Abstract
BACKGROUND AND PURPOSE: The risk of stroke after a transient ischemic attack (TIA) is high. Appropriately directed therapies may reduce this risk. However, sensitive means of detecting the presence of subtle neuronal ischemia are lacking. We investigated the potential use of quantitative diffusion-weighted (DW) MR imaging in the detection of deficits produced by transient cerebral ischemia.
METHODS: Twenty-eight patients who came to the stroke service from the emergency room of a tertiary teaching hospital with the final diagnosis of transient cerebral ischemia underwent conventional MR imaging, MR angiography, and DW MR imaging within 24 hours of presentation. Fifteen patients had normal conventional DW images confirmed by a staff neuroradiologist and neurologist. For these patients, absolute quantitative diffusion values were subsequently calculated for the clinically relevant brain region and were compared with the values calculated for the corresponding contralateral unaffected brain region. Thirteen patients had conventional DW images positive for lesions and were not studied.
RESULTS: Quantitative DW imaging enabled detection of abnormal decreases (9–26%, P < .05) in the diffusion constant in brain regions suspected to be clinically involved by ischemia, when compared with the contralateral clinically unaffected brain tissue as well as with two other internal controls.
CONCLUSION: Quantitative DW imaging depicts diffusion deficit in patients with TIA. Quantitative DW imaging may have better sensitivity compared with conventional DW imaging in detecting transient cerebral ischemia.
A transient ischemic attack (TIA) is an acute neurologic syndrome of vascular etiology that resolves within 24 hours. Other paroxysmal disorders such as focal seizures, migraines, and transient global amnesia can mimic TIA. The accurate diagnosis of TIA is important because, depending on the underlying mechanism, the stroke risk can be as high as 40% in the subsequent 2 years (1, 2).
The diagnosis of TIA may be difficult, however, and misdiagnosis is frequent (3, 4). Because missing a sentinel ischemic attack is a lost opportunity to investigate and prevent cerebral infarction, it would be useful to have sensitive methods to detect these warning events.
Diffusion-weighted (DW) MR imaging has been helpful in the diagnosis of stroke. Conventional DW techniques rely on the principle that the cytotoxic edema induced by cerebral infarction changes the nature of the microscopic diffusion of water. As sodium pump failure is induced by ischemia, progressive intracellular cytotoxic edema occurs. This diffusion deficit is detected as an area of high signal intensity on conventional DW images.
Conventional DW imaging does not have a high yield in the detection of deficits induced by transient ischemia. A recent study of patients with TIA revealed conventional DW images positive for relevant lesions in only 50% of the patients (5). In another study involving patients who presented with symptoms suggestive of TIA and in whom the diagnosis was not made with DW imaging, the authors observed that in 63% of the patients, the best final diagnosis was still ischemia established by means of other vascular studies (6).
The degree of neuronal failure in TIA is probably subtle compared with that of stroke patients. We hypothesized that quantifying diffusion deficits in areas that are clinically involved in the TIA of those patients whose conventional DW images are normal may be more helpful in diagnosing their deficits. We applied this method to study patients with TIA whose conventional DW images failed to reveal any abnormality.
Methods
Inclusion Criteria
During a 1-year period from September 1999 to August 2000, patients who came to the neurology service with the diagnosis of TIA and who underwent conventional MR imaging, DW imaging, and MR angiography within 24 hours of presentation were studied. All patients had a detailed history and a neurologic examination by a neurologist. The clinical diagnosis of TIA was applied if the patient had neurologic symptoms that were sudden in onset and if there was no observed seizure or seizure-like movement, no history of established epilepsy, and no history of migraine with aura or a march of symptoms suggestive of cortical spreading depression (7). Neuroanatomic location was noted for all patients; hemispheric symptoms (eg, aphasia, neglect, isolated hand or arm weakness) and subcortical or brain stem ischemia were included. A neurologist in each individual case confirmed the clinical diagnosis of cerebral ischemia and its appropriate location.
The following clinical data were compiled for all patients: age, sex, handedness, symptoms of TIA, date and time of symptom onset, date and time of symptom resolution, duration of TIA, history of previous clinical TIA or stroke, and vascular risk factors (hypertension, coronary artery disease, diabetes mellitus, hypercholesterolemia, and history of tobacco use). Also noted were the results of all vascular studies performed in the patients (echocardiography and transesophageal echocardiography, carotid Doppler US, aortic arch angiography).
