Abstract
Summary: Primary angiitis of the CNS is histopathologically characterized by ischemic lesions and small petechial hemorrhages. Unlike CT or conventional MR imaging, gradient-echo MR imaging depicts these chronic petechial hemorrhages. We herein report the case of biopsy-proved primary angiitis of the CNS in a 42-year-old man; whom gradient-echo MR imaging revealed multiple petechial hemorrhages in the cortical-subcortical brain regions. The identification of petechial hemorrhages by gradient-echo MR imaging promises to be a valuable surrogate marker supporting the diagnosis of primary angiitis of the CNS.
Primary angiitis of the CNS is a rare form of vasculitis restricted to the CNS with no apparent systemic involvement. The neuropathologic findings consist of granulomatous inflammation or fibrinoid necrosis with disruption of leptomeningeal and parenchymal arteries and veins, leading to discrete foci of hemorrhages (1, 2). Because the involved blood vessels are approximately 200 to 300 μ in diameter, the hemorrhages tend to be small (2). CT and conventional MR imaging may reveal large lobar hemorrhages in primary angiitis of the CNS but cannot capture these petechial hemorrhages.
Gradient-echo MR imaging depicts chronic blood products (hemosiderin deposits) as regions with marked signal intensity loss (3). It has proved efficacious in showing cortical-subcortical petechial hemorrhages not visible on conventional MR images and in monitoring the disease progression in patients with cerebral amyloid angiopathy (4, 5). Moreover, gradient-echo MR imaging, but not conventional MR imaging, reveals coexisting chronic micro-hemorrhages in patients with hypertensive small vessel disease (6). We herein report the case of a patient with primary angiitis of the CNS for whom gradient-echo MR imaging revealed cerebral amyloid angiopathy-like multiple petechial hemorrhages scattered throughout the cortical-subcortical brain regions.
Case Report
A 42-year-old man presented with a history of three stereotypic tingling spells in the right hand. The first had started 9 days previously at the tips of his fourth and fifth fingers, spreading smoothly to the wrist along the ulnar side in approximately 5 minutes, and lasting for 15 minutes. A week later, he had a 10-minute episode with the same onset and spreading characteristics. As the tingling was resolving, difficulty in finding words began, which deteriorated to the level of muteness that lasted 6 hours; his speech gradually recovered within 12 hours. The next day he had a similar attack of tingling lasting 10 minutes.
The patient’s medical history was unremarkable. He had no history of previous drug use or hypertension. Blood pressure at admission was 110/70 mm Hg. Results of the physical examination were normal. A neurologic examination revealed reduced sensation to pain, temperature, and light touch on the ulnar side of the right hand. Astereognosis, graphanesthesia, and sensory extinction were also noted in that hand.
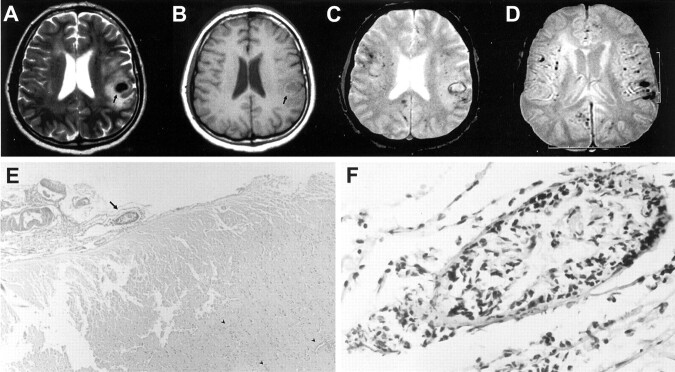
CT of the cranium showed a small hematoma in the left parietal lobe. Conventional MR imaging performed on a 1.5-T unit included T1-weighted, turbo spin-echo T2-weighted, and fluid-attenuated inversion recovery sequences that revealed an acute hemorrhage in the left parietal lobe and a subacute hemorrhage in the right frontal lobe (Fig 1A and B). T2-weighted gradient-echo images showed multiple small hemorrhages scattered throughout the cerebral hemispheres, located in the cortical-subcortical regions (Fig 1C and D). The basal ganglia, thalamus, and brain stem were spared. Blood cell counts, blood chemistry, antibody panel (antiphospholipid, antinuclear, anti-DNA, antineutrophilic-cytoplasmic antibodies), cryoglobulins, serum and CSF angiotensin-converting enzyme levels, thrombocyte function test results, and coagulation parameters (prothrombin time, partial thromboplastin time, proteins C and S, fibrinogen, antithrombin 3, and activated protein C resistance) were normal. The erythrocyte sedimentation rate was 3 mm/h. CSF was clear with normal opening pressure, and no pleocytosis was noted. The protein level was 44 mg/dL (15–40 mg/dL). Echocardiography, renal function test, electromyography (for ulnar nerve entrapment), and electroencephalography results were all normal. Selective cerebral angiography revealed no vascular abnormality.
