Abstract
BACKGROUND AND PURPOSE: Macrocrania is a common pediatric clinical condition affecting up to 5% of the population. The purpose of this study was to determine clinical and imaging predictors that are useful in the differentiation of disorders requiring surgical treatment from those that can be treated medically in children with macrocrania.
METHODS: In a 3-year 7-month retrospective study, 88 patients (median age, 8 months; interquartile range, 5−13 months) with macrocrania and no known underlying neurologic disorder underwent imaging of the brain (sonography, n = 36; CT, n = 31; MR imaging = 21). The study was conducted in a pediatric tertiary care referral center. Clinical and imaging data were correlated to final diagnosis by means of logistic regression and receiver operating characteristic curves.
RESULTS: Sixteen (18%) of the patients had disorders requiring surgery: communicating hydrocephalus, n = 7; noncommunicating hydrocephalus, n = 3; hemorrhagic subdural collections, n = 3; neoplasm, n = 1; encysted cavum septi pellucidi, n = 1; and vein of Galen malformation, n = 1. Clinical predictors of disorders requiring surgery included vomiting (P = .007), labor instrumentation (P = .026), developmental delay (P = .008), and abnormal neurologic findings (P = .028). Imaging predictors of disorders requiring surgery included a focal space-occupying lesion (P < .0001) and moderate-to-severe ventriculomegaly (P < .0001). The diagnostic sensitivity of the combination of independent clinical and imaging predictors was higher than that of independent clinical predictors alone, being 100% (95% confidence interval = 96.9%, 100%) and 93.8% (95% confidence interval = 88.7%, 98.8%), respectively. A trend indicated that the area under the receiver operating characteristic curve for clinical plus imaging findings (0.95) was greater than that for clinical findings alone (0.85) (P = .09). An increase in the number of clinical and imaging predictors was highly correlated with an increased risk of a disorder requiring surgery (P < .0001).
CONCLUSION: Baseline neuroimaging is indicated for children with macrocrania because the combination of clinical and imaging predictors has the best diagnostic performance in determining the need for surgical versus nonsurgical management.
Macrocrania is a common pediatric clinical condition affecting up to 5% of the population (1). Macrocrania, seizure, and headache disorders are the leading causes of pediatric neurologic consultation and neuroimaging. Idiopathic external hydrocephalus, or benign macrocrania, is a common cause of macrocrania in infants (2). By imaging, idiopathic external hydrocephalus is usually characterized by prominent subarachnoid fluid with minimal or no ventricular system dilation (2). Idiopathic external hydrocephalus is a benign self-limiting condition that usually resolves without treatment (3−6). Conversely, an important subgroup of children with macrocrania have hydrocephalus (ie, communicating or noncommunicating), extraaxial collections (ie, subdural effusions or hematomas), or focal space-occupying lesions (ie, neoplasms or vascular malformations) that may warrant surgical intervention (7).
Because macrocrania is a common clinical condition and because hydrocephalus, subdural collections, and other focal space-occupying lesions are important to diagnose and treat in a timely manner, reliable predictors that help differentiate surgical from nonsurgical disorders need to be determined. Appropriate use of clinical predictors and imaging may be important in the evaluation and management of children with macrocrania. Furthermore, appropriateness of imaging children with macrocrania has been questioned in the current cost-conscious health care environment. To our knowledge, no study has adequately addressed these issues thus far.
We performed a 3-year 7-month retrospective study of children with no known underlying CNS disorder who underwent imaging of the brain for presenting signs of macrocrania. The purpose of this study was to identify clinical and imaging predictors that help distinguish patients with lesions requiring surgical intervention from those with disorders that can safely be treated medically.
Methods
Study Cohort
The study was conducted between January 1, 1994, and July 31, 1997, at our institution, which is the primary pediatric tertiary care referral center in the region. The entry criteria for the study included children with a presenting sign of macrocrania and with no known previous CNS disorder or treatment who were referred to the neurosciences (neurosurgery and neurology) departments at our tertiary institution. Macrocrania was defined as a head circumference greater than the 95th percentile on the standard growth curve (1, 2). All patients in the study population underwent imaging of the brain with either sonography, CT, or MR imaging. The patients were identified using the Department of Radiology and Neurosciences databases.
Definition of Variables and Outcomes
The medical records were reviewed in detail for demographic data, complete history, and results of physical and neurologic examinations. The history was obtained and the physical examination was conducted for each patient by a neurosurgeon, a neurologist, or a neurologist-in-training with an attending neurologist or neurosurgeon. Failure to mention the presence of a clinical sign or symptom was interpreted as evidence that the patient did not have that feature. The final diagnosis was determined by a study physician (K.F., D.B.) from a combination of surgical, histopathologic, clinical, and/or imaging follow-up data. Two and a half years after the end of the study period, the medical records of all study patients were reviewed to determine changes in final diagnoses and possibility of late diagnoses of lesions requiring surgery. The study hospital has the only pediatric neurosurgical center in the referral area.