All normal DW images were interpreted as such by a staff neuroradiologist (A.Z.S.) who was aware of the clinical history of each patient. A staff neurologist who was also aware of the history and presumed neuroanatomic location independently confirmed the DW images to be normal by visual analysis. Thus, patients who had a TIA and a nondiagnostic (normal) DW imaging study were selected for further study. Patients with nondiagnostic conventional DW images were all imaged within 6 hours of their symptoms.
All patients (Table 1) included in the study had complete resolution of neurologic symptoms, which was confirmed by repeat history and neurologic examination.
TABLE 1:
Clinical Presentation and Stroke Risk Factors
| Patient no. | Age (y)/Sex | Stroke Risk Factors | Clinical Presentation | Symptom Duration (hrs) |
|---|---|---|---|---|
| 1 | 82/F | HTN, HLD, CAD | Nonfluent aphasia and mild right hemiparesis | 0.5 |
| 2 | 32/F | IDDM | Fluent aphasia, left gaze preference and right hemiparesis | 4 |
| 3 | 91/F | CHF, CAD | Fluent aphasia | 10 |
| 4 | 39/F | Smoking | Face, arm, leg numbness; no weakness. No sensory inattention | 0.25 |
| 5 | 78/F | HTN, DM | Nonfluent aphasia | 3 |
| 6 | 81/F | AF* | vertigo and gait ataxia | 2 |
| 7 | 76/M | CAD | Isolated right hand and arm weakness, no aphasia | 0.75 |
| 8 | 68/F | HTN | Left arm and hand weakness, | 6 |
| sensory neglect | ||||
| 9 | 77/M | HTN, AF* | Left facial weakness, left hand clumsiness, dysarthria and left dysdiadochokinesia | 0.5 |
| 10 | 54/M | Smoking, HTN | Complete speech arrest and mild right pronator drift | 0.5 |
| 11 | 76/M | HTN | Complete speech arrest | 0.17 |
| 12 | 81/M | CAD, HTN | Dysarthria, left facial and left hemiparesis | 0.42 |
Note. AF indicates atrial fibrillation, CAD, coronary artery disease, CHF, congestive heart failure; DM, diabetes mellitus; HLD, hyperlipidaemia, HTN, hypertension; IDDM, insulin-dependent diabetes mellitus.
Not receiving anticoagulation therapy.
Exclusion Criteria
Patients who had abnormal conventional DW images were not included in the study, because they were considered to have an ischemic event with a relevant and obvious lesion and were not representative of patients with nondiagnostic (normal) DW images. Patients with isolated ocular ischemia also were not studied. Patients whose history and clinical examination were nonlocalizing for confusion or syncope were excluded.
Patients
During this 1-year period, 28 patients received a final diagnosis of TIA and underwent DW MR imaging. All patients were examined at admission and followed up subsequently by a stroke neurologist. Thirteen patients had DW images positive for lesions. In the remaining 15 patients, conventional DW imaging was nondiagnostic, and these patients were evaluated in detail. Of these 15 patients, three were excluded from quantitative analysis: the DW images of one patient were of technically inferior quality for the analysis, and another patient had hyperintensity on DW images in regions (contralateral hemisphere) other than that causing his acute symptoms. Since the contralateral hemisphere was not representative of normal brain tissue (infarction by DW imaging), it could not be used for quantitative analysis. The third patient underwent DW imaging more than 6 hours after his TIA; this patient was excluded from the final analysis to ensure a homogeneous patient population.
MR Imaging Methods
In all patients, MR imaging was performed with a 1. 5 T clinical imager (Signa Echo-Speed; GE Medical Systems, Milwaukee, WI). MR imaging studies included a sagittal T1-weighted sequence and axial T2-weighted, T1-weighted, fluid-attenuated inversion-recovery, gradient-echo, and DW sequences. Intracranial and extracranial MR angiography was also performed. The DW MR protocol included single-shot echo planar imaging with data matrix of 128 x 128 pixels, TE of 101 ms, field of view of 22 cm, 30 sections of 5-mm thickness with no gap between sections covering the entire brain. Diffusion weighting was applied separately in three orthogonal directions with a b value of 1000 s/mm2 in each direction, and three DW images (DWIx, DWIy, DWIz) were obtained. Another image without diffusion weighting (So) was also obtained. With use of these three DW images, a combined DW image, DWItrace, that was weighted by the trace of the diffusion tensor was obtained on the imager as follows:
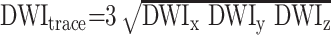 |
This combined image is isotropic, and orientation-dependency of diffusion is removed.
