Abstract
Summary: Intracranial neurenteric cysts are uncommon and usually have low intensity on T1-weighted MR images and high intensity on T2-weighted MR images. We report a case of a neurenteric cyst that was situated in front of the medulla oblongata and the size of which increased with alteration of MR signal from high to isointense compared with that of brain on T1-weighted images obtained 33 months after the initial MR images. We think that the signal change of the cyst was probably caused by a change of protein concentration.
Neurenteric cysts are cystic masses lined by a low columnar or cuboidal epithelium of endodermal origin and are rare in the CNS. Most have high intensity on T2-weighted MR images and isointensity or slightly high intensity compared with CSF on T1-weighted MR images but occasionally have homogeneous very high intensity on T1-weighted MR images (1). We describe a case of neurenteric cyst with alteration of internal signal from hyperintense to isointense compared with brain on T1-weighted MR images.
Case Report
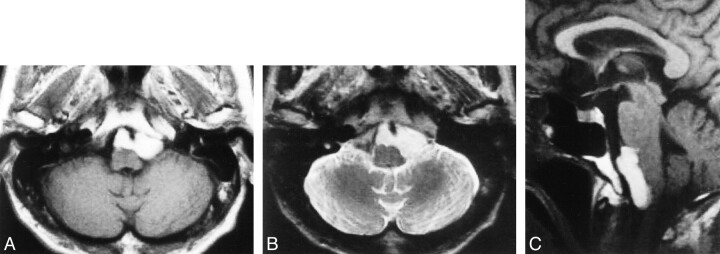
The patient was a 78-year-old man who presented with headache and swallowing disturbance. A neurologic examination revealed no lower cranial nerve deficit. MR images revealed a cystic mass, 3.5 cm in diameter, in front of the medulla oblongata (Fig 1A–C). The mass showed markedly high intensity on T1-weighted MR images (Fig 1A and C) and slightly lower intensity than that of CSF on T2-weighted MR images (Fig 1B).
fig 1.
Initial MR imaging.
A, Axial T1-weighted MR image reveals a high-intensity cystic mass, 3.5 cm in diameter, in front of the medulla oblongata.
B, Axial T2-weighted MR image shows that the mass has higher intensity than brain and lower intensity than CSF.
C, Sagittal T1-weighted MR image reveals that the mass compresses the medulla.
The patient had been followed up without surgical management because he had few complaints and no worsening of swallowing disturbance. MR images obtained 1 year and 4 months later revealed no change in size or intensity on either T1- or T2-weighted MR images.
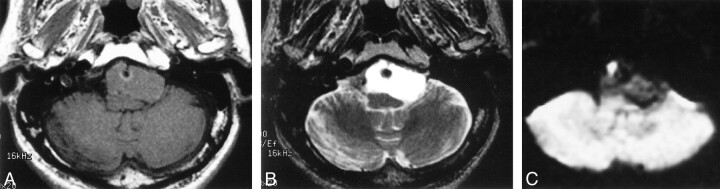
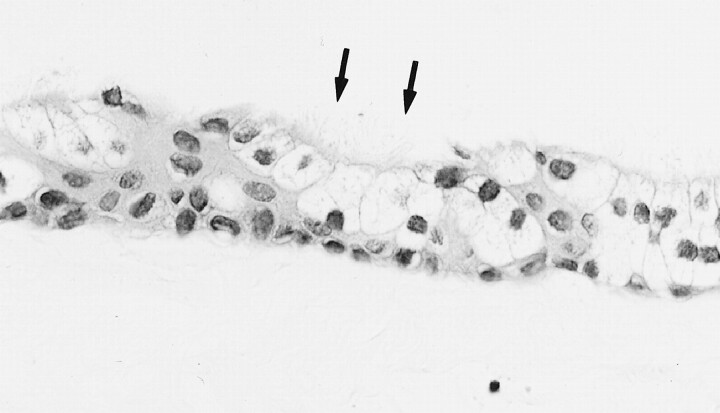
Two years and 9 months after the initial MR examination, the patient manifested loss of taste and worsening of the headache and swallowing disturbance. A neurologic examination revealed absence of gag reflex bilaterally and unskillful tandem gait. MR imaging revealed that the cystic mass had increased in size and compressed the medulla oblongata dorsally. The mass had isointensity compared with brain on T1-weighted MR images (Fig 2A) and higher intensity than CSF on T2-weighted MR images (Fig 2B). On diffusion-weighted images (echo-planar, three orthogonal directions, b = 1000, 5000/99 [TR/TE]), the cyst had low intensity (Fig 2C). Surgery was performed via left suboccipital craniotomy. Just medial to the left IXth and Xth cranial nerves that were displaced laterally, a yellowish cystic mass was found ventral to the brain stem. The yellowish watery fluid of the cyst content was evacuated, and the semilucent white thin wall was partially removed. No hemorrhagic change was observed. Chemical analysis of the aspirated fluid revealed a protein level of 10.1 g/dL and a cholesterol level of 196 mg/dL. A histopathologic examination revealed that the cyst wall was composed of a single layer of ciliated columnar epithelium, which was diagnosed as a neurenteric cyst (Fig 3).
