Abstract
BACKGROUND AND PURPOSE: Mild traumatic brain injury (mTBI) (Glasgow Coma Scale = 14–15) is a common neurologic disorder and a common cause of neurocognitive deficits in the young population. Most patients recover fully from mTBI, but 15% to 29% of patients have persistent neurocognitive problems. Although a partially organic origin is considered likely, little brain imaging evidence exists for this assumption. The aims of the present study were to establish the prevalence of posttraumatic lesions in mTBI patients on MR images and to assess the relation between these imaging findings and posttraumatic symptoms. Secondly, we explored the value of early posttraumatic single-photon emission CT (SPECT) for the evaluation of mTBI.
METHODS: Twenty-one consecutive patients were included in the study. Patients underwent MR examination, technetium-99m hexamethylpropylene amine oxime SPECT, and neurocognitive assessment within 5 days after injury. Neurocognitive follow-up was conducted 2 and 6 months after injury, and MR imaging was repeated after 6 months. Lesion size and brain atrophy were measured on the MR studies.
RESULTS: Twelve (57%) of 21 patients had abnormal MR findings, and 11 (61%) of 18 had abnormal SPECT findings. Patients with abnormal MR or SPECT findings had brain atrophy at follow-up. The mean neurocognitive performance of all subjects was within normal range. There was no difference in neurocognitive performance between patients with normal and abnormal MR findings. Patients with abnormal MR findings only showed significantly slower reaction times during a reaction-time task. Seven patients had persistent neurocognitive complaints and one patient met the criteria for a postconcussional syndrome.
CONCLUSION: Brain lesions are common after mTBI; up to 77% of patients may have abnormal findings either on MR images or SPECT scans, and these lesions may lead to brain atrophy. The association between hypoperfusion seen on acute SPECT and brain atrophy after 6 months suggests the possibility of (secondary) ischemic brain damage. There is only a weak correlation between neuroimaging findings and neurocognitive outcome.
Traumatic brain injury is the neurologic disorder with the highest incidence in the young population and the most common cause of cognitive impairment in this group. The Head Injury Task Force of the National Institute of Neurologic Disorders has estimated that there are 2,000,000 cases of head injury in the United States annually, of which approximately 80% sustain a mild traumatic brain injury (mTBI) (1). Many patients complain of headache, dizziness, memory impairment, and concentration problems after mTBI, but in most patients these initial symptoms subside within a few weeks. Some patients, however, continue to report a variety of symptoms, such as headaches, dizziness, memory and concentration problems, and irritability, long after they sustained an mTBI. It has become clear that mTBI can cause long-lasting sequelae, and after 6 months, 15% to 29% of patients still have appreciable complaints (2). Although the etiology of these postconcussional symptoms is still controversial, the persistent complaints are considered a syndrome and, as such, the postconcussional syndrome (PCS) is under study for classification in DSM-IV (3). The annual incidence of patients with persistent complaints after mTBI is at least 240,000 in the United States, and is estimated at 7000 in the Netherlands. Apart from PCS, there is also evidence for cognitive impairment in healthy individuals who sustained mTBI a long time ago (4). Mild TBI may also have a synergistic effect on the increased risk of Alzheimer disease in patients with the apo E4 genotype (5). A recent animal study also revealed long-term effects of TBI, showing progressive tissue loss over a 1-year period (6).
Detecting patients at risk of developing PCS is of potential interest, because neurobehavioral rehabilitation reduces the risk of persistent symptoms, and treatment failure is common if symptoms persist after 3 to 6 months (7). It is not possible to detect patients at risk of developing PCS on the basis of clinical presentation (8, 9). Although an at least partially organic origin of postconcussional disorder is no longer a matter of debate (10, 11), a biological marker is still missing, and little imaging evidence exists for this assumption. Previous brain imaging studies with CT have focused on moderate-to-severe brain injury. Because of the limited sensitivity of CT, this technique is less suitable for studying mTBI. In the acute phase of white matter injury, for example, CT has a sensitivity of only 20% (12). Several studies have shown MR imaging to be more sensitive than CT (12–15), especially in the detection of nonhemorrhagic contusion and diffuse axonal injury, lesions commonly found in mTBI. Little is known about the relationship between morphologic damage and the outcome of patients after mTBI. Levin et al (16) published a longitudinal study on patients who had sustained a mild-to-moderate head injury and who were evaluated by neuroimaging and neurocognitive follow-up. They found heterogeneity in the relation between MR findings and neurocognitive test results. Since then, new sequences have been introduced in MR imaging, such as fluid-attenuated inversion recovery (FLAIR) (17), and fast field echo (FFE) T2-weighted imaging (15). These new techniques will further enhance the sensitivity of MR imaging for the detection of posttraumatic changes.