Quantitation
The combined DW image (DWtrace) and the image without diffusion weighting (So) were transferred to a computer workstation. The orientation-independent average diffusion constant (Dav) maps, or ADC maps, were calculated pixel by pixel. The average diffusion constant Dav is defined as:
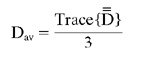 |
where 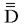 is the diffusion tensor. To calculate Dav, we used a computer program by using the following equation:
is the diffusion tensor. To calculate Dav, we used a computer program by using the following equation:
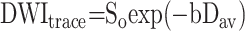 |
where b is the strength of diffusion weighting (8).
Another computer program was used to calculate the diffusion distribution maps from the entire brain, as described in detail elsewhere (8). These distribution maps were then fitted to a triple Gaussian curve,
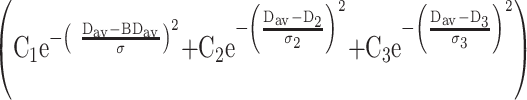 |
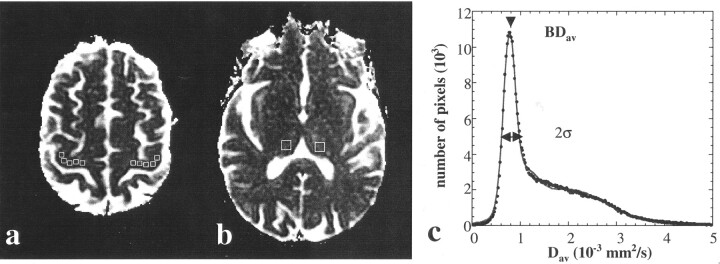
representing the three-compartmental nature of the data: brain tissue, CSF, and an additional compartment for the pixels that contain both brain tissue and CSF (8). From this fit, the mean Dav for the entire brain tissue (BDav) and the width of this distribution (σ) were determined, as previously described (8) (Fig 1).
Fig 1.
A, Quantitative Dav map shows ROIs (boxes) in the location of clinically suspected region of brain (right motor cortex) and contralateral control site. These two measurements were used to calculate the ratio m1.
B, Quantitative Dav map shows regions used for measurements made on right and left thalami (boxes) for calculating the control ratio of c1.
C, Mean value of the brain tissue diffusion constant (BDav) and width of the distribution (σ) were determined from the distribution analysis map by using a three-compartmental model (see Methods). This value was used in determining the ratios m3 and c3. Data are shown as circles, and the fit is shown as a line.
The quantitative Dav maps depicted the TIA-affected tissue in the clinically suspected areas of the brain in nine of 12 cases. By placing regions-of-interest (ROIs) on these Dav maps, we determined the diffusion constants in the region of TIA (Fig 1). For example, in a patient with right limb weakness, ROIs were placed on the left motor cortex. The size of the ROIs were dictated by the relevant anatomy. Brain stem ROIs were smaller compared with those of the the motor cortex. We compared these ROI measurements with a similar region on the contralateral side, as suggested previously (9). In addition, we measured the diffusion constants in both thalami. We compared the diffusion results from the TIA-affected region with the diffusion results from both thalami. We also compared the diffusion results of the thalamus in the ipsilateral hemisphere (hemisphere involved by TIA) with that of the thalamus in the contralateral hemisphere to rule out any hemispheric difference that may arise from reasons other than TIA. As a third independent quantitation measure, we compared the diffusion values measured from TIA-affected regions with the mean diffusion constant of the entire brain (BDav) determined from the distribution analysis and the fit.
Statistical Analysis
We used the mean and standard deviation (SD) of measurements and a two-tailed t test in determining statistical significance of the results (Table 2). The statistical significance was set at the P equals .05 level.
