T, Tofuku Y. An 85 year old case of Basedow’s disease associated with high output heart failure and angina pectoris. Nippon Ronen Igakkaiz Asshi 1996;33:191–195 [DOI] [PubMed] [Google Scholar]
- 35.Cordoba J, Crespin J, Gottstein J, et al. Mild hypothermia modifies ammonia-induced brain edema in rats after portacaval anastomosis. Gastroenterology 1999;116:686–693 [DOI] [PubMed] [Google Scholar]
- 36.Menig G. Induction of filtration oedema by extreme reduction of cerebrovascular resistance associated with hypertension. Euro Neurol 1972;8:97–103 [DOI] [PubMed] [Google Scholar]