MR imaging provides information on structural cerebral damage, whereas techniques such as single-photon emission CT (SPECT) and positron emission tomography may provide insight into functional effects of brain injury. These techniques can identify abnormalities of cerebral perfusion, and therefore detect lesions not seen on MR images. Subacute and late SPECT brain imaging has been found to reveal more abnormal findings than CT and MR in mTBI patients (18–20), and Jacobs et al (21) found a correlation between postconcussional symptoms in mTBI patients and abnormal SPECT findings for up to 1 year after trauma. However, to date no data are available on the value of early SPECT imaging in mTBI patients. The aims of the present study were to establish the prevalence of posttraumatic lesions in mTBI patients on MR images and to assess the relationship between these imaging findings and posttraumatic symptoms. In addition, the value of early posttraumatic brain SPECT in the assessment of mTBI was explored.
Methods
Our study included consecutive patients who were under the age of 50 years and who presented at our emergency department with uncomplicated mTBI. Patients had to meet the following criteria: closed head injury, Glasgow Coma Scale (GCS) score of 14 or 15 (22), loss of consciousness for less than 20 minutes, and posttraumatic amnesia for less than 6 hours. Patients who underwent anesthesia or who had sustained significant extracranial injury were excluded. Patients with previous head injuries, alcohol or other substance abuse history, and patients with major psychiatric, neurologic, or medical problems were also excluded. To prevent interference from aspecific white matter lesions, patients with diabetes mellitus or hypertension were excluded as well. The protocol specified completion of a cerebral MR examination, technetium-99m hexamethylpropylene amine oxime (Tc99m-HMPAO) brain SPECT and neurocognitive assessment within 5 days, but not earlier than 2 days after the trauma. All studies were performed on the same day, always beginning with the neurocognitive examination. Neurocognitive follow-up times were at 2 and 6 months after injury, and MR imaging was repeated after 6 months. The local medical ethics committee approved the study, and all patients gave their informed consent.
Image Acquisition
MR images were acquired on a Philips ACS system operating at 1.5 T (Philips Medical Systems, Eindhoven, The Netherlands). A scout sequence was used to align the subsequent scans. The MR examination protocol consisted of three pulse sequences: axial dual T2-weighted fast spin-echo (3000/23–120/2 [TR/TE/excitations]; echo train length, 12; matrix, 225 × 186) with 24 5-mm-thick slices and a 10% interslice gap; axial T2-weighted fluid-attenuated inversion recovery (FLAIR) (6000/150/4; echo train length, 22; matrix, 231 × 256) with 24 5-mm-thick slices and a 20% interslice gap; and, to detect hemosiderin deposits, axial T2*-weighted gradient-echo sequences (750/40/2; flip angle, 10°; matrix, 256 × 204) with 25 5-mm-thick slices and a 10% interslice gap.
SPECT acquisition and quantification was performed as described by Matsuda et al (23). The patient was left for 15 minutes in a quiet, half-dark room, and after the pulse rate had dropped below 100 beats per minute, 740MBq of Tc99m-HMPAO (Ceretec, Amersham, U.K.) was IV administered. On a single-head camera (Siemens Gammasonics, Hoffman Estates, IL), a 128 × 128 matrix was used to image the patient's head and heart, and 110 images of 1 second's duration were obtained. These images were used to calculate the brain perfusion index according to Matsuda et al (23). Fifteen minutes later, brain SPECT was performed with a triple-head gamma camera (Siemens Gammasonics) (matrix size, 128 × 128; high-resolution collimators, 3 × 30 views). Images were reconstructed using a Butterworth filter of the order of 5 or 6, and applied, using an attenuation coefficient of 0.12/cm. Transverse images of the brain were reconstructed parallel to the orbitomeatal line. Applying the brain perfusion index allowed hemispheric blood flow and regional CBF to be calculated with a program based on the method described by Matsuda et al (23). The Siemens brain quantification program was used to determine mean blood flow. A side-to-side difference between regions of more than 10% was taken as a significant sign of reduced or luxury blood flow attributable to trauma. Two neuroradiologists independently read MR images, and a neuroradiologist and nuclear physician read the SPECT studies.
Image Postprocessing
The axial T2-weighted fast spin-echo images were processed using BrainImage (24) on a Macintosh G3 computer (Apple Computer, Cupertino, CA). The raw data were imported and corrected for signal inhomogeneity. Subsequently, the extracranial tissue and the calvaria were removed from the images by using processing tools available in BrainImage. The inner table of the skull was used as the landmark for the separation. The resulting image contained only brain parenchyma and CSF, and all pixels were assigned to one of these two categories. Subregions of interest included the lateral ventricles, including the temporal horn. Because the initial segmentation step had already identified the interface between CSF and brain parenchyma, precise tracing of the ventricular system was not necessary as long as no other CSF-containing structures were included in the region of interest. Care was taken to analyze comparable slices, because subjects were scanned in an interval of 6 months. Fifteen consecutive slices were analyzed from the floor of the anterior skull base to the vertex. The total brain volume was determined by summing all brain pixels and multiplying that by the voxel dimensions. The same procedure was used to determine the ventricular volume. The ventricle-to-brain ratio (VBR) was calculated, and the ratio measured on the initial scan was divided by the ratio from the scan performed at 6 months (tVBR). In five patients, tVBR could not be determined; three patients were lost to follow-up, and in two patients, the raw data of the axial T2-weighted images were unavailable. A decrease in tVBR is a measure of brain atrophy. The intraobserver variation for VBR determined on a subset of four scans was 0.99.