TABLE 2:
Diffusion Measurements
| Patient No. | TIA Location | ROI Measurements* (10−3 mm2/s) |
Fit† (10−3 mm2/s) |
||||
|---|---|---|---|---|---|---|---|
| Ipsilateral ROI | Contralateral ROI | Ipsilateral Thalamus | Contralateral Thalamus | BDav | σ | ||
| 1 | Broca | 0.620 ± 0.075 | 0.796 ± 0.077 | 0.777 ± 0.008 | 0.771 ± 0.042 | 0.766 | 0.189 |
| 2 | Wernicke | 0.636 ± 0.005 | 0.713 ± 0.053 | 0.720 ± 0.046 | 0.738 ± 0.082 | 0.765 | 0.180 |
| 3 | Wernicke | 0.716 ± 0.067 | 0.852 ± 0.084 | 0.855 ± 0.060 | 0.873 ± 0.064 | 0.837 | 0.181 |
| 4 | R thalamus | 0.619 ± 0.029 | 0.730 ± 0.484 | (0.770 ± 0.090)‡ | (0.772 ± 0.079)‡ | 0.749 | 0.188 |
| 5 | Broca | 0.834 ± 0.062 | 1.111 ± 0.105 | 1.204 ± 0.102 | 1.162 ± 0.084 | 0.854 | 0.218 |
| 6 | L pons | 0.606 ± 0.087 | 0.806 ± 0.031 | 0.736 ± 0.083 | 0.725 ± 0.079 | 0.787 | 0.202 |
| 7 | L motor cortex | 0.685 ± 0.062 | 0.795 ± 0.045 | 0.743 ± 0.090 | 0.743 ± 0.067 | 0.768 | 0.191 |
| 8 | R motor cortex | 0.677 ± 0.044 | 0.780 ± 0.059 | 0.790 ± 0.103 | 0.770 ± 0.086 | 0.768 | 0.174 |
| 9 | L pons | 0.606 ± 0.011 | 0.745 ± 0.028 | 0.774 ± 0.051 | 0.783 ± 0.076 | 0.785 | 0.207 |
| 10 | Broca | 0.613 ± 0.037 | 0.681 ± 0.050 | 0.750 ± 0.107 | 0.745 ± 0.091 | 0.739 | 0.174 |
| 11 | Broca | 0.724 ± 0.055 | 0.799 ± 0.078 | 0.721 ± 0.087 | 0.717 ± 0.059 | 0.773 | 0.183 |
| 12 | R pons | 0.518 ± 0.059 | 0.699 ± 0.003 | 0.800 ± 0.107 | 0.802 ± 0.106 | 0.809 | 0.212 |
| Mean | 0.654 | 0.792 | 0.803 | 0.800 | 0.783 | 0.192 | |
| SD | 0.080 | 0.112 | 0.132 | 0.250 | 0.034 | 0.015 | |
Note.— L indicates left; R, right.
Data are the mean ± SD of the measurements.
Error of fit is 0.01 10−3 mm2/s. BDav is the mean of the average diffusion constant distribution measured from the entire brain tissue. σ is the width of this distribution (8).
Instead of thamali, caudate nuclei were used as control in this patient because TIA involved the right thalamus.
Using the diffusion results of ROI measurements, we first calculated three ratios in each patient: m1, TIA-affected region to contralateral region; m2, TIA-affected region to thalamic average; and m3, TIA-affected region to mean brain diffusion value (BDav) determined by the fit. We then calculated three control ratios as follows: c1, ipsilateral thalamus to contralateral thalamus; c2, contralateral ROI to thalamic average; and c3, contralateral ROI to mean brain diffusion value (BDav) determined by the fit.
The control ratio c1 was calculated to determine the extent of any possible hemispheric difference that might have arisen from physiologic causes or data processing. The control ratios c2 and c3 were calculated to ensure that the chosen contralateral ROI was not abnormally different from the internal reference values of Dav of thalamus and BDav, which in turn further guaranteed the significance of the difference of the ipsilateral ROI measurements’ abnormality when it was compared with the contralateral ROI and two internal controls.
Using the above ratios from all patients, we performed a two-tailed t test to determine the statistical significance of the difference in the measurement ratios versus control ratios. We compared m1 with c1, m2 with c2, and m3 with c3 ratios for all patients. The results were all significant at P less than .05 level. We also determined and reported the mean and SD of the measurements for each ratio group.