Fig 1.
Images obtained in a 42-year-old man who presented with a history of three stereotypic tingling spells in the right hand.
A, Turbo spin-echo T2-weighted image, obtained through the level of lateral ventricles, shows an acute hematoma with surrounding edema (arrow) in the suprasylvian subcortical region on the left. Additional periventricular hyperintense lesions can be seen, possibly representing ischemic changes of indeterminate age.
B, Corresponding T1-weighted image also shows an acute hematoma with surrounding edema (arrow) in the suprasylvian subcortical region on the left.
C, Gradient-echo T2-weighted image, obtained through the same level as that of the images shown in A and B, shows additional millimetric hypointense foci, consistent with chronic hemorrhages.
D, Gradient-echo image (520/12.9 [TR/TE]; flip angle, 30 degrees), obtained through the level of the basal ganglia, clearly shows multiple hypointense foci of chronic hemorrhages located in the cortical-subcortical region in both hemispheres, with a widespread distribution, whereas the deep gray matter appears to be spared.
E, Pathologic examination of the biopsy material reveals leptomeningeal fibrosis and scattered lymphocytic infiltration with destruction of the vessel wall (arrow) (hematoxylin and eosin, original magnification ×40). Also note petechial hemorrhages with predilection around vessels (arrowheads).
F, Higher magnification of a leptomeningeal blood vessel shows severe destruction of the wall with a mixed inflammatory cell infiltrate (original magnification, ×400).
The pathologic examination of biopsy material, including the leptomeninges and the underlying gray and white matter, showed multiple foci of petechial hemorrhages. There was mural destruction of the medium sized leptomeningeal vessels with mixed cellular infiltrate (Fig 1). Scattered lymphocytes around the parenchymal vessels were also noticed. No granulomatous change, cavernous angioma, or calcification was detected. Congo red staining was negative for amyloid deposition under polarized microscopy. On the basis of histopathologic features and the lack of evidence of systemic vasculitis, the patient was considered to have primary angiitis of the CNS. Cyclophosphamide and corticosteroid therapies were administered. During a 6-month follow-up period, the patient remained totally symptom-free with no apparent disease activation.
Discussion
Primary angiitis of the CNS currently is a diagnosis of exclusion. There is no readily available noninvasive diagnostic test. The neurologic signs and symptoms are typically nonspecific. There are a number of conditions that may share the same clinical, imaging, and angiographic characteristics with primary angiitis of the CNS, such as vasospasm, fibromuscular dysplasia, diffuse cerebrovascular atherosclerosis, and multiple embolisms. The cardinal feature of primary angiitis of the CNS is the occurrence of ischemic lesions throughout the CNS (7). However, unlike other conditions, in primary angiitis of the CNS, discrete foci of hemorrhages may accompany the ischemic lesions (2, 8). In a report of 108 patients, presented by Calabrese et al (7), frank intracerebral hemorrhages were observed in 4%. A recent review by Schmidley (2) reported 14 patients with biopsy-proved hemorrhages. In these patients, the hemorrhages often involved cortical and subcortical regions and no risk factors predisposing to hemorrhages were identified. The current report is the first to show that gradient-echo MR imaging can successfully capture widespread petechial hemorrhages in primary angiitis of the CNS. This gradient-echo MR imaging finding, when combined with the evidence of coexisting ischemic lesions on other MR imaging sequences, may be a powerful aid to diagnosis in a subset of patients with clinical suspicion of primary angiitis of the CNS.