The volume of parenchymal lesions was assessed on the T2-weighted FLAIR and T2*-weighted FFE images. A local threshold algorithm of BrainImage was used to segment the lesions. High-signal lesions were measured on the T2-weighted FLAIR images, whereas low-signal lesions, containing hemosiderin, were measured on the T2*-weighted FFE images. The lesion volumes were determined using a method analogous to that used for the brain volumes.
Neurocognitive Testing
The choice of neurocognitive tests was based upon earlier studies in patients with mTBI (4, 25, 26). The following tests were used.
The Visual Verbal Learning Test (VVLT)
This memory test is a visual version of the Rey Auditory Verbal Learning Test (27). In three consecutive trials, a list of 15 words has to be memorized and reproduced. The VVLT also involves a delayed recall after 20 minutes, thus enabling measurement of memory retrieval. The dependent variables are the total number of words recalled over the three trials (VVLTtot), and the recall performance after 20 minutes (VVLTrec).
Stroop Color Word Test (SCWT) (27)
The SCWT has often been used to test selective attention, mental speed, and interference susceptibility (28). The test uses three cards displaying forty stimuli each: color names (SCWT I), color patches (SCWT II), and color names printed in incongruously colored ink (SCWT III). The dependent variables are the times needed to read (SCWT I) or to name the color of the patches (SCWT II) or the printing ink (SCWT III).
Concept Shifting Test (CST)
This test is an adaptation of the Trail Making Test, which is a test of visual conceptual and visuomotor tracking and has been used to measure the ease of shifting between concepts in ongoing behavior (28). The CST consists of three subtasks. In each task, 16 small circles are grouped in a large circle. In the first part, the circles contain numbers (CSTA), in the second part letters (CSTB), and in the third part both numbers and letters (CSTC). The circles are in random order and in each subtask subjects are asked to cross out items in the correct ascending order. The depended variable is the time needed to complete the task.
Letter Digit Substitution Test (LDST)
This test is a modification of the procedurally identical Symbol-Digit-Modalities Test (27, 29). The subjects are supplied with a code at the top of a page, which links a digit to a letter. Subjects have limited time to fill in blanks that correspond to the correct codes. The coding test is used to measure the speed of processing of general information. The dependent variable is the total number of digits written correctly in 60 seconds.
Fluency (29)
This test can be regarded as a measure of the adequate, strategy-driven retrieval of information from semantic memory. Fluency is defined as the ability to produce as many words as possible in a given category, within a fixed time span. Subjects are asked to name as many animals as possible within 1 minute (dependent variable).
The Motor Choice Reaction Test (MCRT)
This test is a computer task in which reaction times are studied as a function of the complexity of the task requirements (30). Briefly, a button is lit, in response of which those or another button has to be pressed. The dependent variable is the median incompatible choice reaction time in milliseconds, a condition in which stimulus-response incompatibility was added in contrast to choice reaction time and simple reaction time conditions. This test was only performed at the last follow-up, after 6 months.
The results of the various tests were transformed to a standard z score (27, 31) by using reference values from the Maastricht Memory Study (32) and the Maastricht Aging Study (29). In order to prevent type I error, we combined the scores of the different tests into a single z score by using the following formula:
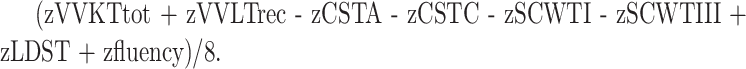 |
Combined z scores for the initial assessment, as well as for the follow-up at 2 and 6 months, were calculated.
At 2 and 6 months, patients filled out a 28-item questionnaire on posttraumatic symptoms (33). The results were reduced to a single score. The outcome has been found to correlate with persistent symptoms after mTBI (33)
Results
Twenty-one subjects were included in the study (nine female, 12 male). The mean age was 22.8 years (SD, 7.65; range, 15–42 years). The mean education level was 5 on a scale from 1 (only primary school) to 8 (university education), and the mean number of educational years was 13.5 (range, 9–23 years). The mean GCS score at presentation was 14.48 (SD, 0.6; range, 14–15) with a mean duration of unconsciousness of 4 minutes (SD 4.40; range, 0–15 minutes), and a posttraumatic amnesia period of 67 minutes (SD, 83.76; range, 0–300 minutes) (Table 1). The accidents were traffic-related in 16 cases and sports-related in four. One patient had fallen down the stairs.