Results
By neuroanatomic and vascular localization in the 12 patients, eight patients had cortical hemispheric symptoms, one had a posterior circulation TIA, and three presented with symptoms of subcortical ischemia. The longest time of persistent clinical symptoms was 10 hours in one patient, and the shortest time reported was 10 minutes. The median duration of clinical symptoms was 38 minutes (mean duration, 140 minutes). The time from symptom onset to imaging was less than 6 hours in all patients who were analyzed. Except for one patient in whom cerebrovascular risk factors could not be identified, all the patients had risk-factor profiles that would place them at high risk for cerebral ischemia. Seven patients had hypertension, four had coronary artery disease, two had atrial fibrillation and were not receiving anticoagulation, and one had hyperlipidemia.
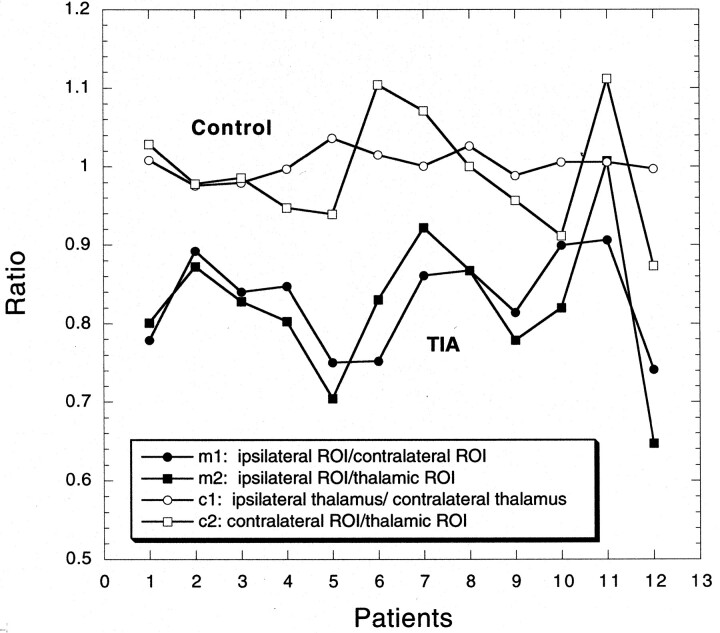
Quantitation of average diffusion constant (Dav) showed that all patients had decreased diffusion in the range of 9% to 26% in the TIA-affected regions of the brain when compared with the contralateral side (Table 2, Fig 2). This decrease in Dav is about half of what is reported in patients with acute stroke (9, 10). Although this decrease was visually evident in most cases (nine of 12) on Dav images (Fig 3), the conventional DW images did not depict the regions and were interpreted as normal by the staff radiologist and neurologist in all cases. In three of 12 cases in which visual abnormality was not obvious, quantitation revealed low diffusion in the areas of brain suspected to be involved in TIA. Patient 11 who had the shortest TIA symptom duration had the least diffusion decline in the clinically affected region when compared with the contralateral side (Fig 2).
Fig 2.
Four measured ratios (m1, m2, c1, c2) are shown for all 12 patients. TIA-affected regions are compared with corresponding contralateral brain regions (m1) and to thalamic controls (m2) not affected by TIA (solid symbols). The resultant ratios are consistently below unity and are around 0.82. However, when regions of brain not affected by the TIA are compared (c1, c2), no interhemisperic difference is noted and the ratios approach unity (open symbols).
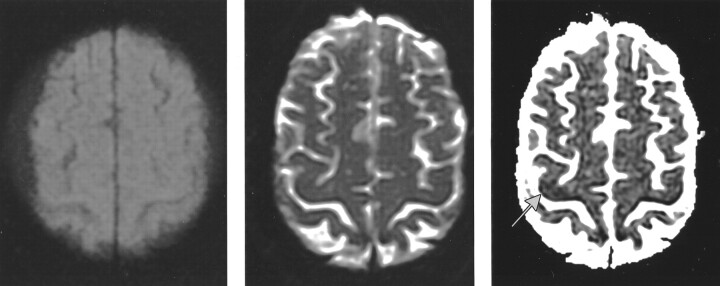
Fig 3.
Patient with left hand and arm weakness (patient 8).
A and B, DW image (A) and T2-weighted image (B) of an axial section through the motor cortex do not show any abnormality.
C, Quantitative Dav map shows a dark region (arrow) in the right motor cortex.