The histologic proof of vascular inflammation is currently the reference standard for diagnosis. However, biopsy in cases of primary angiitis of the CNS may still be falsely negative in ≤50% of patients because of inappropriate sampling as a result of patchy involvement and the multifocal nature of the disease (9). The identification by gradient-echo MR imaging of petechial hemorrhages does not, per se, eliminate the need for biopsy, because several neurologic conditions, such as cerebral amyloid angiopathy, multiple cavernous angiomas, and hypertensive small vessel disease, are also associated with widespread petechial hemorrhages revealed by gradient-echo MR imaging (4, 6). The finding of widespread and predominantly cortical-subcortical location of hemorrhages, however, may have a distinctive diagnostic value, because it overlaps with the distribution of histopathologic findings of vascular inflammation in primary angiitis of the CNS.
Patients with primary angiitis of the CNS may occasionally present with transient neurologic symptoms. Transient spells of tingling spreading to the contagious body parts and one episode of transient aphasia were notable in our patient with hemorrhages. Greenberg et al (10) emphasized that patients with small cortical hemorrhages due to cerebral amyloid angiopathy may present with spreading neurologic dysfunction mimicking transient ischemic attacks. A spreading depression-like mechanism elicited by the cortical bleed has been suspected to cause these transient spells. Although one should always be cautious in interpreting intermittent neurologic symptoms that recur over time as transient ischemic attacks, the recurrent nature of these stereotypic spells in a patient with a known vasculitis may sometimes justify the use of risky treatments such as anticoagulants or anti-aggregants. The gradient-echo MR imaging evidence of cortical hemorrhages in our patient emphasizes that recurrent transient neurologic spells in a patient with vasculitis are not synonymous with ischemic attacks.
Conclusion
Chronic, silent, petechial hemorrhages along with multifocal ischemic lesions raise a suspicion of primary angiitis of the CNS. In this respect, gradient-echo MR imaging seems to be a useful addition to the evaluation of patients with primary angiitis of the CNS. Whether the number and extent of these hemorrhages correlate with the disease activity remains to be elucidated.
References
- 1.Lie JT. Primary (granulomatous) angiitis of the central nervous system: a clinicopathologic analysis of 15 new cases and a review of the literature. Hum Pathol 1992;23:164–171 [DOI] [PubMed] [Google Scholar]
- 2.Schmidley JW. Isolated CNS angiitis: pathology. In: Schmidley JW, ed. Central Nervous System Angiitis. Woburn: Butterworth-Heinemann;2000. :1–28
- 3.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–642 [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology 1996;46:1751–1754 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SM, O’Donnell HC, Schaefer PW, Kraft E. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology 1999;53:1135–1138 [DOI] [PubMed] [Google Scholar]
- 6.Kwa WI, Franke CL, Verbeeten B, Stam J. Silent intracerebral microhemorrhages in patients with ischemic stroke. Ann Neurol 1998;44:372–377 [DOI] [PubMed] [Google Scholar]
- 7.Calabrese LH, Furlan AJ, Gragg LA, Ropos TJ. Primary angiitis of the central nervous system: diagnostic criteria and clinical approach. Cleve Clin J Med 1992;59:293–306 [DOI] [PubMed] [Google Scholar]
- 8.Hunn M, Robinson S, Wakefield L, Mossman S, Abernethy D. Granulomatous angiitis of the CNS causing spontaneous intracerebral haemorrhage: the importance of leptomeningeal biopsy. J Neurol Neurosurg Psychiatry 1998;65:956–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmer TL, Guamaccia J, Harrington W, Pacia SV, Petroff OA. Idiopathic granulomatous angiitis of the central nervous system: diagnostic challenges. Arch Neurol 1993;50:925–930 [DOI] [PubMed] [Google Scholar]
- 10.Greenberg SM, Vonsattel JP, Stakes JW, Gruber M, Finklestein SP. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology 1993;43:2073–2079 [DOI] [PubMed] [Google Scholar]