TABLE 1:
Demographic data, trauma characteristics, imaging, and neurocognitive results of the mTBI patients

Twenty-one patients underwent initial MR examination and 18 patients underwent HMPAO SPECT. No patient required neurosurgical intervention. One subject only had a follow-up examination at 6 months, whereas three subjects were lost to follow-up.
Acute Imaging
Eleven patients (61%) had abnormal SPECT findings and 12 (57%) had abnormal MR findings. Four patients had normal MR and SPECT results, and both examinations were abnormal in seven patients (Fig 1–3). The SPECT study alone was abnormal in an additional four patients. In these patients, the SPECT images showed hypoperfusion in the frontal and parietal lobes and also in the thalamus in one subject. MR imaging revealed three additional abnormal subjects. In two of these patients, MR imaging showed subtle abnormalities on either the T2*-weighted or FLAIR images. The third patient had multiple posttraumatic lesions. The agreement between MR and SPECT studies was, on the whole, poor. The kappa value was 0.20. To assess the correlation between MR imaging and SPECT with respect to lesion location, all lesions were assigned to one of four brain quadrants: frontobasal; high frontal; temporal; or parietal. Lesions in other locations were disregarded. If at least one lesion in a quadrant was seen, both on MR and SPECT studies, it was rated as an agreement. The kappa values of the four quadrants were 0.35 for the frontobasal region, 0.44 for the high frontal region, 0.42 for the temporal region, and 0.22 for the parietal region. The mean kappa for the four regions was 0.36.
fig 1.
A and B, FLAIR images of a 36-year-old man who fell from a bicycle. Contusions are seen in the left temporal lobe as well as in the left frontal lobe. Note also the right frontal extracerebral hemorrhage
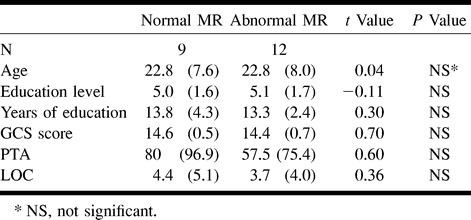
The FLAIR images revealed more and larger lesions than did the T2*-weighted images. In some instances, small hemorrhagic lesions located in the white matter (diffuse axonal injuries) were seen only on the T2*-weighted images. The mean lesion volume for patients with abnormalities was 3.61 ml on the FLAIR images (SD, 5.9 ml; range, 0.00–18.46 ml) and 1.72 ml on the T2*-weighted images (SD, 2.93 ml; range 0.00–9.44 ml). This difference in lesion volume was statistically significant (paired t test, P = .05). Two patients had extracerebral hemorrhage. Most lesions were found in the frontal lobe (42 lesions) and temporal lobe (16 lesions). One cerebellar lesion was found, but there were no brain stem lesions. Patients with abnormal MR findings did not differ from patients with normal MR findings with regard to age, education, and trauma severity parameters (Table 2).
TABLE 2:
Independent sample t test on demographic variables and injury severity characteristics for patients with normal and abnormal MR findings
No new lesions were detected at follow-up. There was a statistically significant reduction in lesion volume on both the FLAIR images (mean, 0.52 ml; SD, 0.995 ml; P = .02) and the FFE images (mean, 0.50 ml; SD, 0.84 ml; P = .04). Patients with abnormal imaging studies showed brain atrophy at follow-up, as expressed by a decreased tVBR. tVBR was significantly lower in patients with abnormal MR findings than in those with a normal MR examination (normal, 1.04 [SD, 0.03]); abnormal, 0.94 [SD, 0.08]; Student's t test, P = .02). Division of the patients into two groups based on SPECT findings also yielded the same difference in tVBR (normal, 1.04 [SD 0.02]; abnormal, 0.95 [SD 0.09]; Student's t test, P = .03).
Neurocognitive Examinations
A 22-year-old woman was excluded from the analysis of the neurocognitive data because of a high probability of motivationally impaired performance.
The mean z score for neurocognitive performance was −0.21 (SD 0.75) at the initial assessment, 0.14 (SD 0.60) after 2 months, and 0.45 (SD (0.52) after 6 months. This improvement was statistically significant for both intervals (MANOVA for repeated measurements F [2, 30] = 12.711, P < .001) (Fig 4). Patients with abnormal MR findings had a lower z score at initial assessment and after 2 months (Fig 4, Table 3). However, these differences were not statistically significant. Measures of cerebral damage (lesion volume and tVBR) did not correlate with the z score of neurocognitive performance.
fig 4.
Combined z scores of the patients with normal (○) and abnormal (•) MR findings over the 6-month follow-up period
TABLE 3:
Mean z score and SD (between brackets) of the neu~rocognitive examination (NCE) of patients with normal and ab~normal MR findings

The reaction time task (MCRT) after 6 months showed a difference between patients with abnormal and those with normal MR findings. Patients with abnormal MR findings had a significantly slower reaction time on the incompatible reaction time task of MCRT (t test, t [14] = −3.11, P = .008).