fig 2.
Images obtained 2 years 9 months after initial MR examination.
A, Axial T1-weighted MR image shows that the cystic mass has isointensity and has increased in size.
B, T2-weighted MR image shows that the cyst has higher intensity than CSF.
C, Diffusion-weighted image shows that the mass has low intensity. The finding may enable differentiation from epidermoid cyst, which can show similar intensities on T1- and T2-weighted MR images but shows iso- or hyperintensity compared with brain on diffusion-weighted images.
fig 3.
Histopathologic sections (hematoxylin and eosin, original magnification ×400). The cyst wall is composed of a single layer of ciliated (arrows) columnar epithelium and was diagnosed as a neurenteric cyst
Discussion
Neurenteric cysts are cystic masses lined by a mucin-producing, low columnar or cuboidal epithelium, are of endodermal origin, and develop during or shortly after the third week of embryogenesis, the period of notochordal development (1). They are known to occur in the posterior mediastinum and abdomen. Occurrence adjacent to the CNS is rare but is predominantly in the cervical and thoracic spinal canal, located intradurally and extramedullarly. It is uncommon to find enterogenous cysts located intracranially (2).
Neurenteric cysts are isointense to hyperintense relative to CSF on T2-weighted MR images (1, 3). On T1-weighted MR images, most neurenteric cysts are isointense or slightly hyperintense relative to CSF (1, 3). Occasionally, homogeneous, very bright signal intensity on T1-weighted MR images and/or very low intensity on T2-weighted MR images may be seen (1, 2, 4). These signal characteristics are thought to correlate with protein content or hemorrhage within the cysts (1, 2, 4).
There are several reports concerning the relationship between cyst protein concentration and signal intensity on T1-weighted MR images (5, 6). Hayashi et al (6) examined the relationship between signal intensity relative to white matter and protein concentration in plastic tubes. They found low intensity on T1-weighted MR images and high intensity on T2-weighted MR images at concentrations of ≤10 g/dL, high intensity on both T1- and T2-weighted MR images at concentrations between 10 and 17 g/dL, and high intensity on T1-weighted MR images and low intensity on T2-weighted MR images at concentrations of ≥17 g/dL. In the text of their article, they did not describe how much concentration of protein showed isointensity on T1-weighted MR images. In their figure, however, a plastic tube at the concentration of 10 g/dL showed isointensity on the T1-weighted MR image and hyperintensity on the T2-weighted MR image. They also examined the contents of Rathke cleft cysts that were hyperintense on T1-weighted MR images and observed that all five cysts had protein concentrations >11 g/dL. Ahmadi et al (5) reported that the cyst of a craniopharyngioma with a protein level of 12.4 g/dL had high intensity on T1-weighted MR images, a cyst with a protein level of 9.0 g/dL had isointensity with brain, and a cyst with a protein level of 4.1 to 6.1 g/dL showed low intensity. In our case, the protein level was 10.1 g/dL and the cyst had isointensity on T1-weighted MR images and high intensity on T2-weighted MR images, in accordance with both of these reports. The high intensity on T1-weighted MR images and lower intensity on T2-weighted MR images from the initial MR study were thus probably not caused by cholesterol content but by high concentrations of protein in cyst fluid. In an in vitro study, cholesterol levels did not affect MR signals, at least ≤3000 mg/dL (6); this suggests that the cholesterol level of 196 mg/dL in our case must have had no effect on MR signal.