Comparison of the diffusion value of the TIA-affected region with the diffusion value of either the corresponding contralateral brain or thalamus or the mean diffusion value measured from the entire brain (BDav) provided similar results. On average, the diffusion ratio of TIA-affected region to contralateral region was 0.83 ± 0.06, to thalamus was 0.82 ± 0.09, and to BDav was 0.84 ± 0.09.
In all patients, the contralateral control region that we chose had a Dav value similar to that of the rest of the brain. This was determined by comparing the contralateral region with the thalamic measurement and BDav. The ratio of the Dav of the contralateral region to thalamus was 0.99 ± 0. 07 and to BDav was 1.01 ± 0.11.
We also compared the diffusion value of the thalamus in the ipsilateral hemisphere with that in the other thalamus. The value of this ratio was 1.00 ± 0.02, which ruled out the possibility of hemispheric difference for diffusion values.
All three quantitative measurements were significantly different (P < .05) when compared with controls, ensuring the validity of the quantitation methods used.
Discussion
By the application of quantitative DW imaging, we were able to detect decreases in the range of 9% to 26% in patients with TIA who had negative conventional DW images. In these patients, we found abnormal values even if the duration of the reported cerebral ischemia was as low as 10 minutes (patient 11). Presumably the short duration of ischemia causes measurable diffusion decline but not to a degree to cause visible lesions on DW images at the current b value used. A recent study (11) used high b-value imaging in patients with cerebral ischemia. High b values allowed old cortical strokes and lacunes to become more hypointense and more obvious but did not increase the yield for any “new” diffusion-positive lesions (11). The small magnitude of the diffusion declines seen in our patients may not translate into a high signal intensity on DW images even if high b-value imaging is used. The utility of high b-value imaging in TIA still remains unanswered.
Clinical localization helped guide the placement of ROIs. Patients who have an unclear history or diffuse hemispheric dysfunction cannot be studied with this technique, as their symptoms cannot be ascribed to focal cortical dysfunction. ROI analysis also may be difficult when analyzing loss of complex brain function, for example aphasia or neglect. There are limitations in the clinical assessment of TIA (3). Although reported interobserver reliability for the diagnosis of TIA is substantial (12), determination of the location of TIA is only fair (12). This is an inherent limitation in all studies investigating TIA in which symptoms resolve quickly. In our study, we attempted to overcome this limitation by examining patients in the short term (< 6 hours). We adhered to the neurologic definition of TIA (13). Misplacements of ROIs would be expected to bias toward negative results. Despite this, decreases in quantitative diffusion were found among all our patients.
Studies of controlled middle cerebral artery occlusions and reperfusion in animals have shown that there are two phases of diffusion constant decline: a relatively acute decline ascribed to pump failure and acute cytotoxic edema, and a second steady and delayed slow decline (14, 15). The latter decline has been ascribed to reperfusion injury, calcium influx, and secondary neuronal failure and apoptosis. In human stroke studies, the magnitude of the diffusion decrease is approximately 60% when the clinical picture is completed stroke (10) compared with 40% in a diffusion-positive TIA (5). We found diffusion decreases in the range of 20% when the clinical presentation was consistent with a TIA and conventional DW imaging failed to reveal any abnormality.
Migraine auras represent another classic model of paroxysmal neurologic deficit that is almost always associated with complete neurologic recovery. Recent animal models of cortical spreading depression (a spreading wave of cerebral depolarization with increased intracellular sodium, cell swelling) have shown that there are 20–30% measurable decreases in diffusion (16). These animal studies measured a degree of neuronal failure that is spontaneously reversible. We find it interesting that in both clinical contexts of spontaneous recovery, the measured degree of diffusion deficit is of the same magnitude.
In most patients with TIA, the duration of ischemia is short, usually less than 1 hour. The reported median duration of a carotid distribution TIA is 14 minutes and that of a vertebrobasilar TIA is 8 minutes (17). In most patients, symptoms have resolved by the time they see a neurologist or are rapidly improving. These typical patients would not be expected to have lesions on DW images. Indeed, in a recent study of patients with TIA, only 48% had diffusion-positive lesions (5). In this study, prolonged duration of symptoms was highly predictive of positive DW images; patients with positive DW images had more persistent symptoms (mean duration, 7.3 hours) than those without lesions on DW images (3.2 hours) (5). In our patients, the median duration of symptoms was 0.5 hours and the mean duration of symptoms was 2.2 hours. This is more representative of the typical TIA patient, and based on the above study, conventional DW images would be expected to be normal (5).