There was no statistically significant difference between the number of symptoms scored on the questionnaire by patients with abnormal and normal MR findings (Table 4).
TABLE 4:
Mean number of posttraumatic complaints, as rated on a 28-item questionnaire

Six months after injury, seven patients still had subjective cognitive complaints. Complaints of forgetfulness (39%) and difficulty in concentrating (33%) were the most common, followed by mild problems with word finding (28%) and mental slowness (22%). One patient complained about difficulty in mental planning. One of the patients fulfilled the DSM-IV criteria of a PCS, and this patient had both abnormal SPECT and MR findings. There was no statistically significant association between SPECT findings and neurocognitive data.
Discussion
The present prospective study examined the prevalence of posttraumatic lesions in mTBI patients by use of MR imaging and HMPAO SPECT, and the relation between imaging findings and posttraumatic symptoms. This study is the largest reported prospective study of patients with mTBI to use both neurocognitive investigations and neuroimaging. The most important finding in our series of mTBI patients was the high prevalence of brain lesions: 77% of the mTBI patients had either an abnormal MR or an abnormal SPECT study. A recent metaanalysis has established the prevalence of hemorrhagic lesions in the mTBI patients to be approximately 8% (34). This estimation was based on CT findings. The prevalence of posttraumatic lesions may be somewhat high, because not all lesions are hemorrhagic. Nonetheless, our findings show the relatively high sensitivity of MR and SPECT for posttraumatic lesions. Patients with lesions revealed by initial MR had brain atrophy at 6 months, as illustrated by the decrease in tVBR. This has also been shown in patients who sustained a moderate-to-severe head injury (35), but has not been reported previously in mTBI patients. These two findings illustrate that mTBI is accompanied by organic brain damage in a large percentage of patients. In the current study, no pathologic-anatomic confirmation is available for the MR lesions. However, on the basis of the location and MR characteristics, lesions can be attributed to trauma. Small extraaxial hematoma, nonhemorrhagic contusions, and hemorrhagic contusions were found. We also identified lesion characteristics for diffuse axonal injury (15, 36).
During the chronic phase, SPECT is considered more sensitive than CT or MR imaging (18–20). However, in our series, three patients had normal SPECT findings, whereas MR imaging revealed posttraumatic lesions. SPECT, on the other hand, showed posttraumatic alterations in four patients with normal MR findings. Furthermore, the correlation between brain atrophy and the initial SPECT findings showed a lower P value than the correlation with the initial MR finding. Therefore, in this study, HMPAO SPECT was not more sensitive to posttraumatic changes than MR but it was sensitive to different types of change. This was corroborated by the low anatomic correlation between SPECT and MR imaging.
The majority of patients with abnormal SPECT findings showed areas of hypoperfusion. This finding has been described previously (37, 38). This hypoperfusion may result from vasospasm, direct vascular injury, and perfusion changes due to alterations in remote neuronal activity (diaschisis). Therefore, the characterization of an area of asymmetrical activity as a lesion on a brain SPECT scan requires more fundamental knowledge of the relationship between pathologic abnormalities of the brain and SPECT. However, the cerebral autoregulation is diminished in some patients after mTBI (39), and animal studies have shown that the brain is more vulnerable to ischemic injury after mTBI (40). It is, therefore, tempting to hypothesize that the SPECT findings represent hypoperfusion, which may lead to secondary ischemic injury. This is a well-known phenomenon in severe brain injury. This hypothesis is supported by the association of early SPECT abnormalities and brain atrophy after 6 months. The current study does not provide evidence for this hypothesis, but if it could be proven, it would open a therapeutic window for mTBI patients. Considering the long-term effects of mTBI, this would be of great impact.
The heterogeneity of the head-injured population, and the limited number of patients, makes it difficult to demonstrate a relation between lesions and specific neurocognitive deficits. It is more feasible to demonstrate a correlation between measures that represent the overall severity of brain damage and general neurocognitive function (41). We, therefore, combined the results of neurocognitive tests into a single z score. In the acute phase and after 2 months, patients with abnormal MR findings performed less well on neurocognitive testing. After 6 months, these patients also performed less well on a reaction time task. That only the MCRT shows a significant difference between patients with a normal and abnormal MR study may be due to the fact the MCRT was the only task that required a response to externally presented paced stimuli. These types of tasks have been shown to be more closely related to overall neuropsychological impairment after traumatic brain injury than self-paced tasks (42). These findings suggest an association between cerebral damage and neurocognitive performance. On the other hand, patients with normal MR findings tended to have more subjective symptoms. A larger prospective study is needed to elucidate this issue.
We did not find a clear recovery of patients regarding neurocognitive measures as well as imaging findings. Although lesions were located predominately in the frontal and temporal lobes, the size and precise location differed considerably among patients. This heterogeneity is most probably due to differences in trauma mechanisms. The neurocognitive data also showed a heterogeneous image; different cognitive domains were more or less severely affected. It is likely that there is an association between the site of the lesions and the neurocognitive deficit. Levin et al (16) showed an association between lesion location and performance on memory and planning tasks in a group of head-injured patients. The majority of patients in that series, however, had sustained moderate head injury. The heterogeneity of both organic lesions and neurocognitive deficits may explain why an association between these was not demonstrated. Another causative factor may be the age of the patients.