There is no good explanation concerning the cause of the expansion and the MR signal change of this cyst. These changes might have been due to the breakdown of balance between epithelial secretion and absorption. Another possible explanation is dilution of the cyst contents by CSF. Thinning of the cyst wall and an osmotic response to cyst content may cause inward permeation of CSF, resulting in dilution of cyst content. There is, however, no evidence to support these explanations from the surgical findings or histologic examinations of the cyst wall. Recent hemorrhage may have caused this expansion and signal change on the MR images, but there was no evidence of previous hemorrhage at surgery.
There is only one reported case in which follow-up MR imaging revealed expansion of an intracranial neurenteric cyst, the signal intensity of which changed from slightly low to lower intensity on T1-weighted MR images obtained 4 years after the initial MR images (7). In our case, the intracranial neurenteric cyst both increased in size and changed in signal intensity from high to isointense on T1-weighted MR images.
The differential diagnoses of cystic masses in the basal cistern include arachnoid cyst and epidermoid cyst. Arachnoid cyst has signal intensity almost identical to that of CSF. The high intensity on T1-weighted MR images made the diagnosis of an arachnoid cyst unlikely in this case. Epidermoid cyst is another possibility. Epidermoid cysts are epithelial inclusion cysts that commonly develop in the cerebellopontine cistern and parasellar region intracranially. Epidermoid cysts usually have low signal on T1-weighted MR images and high signal on T2-weighted MR images, although high signal epidermoid cysts on T1-weighted MR images have been reported (8). On diffusion-weighted images, however, epidermoid cysts show iso- or hyperintensity compared with brain and can be distinguished from other cysts (9). The low signal intensity of this neurenteric cyst on diffusion-weighted images may enable differentiation from epidermoid cysts even if these cysts exhibit similar intensities on conventional MR images.
Acknowledgments
We thank Keiko Nakayama, MD, Hideo Daikokuya, MD, Akira Hagiwara, MD, and Kinuko Kono, MD, for acquiring MR studies and reviewing the manuscript.
Footnotes
Address reprint requests to Miyuki Shakudo, MD, Department of Radiology, Osaka City General Hospital, 2–13–22, Miyakojimahondori, Miyakojima-ku, Osaka 534–0021, Japan.
References
- 1.Brooks BS, Duvall ER, el Gamma T, Garcia JH, Gupta KL, Kapila A. Neuroimaging features of neurenteric cysts: analysis of nine cases and review of the literature. AJNR Am J Neuroradiol 1993;14:735-746 [PMC free article] [PubMed] [Google Scholar]
- 2.Kak VK, Gupta RK, Sharma BS, Banerjee AK. Craniospinal enterogenous cyst: MR findings. J Comput Assist Tomogr 1990;14:470-472 [DOI] [PubMed] [Google Scholar]
- 3.Pierot L, Dormont D, Queslati S, Cornu P, Rivierez M, Bories J. Gadolinium-DTPA enhanced MR imaging of intradural neurenteric cysts. J Comput Assist Tomogr 1988;12:762-764 [DOI] [PubMed] [Google Scholar]
- 4.Bejjani GK, Wright DC, Schessel D, Sekhar LN. Endodermal cysts of the posterior fossa: report of three cases and review of the literature. J Neurosurg 1998;89:326-335 [DOI] [PubMed] [Google Scholar]
- 5.Ahmadi J, Destian S, Apuzzo ALJ, Segall HD, Zee CS. Cystic fluid in craniopharyngiomas: MR imaging and quantitative analysis. Radiology 1992;182:783-785 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi Y, Tachibana O, Muramatsu N, et al. Rathke cleft cyst: MR and biomedical analysis of cyst content. J Comput Assist Tomogr 1999;23:34-38 [DOI] [PubMed] [Google Scholar]
- 7.Fuse T, Yamada K, Kamiya K, Inagaki H. Neurenteric cyst at the craniovertebral junction: report of two cases. Surg Neurol 1998;50:431-436 [DOI] [PubMed] [Google Scholar]
- 8.Ochi M, Hayashi K, Hayashi T, et al. Unusual CT and MR appearance of an epidermoid tumor of the cerebellopontine angle. AJNR Am J Neuroradiol 1998;19:1113-1115 [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuruda JS, Chew WM, Moseley ME, Norman D. Diffusion-weighted MR imaging of the brain: value of differentiating between extraaxial cysts and epidermoid tumors. AJR Am J Roentgenol 1990;155:1059-1065 [DOI] [PubMed] [Google Scholar]