Definition of Important Variables
The relevant clinical and imaging variables used in this study were as follows: 1) developmental delay, defined as unfulfilled motor, social, adaptive, and/or language milestones for the patient's age and gender (8); 2) family history of macrocrania in a first degree (ie, parent or sibling) or second degree (ie, grandparent, uncle, aunt, or cousin) relative; 3) labor instrumentation, defined as vacuum extraction or forceps during labor or delivery; 4) accelerated head growth, defined as an accelerated increase over time of the patient's head circumference according to age and gender; 5) abnormal fontanel, defined as a bulging, tense, or full anterior fontanel; 6) focal space-occupying lesion, defined as a focal intracranial lesion, including neoplasm, vascular malformation, encysted cavum septi pellucidi et vergae, or subdural collection; and 7) degree of ventriculomegaly (transverse millimeters at lateral ventricle atrium ÷ transverse millimeters of ipsilateral hemisphere [medial-to-lateral margin] at same level × 100%), defined as no ventriculomegaly if less than 20%, mild if 20% to less than 30%, moderate if 30% to less than 40%, and severe if greater than or equal to 40%.
Definition of Important Clinical Outcomes
The important clinical outcomes that were assessed in this study were disorders requiring surgical treatment and idiopathic external hydrocephalus, or benign macrocrania. Disorders requiring surgical treatment were defined as intracranial lesions, including hydrocephalus, subdural collections, neoplasm, vascular malformation, and encysted cavum septi pellucidi, that necessitated surgical intervention as part of treatment. Idiopathic external hydrocephalus, or benign macrocrania, was defined as a self-limited external hydrocephalus that resolved without treatment and was closely related to benign familial macrocrania (2).
Imaging of the Brain
Cranial sonography was performed with an XP10-ART or Sequoia machine (Acuson, Mount View, CA) using a combination of a 5- to 7-MHz sector and a 7-MHz linear probe through the anterior fontanel. Serial images of the brain were acquired in the coronal and sagittal planes, including magnified coronal views of the interhemispheric tissue and medial convexities to evaluate for extraaxial collections.
CT was performed with the High Speed Advantage CT (GE Medical Systems, Milwaukee, WI). All CT scans were obtained with a 5- to 10-mm section thickness, 512 × 512 matrix, and 20- to 24-cm field of view. Contrast-enhanced CT was performed with 2 ml/kg IV administered iopamidol (Bracco Diagnostics, Princeton, NJ) or ioversol (Mallinckrodt Medical, St. Louis, MO).
MR imaging was performed with a 1.5-T Signa system (GE Medical Systems). The examination included at least the following three sequences: sagittal T1-weighted conventional spin-echo (500/16 [TR/TE]), axial proton density–weighted conventional spin-echo (2500/30), and axial T2-weighted conventional spin-echo (2500/100) images. In some patients, coronal T2-weighted fast spin-echo (5000/125) and enhanced T1-weighted conventional spin-echo images were obtained after the IV administration of 0.2 ml/kg gadopentetate dimeglumine (Berlex Laboratories, Wayne, NJ). For all sequences, 5-mm-thick sections were obtained with at least a 256 × 192 matrix, 18- to 24-cm field of view, and two signal acquisitions.
All MR images and CT scans were interpreted by an experienced pediatric neuroradiologist, and all cranial sonograms were interpreted by an experienced pediatric radiologist. The imaging studies were interpreted with knowledge of the presenting sign of macrocrania but not of the final diagnosis. The prospectively generated written report was used as the official interpretation. No retrospective reevaluation of the imaging or clinical (history and physical examination) interpretation was conducted by other physicians.
Analysis
All clinical data from the charts were reviewed. Twenty-five variables were consistently found. These clinical variables were studied with logistic regression analysis to compare patients with disorders requiring surgery with those who had medically managed disorders. Univariate analysis was conducted with the likelihood ratio test, distributed as a χ2 statistic (9). Stepwise multiple logistic regression was used to identify the significant independent predictors with regression coefficients estimated by maximum likelihood estimation (10). Variables with a P value <.20 in the univariate analysis were chosen as candidates for the multivariate model. Final selection of multivariate predictors was determined using the backward elimination method with criteria of P <.10 for entry into the model and P <.05 for removal (10). All models were examined with forward selection and backward elimination, with comparable results. Model fit was evaluated by means of the Hosmer-Lemeshow goodness-of-fit test (11). Statistical analysis was conducted using the SAS statistical package, version 6.12 (SAS Institute, Cary, NC). A Pearson χ2 test for linear trend was used to evaluate the proportion of surgical lesions on the basis of the number of independent multivariate predictors (12).
Receiver operating characteristic curve analysis was conducted because it provided a description of the disease detectability (disorders requiring surgical versus nonsurgical treatment) independent from both disease prevalence and decision threshold effects (13). The receiver operating characteristic curves were constructed to compare the diagnostic performance of the multivariate clinical predictors alone versus clinical and imaging predictors. GraphROC, version 2.0, was used to compare areas under the receiver operating characteristic curves based on the Wilcoxon statistic (14).