At a mean age of 23 years, patients may have the cognitive flexibility to compensate for small deficits. However, it has been suggested that biological life events, such as mTBI, could aggravate the effect of “normal” cognitive aging (4, 30). Considering the high prevalence of posttraumatic abnormalities, these patients could be at risk of earlier or more rapid cognitive decline when the normal biological aging process becomes evident. Furthermore, psychological and socioeconomic factors may also influence recovery after mTBI.
Seven patients presented with persistent cognitive complaints, and one of these patients had PCS according to DSM-IV criteria (3). This prevalence of 5% is low relative to previously reported figures, which may be due to the age of the subjects as well. In a recent prospective study of patients with mTBI, 25% had PCS at 6 months. In their control group of non-head-injured patients, however, 34% of subjects also presented with complaints that met the PCS criteria (43). Another study also found PCS-like complaints in 11% of patients not diagnosed with head injury (44).
The value of acute MR imaging in the management of head injury has not been determined. Previously, MR imaging was considered insensitive to early hemorrhage, which is the primary indication for neurosurgical intervention in head trauma patients. However, new techniques have also made MR imaging sensitive to acute hemorrhage. To date, however, CT is the technique of choice for evaluating head trauma; it is more economical than MR, it is easily accessible in most hospitals, and is simple to perform in agitated patients. Our data do not support the routine application of MR imaging in the management of mTBI, because no clear prognostic value could be demonstrated. However, the correlation between MR findings and neurocognitive performance does justify further study. New MR techniques, such as magnetization transfer imaging (45–47), diffusion-weighted imaging (48, 49), and MR spectroscopy (50, 51) may increase the sensitivity of MR imaging for traumatic lesions and increase correlation to neurocognitive deficits and, eventually, to long-term outcome.
Conclusion
The majority in a series of consecutive patients with mTBI showed abnormalities on neuroimages, and patients with abnormal neuroimaging findings had mild brain atrophy after 6 months. The correlation between MR and SPECT results was poor, and more fundamental knowledge is needed to interpret brain perfusion SPECT studies. The mean neurocognitive performance of all subjects was within the normal range, and there was no difference in neurocognitive performance between patients with normal and abnormal MR findings. Although our data suggest an association between MR findings and neurocognitive performance, no significant association could be demonstrated. Other factors, such as the location of the lesions, psychological factors, and educational level, might determine the outcome of mTBI at least as much as brain lesions.
Further research is needed to establish the relation between the apparent cerebral damage and its neurocognitive consequences, but a wider application of MR imaging and SPECT in patients with mTBI seems justifiable, considering the sensitivity of these techniques to posttraumatic brain lesions.
fig 2.
Images obtained from a 23-year-old man who was involved in a car accident.
A, SPECT image shows a left frontal perfusion deficit.
B, FLAIR image shows no lesions at this location.
C, Owing to diffuse axonal injury, T2*-weighted image shows two deep hemorrhagic lesions.
fig 3.
Images obtained from a 37-year-old bicyclist.
A, SPECT image shows a right frontal perfusion deficit.
B, Corresponding FLAIR image shows a hemorrhagic contusion.
Footnotes
Address reprint requests to Paul A.M. Hofman, Department of Radiology, University Hospital Maastricht, P.O. Box 5800, 6202 AZ Maastricht, The Netherlands.