The usual short duration of ischemia and the rapid clinical resolution suggest that the ischemic insult may not be of the magnitude to cause severe membrane pump failure and thus does not cause diffusion-positive lesions on MR images. Even in the patients with TIA who had positive DW images and whose diffusion values were decreased, the magnitude of this decrease was not as large as in completed cerebral infarction (5). Ideally, these patients could be reimaged to determine if the regions with decreased diffusion constants become normal, but with clinical resolution of symptoms, reimaging is not clinically justified.
These observations suggest that by measuring diffusion constants and quantifying diffusion deficits in patients with TIA, we may be able to establish quantitative measures of neuronal damage that can be used to predict the threshold for reversibility if these results can be verified by a larger study. Detection of these small but measurable insults may be supportive evidence for the diagnosis of ischemia. This methodology may ultimately provide a deeper pathophysiologic understanding of the neuronal events underlying TIA.
Acknowledgments
We thank Peter B. Barker for critically reading the manuscript. We are also thankful for his IMAX2 data analysis program that we used for ROI analysis.
References
- 1.American Heart Association medical statement: guidelines for the management of transient ischemic attacks. Available at: http://www. americanheart.org/Scientific/statements/1994/069401.htm. Accessed [DATE]
- 2.Caplan LR. Are terms such as completed stroke or RIND of continued usefulness? Stroke 1983;14:431–433 [DOI] [PubMed] [Google Scholar]
- 3.Ferro JM, Falcao I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist: a validation study. Stroke 1996;27:2225–2229 [DOI] [PubMed] [Google Scholar]
- 4.Calanchini PR, Swanson PD, Gotshall RA, et al. Cooperative study of hospital frequency and character of transient ischemic attacks, IV: the reliability of diagnosis. JAMA 1977;238:2029–2033 [PubMed] [Google Scholar]
- 5.Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999;30:1174–1180 [DOI] [PubMed] [Google Scholar]
- 6.Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology 1999;52:1784–1792 [DOI] [PubMed] [Google Scholar]
- 7.Caplan LR. Nonatherosclerotic vasculopathies. In: Caplan LR, ed. Stroke. A Clinical Approach. 3rd ed. Woburn, Mass: Butterworth-Heinemann;2000. :323–324
- 8.Chun T, Filippi CG, Zimmerman RD, Uluğ AM. Diffusion changes in the aging human brain. AJNR Am J Neuroradiol 2000;21:1078–1083 [PMC free article] [PubMed] [Google Scholar]
- 9.Uluğ AM, Beauchamp N Jr, Bryan RN, van Zijl PCM. Absolute quantitation of diffusion constants in human stroke. Stroke 1997;28:483–490 [DOI] [PubMed] [Google Scholar]
- 10.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231–241 [DOI] [PubMed] [Google Scholar]
- 11.Meyer JR, Gutierrez A, Mock B, et al. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol 2000;21:1821–1829 [PMC free article] [PubMed] [Google Scholar]
- 12.Shinar D, Gross CR, Mohr JP, et al. Interobserver variability in the assessment of neurologic history and examination in the stroke data bank. Arch Neurol 1985;42:557–565 [DOI] [PubMed] [Google Scholar]
- 13.Millikan CH. Ad hoc committee on cerebrovascular disease: a classification and outline of cerebrovascular disease, II. Stroke 1975;6:565–616 [Google Scholar]
- 14.Li F, Silva MD, Liu KF, et al. Secondary decline in apparent diffusion coefficient and neurological outcomes after a short period of focal brain ischemia in rats. Ann Neurol 2000;48:236–244 [PubMed] [Google Scholar]
- 15.Dijkhuizen RM, Knollema S, van der Worp HB, et al. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: evidence for region-specific sensitivity and delayed damage. Stroke 1998;29:695–704 [DOI] [PubMed] [Google Scholar]
- 16.Roether J, de Crespigny AJ, D’Arceuil H, Mosley ME. MR detection of cortical spreading depression immediately after focal ischemia in the rat. J Cereb Blood Flow Metab 1996;16:214–220 [DOI] [PubMed] [Google Scholar]
- 17.Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999;52:976–980 [DOI] [PubMed] [Google Scholar]