References
- 1.Kraus J, Nourjah P. The epidemiology of mild head injury. In: Levin H, Eisenberg H, Benton A, eds. Mild Head Injury Oxford: Oxford University Press 1989;8-22
- 2.Bohnen N, Twijnstra A, Jolles J. Persistence of postconcussional symptoms in uncomplicated, mildly head-injured patients: a prospective cohort study. Neuropsychiatry Neuropsychol Behav Neurol 1993;6:193-200 [Google Scholar]
- 3.Frances CA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington: American Psychiatric Association 1994;
- 4.Klein M, Houx P, Jolles J. Long-term persisting cognitive sequelae of traumatic brain injury and the effect of age. J Nerv Ment Dis 1996;184:459-467 [DOI] [PubMed] [Google Scholar]
- 5.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-e4 in patients with Alzheimer's disease. Neurology 1995;45:555-557 [DOI] [PubMed] [Google Scholar]
- 6.Smith DH, Meaney DF, Lenkinski RE, et al. New magnetic resonance imaging techniques for the evaluation of traumatic brain injury. J Neurotrauma 1995;12:573-577 [DOI] [PubMed] [Google Scholar]
- 7.Mittenberg W, Tremont G, Zielinski RE, Fichera S, Rayls KR. Cognitive-behavioral prevention of postconcussion syndrome. Arch Clin Neuropsychol 1996;11:139-145 [PubMed] [Google Scholar]
- 8.Levin HS, Amparo E, Eisenberg HM, et al. Magnetic resonance imaging and computerized tomography in relation to neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg 1987;66:706-713 [DOI] [PubMed] [Google Scholar]
- 9.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery 1990;27:422-428 [DOI] [PubMed] [Google Scholar]
- 10.Lishman WA. Physiogenesis and psychogenesis disorder in the ‘post-concussional syndrome.’. Br J Psychiatry 1988;153:460-469 [DOI] [PubMed] [Google Scholar]
- 11.Brown SJ, Fann JR, Grant I. Postconcussional disorder: time to acknowledge a common source of neurobehavioral morbidity. J Neuropsychiatry Clin Neurosci 1994;6:15-22 [DOI] [PubMed] [Google Scholar]
- 12.Orrison WW, Gentry LR, Stimac GK, Tarrel RM, Espinosa MC, Cobb LC. Blinded comparison of cranial CT and MR in closed head injury evaluation. AJNR Am J Neuroradiol 1994;15:351-356 [PMC free article] [PubMed] [Google Scholar]
- 13.Hesselink JR, Dowd CF, Healy ME, Hajek P, Baker LL, Luerssen TG. MR imaging of brain contusions: a comparative study with CT. AJR Am J Roentgenol 1988;150:1133-1142 [DOI] [PubMed] [Google Scholar]
- 14.Yokota H, Korokawa A, Otsuka T, Kobayashi S, Nakazawa S. Significance of magnetic resonance imaging in acute head injury. J Trauma 1991;31:351-357 [DOI] [PubMed] [Google Scholar]
- 15.Mittl RL, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal CT findings. AJNR Am J Neuroradiol 1993;15:1583-1589 [PMC free article] [PubMed] [Google Scholar]
- 16.Levin HS, Williams DH, Eisenberg HM, High WM, Guinto FC. Serial MRI and neurobehavioral findings after mild to moderate head injury. J Neurol Neurosurg Psychiatry 1992;55:255-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashikaga R, Araki Y, Ishida O. MRI of head injury using FLAIR. Neuroradiology 1997;39:239-242 [DOI] [PubMed] [Google Scholar]
- 18.Kant R, Smith-Seemiller L, Isaac G, Duffy J. Tc-HMPAO SPECT in persistent post-concussion syndrome after mild head injury: comparison with MRI/CT. Brain Inj 1997;11:115-124 [DOI] [PubMed] [Google Scholar]
- 19.Roper SN, Mena I, King WA, et al. An analysis of cerebral blood flow in acute closed-head injury using technetium-99m-HMPAO SPECT and computed tomography [published erratum appears in J Nucl Med 1991;11:2070]. J Nucl Med 1991;32:1684-1687 [PubMed] [Google Scholar]
- 20.Ichise M, Chung DG, Wang P, Wortzman G, Gray BG, Franks W. Technetium-99m-HMPAO SPECT in the evaluation of patients with chronic traumatic brain injury: a correlation with neuropsychological performance [see comments]. J Nucl Med 1994;35:217-226 [PubMed] [Google Scholar]
- 21.Jacobs A, Put E, Ingels M, Put T, Bossuyt A. One-year follow-up of technetium-99m-HMPAO SPECT in mild head injury. J Nucl Med 1996;37:1605-1609 [PubMed] [Google Scholar]
- 22.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-84 [DOI] [PubMed] [Google Scholar]
- 23.Matsuda H, Tsuji S, Shuke N, Sumiya H, Tonami N, Hisada K. A quantitative approach to technetium-99m hexamethylpropylene amine oxime. Eur J Nucl Med 1992;19:195-200 [DOI] [PubMed] [Google Scholar]
- 24.Reiss AL. BrainImage 3.1.. Stanford: Stanford University Psychiatry Neuroimaging Lab 1999;
- 25.Bohnen N, Jolles J. Neurobehavioral aspects of postconcussive symptoms after mild head injury. J Nerv Ment Dis 1992;180:683-692 [DOI] [PubMed] [Google Scholar]
- 26.Houx PJ, Vreeling FW, Jolles J. Age-associated cognitive decline is related to biological life events. In: Iqbal K, McLachlan DRC, Winblad B, Wisniewski HM, eds. Alzheimer's Disease: Basic Mechanisms, Diagnosis and Therapeutic Strategies Chichester, UK: John Wiley & Sons 1991;353-358
- 27.Lezak MD, Neuropsychological Assessment. 3rd ed. Oxford: Oxford University Press 1995;
- 28.Houx PJ, Jolles J. Vulnerability factors for age-related cognitive decline. In: Isaacson RL, Jensen KF, eds. The Vulnerable Brain and Environmental Risks. New York: Plenum Press 1994;25-41
- 29.Jolles J, Houx PJ, van Boxtel MPJ, Ponds RWHM. The Maastricht Aging Study. Determinants of Cognitive Aging.. Maastricht: Neuropsych Publishers 1995;
- 30.Houx PJ, Jolles J. Age-related decline of psychomotor speed: effects of age, brain, health, sex, and education. Percept Mot Skills 1993;76:195-211 [DOI] [PubMed] [Google Scholar]
- 31.van Boxtel MPJ, Langerak K, Houx PJ, Jolles J. Self-reported physical activity, subjective health, and cognitive performance in older adults. Exp Aging Res 1996;22:363-379 [DOI] [PubMed] [Google Scholar]
- 32.Houx PJ. Cognitive Aging and Health-related Factors. Thesis, Maastricht University, 1991;
- 33.Bohnen N, Jolles J, Twinjnstra A. Posttraumatic and emotional symptoms in different subgroups of patients with mild head injury. Brain Inj 1992;6:481-487 [DOI] [PubMed] [Google Scholar]
- 34.Hofman PAM, Nelemans P, Kemerink GJ, Wilmink JT. Value of radiological diagnosis of skull fracture in the management of mild head injury: a meta-analysis. J Neurol Neurosurg Psychiatry 2000;68:416-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blatter DD, Bigler ED, Gale SD, et al. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. AJNR Am J Neuroradiol 1997;18:1-10 [PMC free article] [PubMed] [Google Scholar]
- 36.Gean AD. Imaging of Head Trauma.. New York: Raven Press; 1994;
- 37.Masdeu JC, Heertum RLV, Kleiman A, et al. Early single-photon emission computed tomography in mild head trauma. J Neuroimaging 1994;4:177-181 [DOI] [PubMed] [Google Scholar]
- 38.Arvigo F, Cossu M, Fazio B, et al. Cerebral blood flow in minor cerebral contusion. Surg Neurol 1985;24:211-217 [DOI] [PubMed] [Google Scholar]
- 39.Junger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. J Neurosurg 1997;86:425-432 [DOI] [PubMed] [Google Scholar]
- 40.Jenkins LW, Lu Y, Johnston WE, Lyeth BG, Prough DS. Combined therapy affects outcomes differentially after mild traumatic brain injury and secondary forebrain ischemia in rats. Brain Res 1999;817:132-144 [DOI] [PubMed] [Google Scholar]
- 41.Wilson JTL. The relationship between neuropsychological function and brain damage detected by neuroimaging after closed head injury. Brain Inj 1990;4:349-363 [DOI] [PubMed] [Google Scholar]
- 42.Ponsford J, Kinsella G. Attentional deficits following closed-head injury. J Clin Exp Neuropsychol 1992;14:822-838 [DOI] [PubMed] [Google Scholar]
- 43.Bazarian JJ, Wong T, Harris M, Leahey N, Mookerjee S, Dombovy M. Epidemiology and predictors of postconcussive syndrome after minor head injury in an emergency population. Brain Inj 1999;13:173-189 [DOI] [PubMed] [Google Scholar]
- 44.Chambers J, Cohen SS, Hemminger L, Prall JA, Nichols JS. Mild traumatic brain injuries in low-risk trauma patients. J Trauma 1996;41:976-980 [DOI] [PubMed] [Google Scholar]
- 45.Hofman PAM, Kemerink G, Jolles J, Wilmink JT. Quantiative analysis of magnetization transfer images of the brain: the effect of closed head injury, age and sex on white matter. Magn Reson Med 1999;42:803-806 [DOI] [PubMed] [Google Scholar]
- 46.Kimura H, Meaney DF, McGowan JC, et al. Magnetization transfer imaging of diffuse axonal injury following experimental brain injury in the pig: characterization by magnetization transfer ratio with histopathologic correlation. J Comput Assist Tomogr 1996;20:540-546 [DOI] [PubMed] [Google Scholar]
- 47.McGowan JC, Yang JH, Plotkin RC, et al. Magnetization transfer imaging in the detection of injury associated with mild head trauma. AJNR Am J Neuroradiol 2000;21:875-880 [PMC free article] [PubMed] [Google Scholar]
- 48.Assaf Y, Holokovsky A, Berman E, Shapira Y, Shohami E, Cohen Y. Diffusion and perfusion magnetic resonance imaging following closed head injury in rats. J Neurotrauma 1999;16:1165-1176 [DOI] [PubMed] [Google Scholar]
- 49.Liu AY, Maldjian JA, Bagley LJ, Sinson GP, Grossman RI. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol 1999;20:1636-1641 [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman SD, Brooks WM, Jung RE, Hart BL, Yeo RA. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR Am J Neuroradiol 1998;19:1879-1885 [PMC free article] [PubMed] [Google Scholar]
- 51.Smith DH, Cecil KM, Meaney DF, et al. Magnetic resonance spectroscopy of diffuse brain trauma in the pig. J Neurotrauma 1998;15:665-674 [DOI] [PubMed] [Google Scholar]