Results
During a 3-year 7-month period, 134 patients with macrocrania who underwent imaging of the brain were identified. Forty-six patients were excluded because of known previous CNS disorders or therapy. Therefore, 88 patients were enrolled in the study. Of these patients, 16 (18%) had surgically treated lesions and 72 (82%) had disorders that were nonsurgically treated.
Characteristics of the Study Population
The median age at time of presentation was 8 months (interquartile range, 5−13 months). There were 59 (67%) male and 29 (33%) female patients. All 88 patients had complete medical histories and had undergone physical examinations that included a detailed neurologic examination. All patients had at least a 3-month follow-up duration. Fifty-four (61%) of the patients had a 6-month follow-up duration. A review of the medical charts in February 2000 (2.5 years after the end of the study period) revealed that no patient had been referred to neurosurgery for delay diagnosis of a surgical disorder.
All patients had undergone initial imaging evaluation with either sonography, CT, or MR imaging. The type of imaging study was determined by the referring clinician.
Course of Patients Requiring Surgery
Sixteen (18%) of the study population had surgical lesions requiring either neurosurgical or neurointerventional procedures (Table 1). All patients who received surgical treatment underwent intervention within 1 week of the imaging study. These included 10 CSF shunting procedures, three subdural collections requiring drainage, and treatment of three focal space-occupying lesions.
TABLE 1:
Final diagnosis among the children with macrocrania
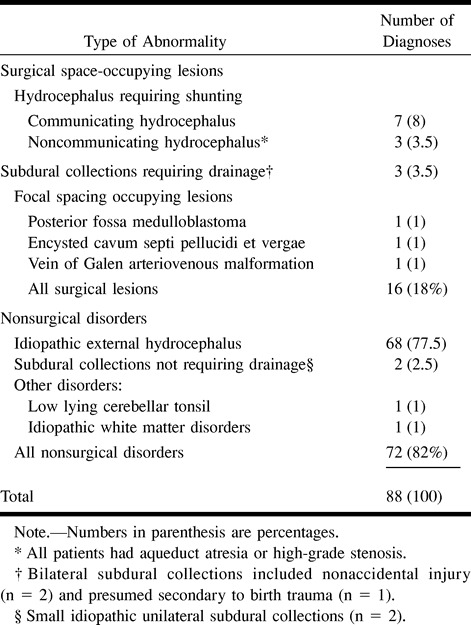
The 10 patients who underwent shunting had hydrocephalus. Seven of these patients had communicating hydrocephalus treated with ventriculoperitoneal shunting (n = 6) or lumboperitoneal shunting (n = 1). Three patients had noncommunicating hydrocephalus with obstruction at the sylvian aqueduct due to atresia or high-grade stenosis. The cases of noncommunicating hydrocephalus were treated with either ventriculoperitoneal shunting (n = 2) or third ventriculostomy (n = 1).
Of the three patients with subdural collections requiring drainage, two were because of child abuse and one was presumed to be secondary to birth trauma. Neurosurgical intervention included percutaneous evacuation of bloody effusions in two patients and open surgical drainage in one. Two patients had small idiopathic subdural collections with no mass effect revealed by the initial or follow-up studies. Both patients were treated conservatively, requiring no intervention.
The three focal surgical space-occupying lesions included posterior fossa medulloblastoma (n = 1), encysted cavum septi pellucidi et vergae (n = 1), and vein of Galen arteriovenous malformation (n = 1). The posterior fossa medulloblastoma with associated supratentorial hydrocephalus underwent surgical resection and then craniospinal radiation therapy. The encysted cavum septi pellucidi et vergae with mass effect causing effacement of the lateral ventricles and cerebellar tonsillar herniation underwent endoscopic fenestration. The patient with a vein of Galen arteriovenous malformation and moderate enlargement of the lateral and third ventricle underwent percutaneous neurointerventional embolization.
Neuroimaging Studies
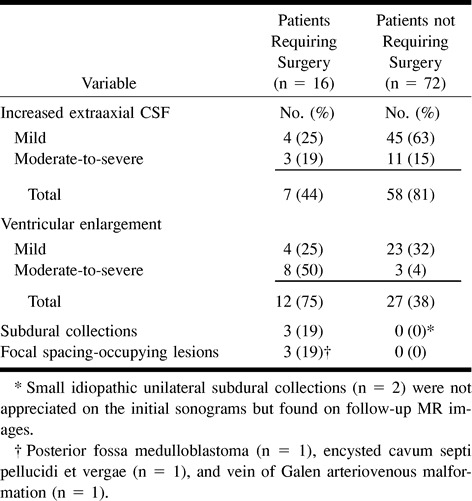
Thirty-six (41%) of the patients had undergone sonography, 31 (35%) had undergone CT, and the remaining 21 (24%) had undergone MR imaging as their initial diagnostic imaging examination. Thirty-eight (43%) underwent follow-up imaging examinations, including five (6%) who underwent sonography, 10 (11%) who underwent CT, and 23 (26%) who underwent MR imaging. The imaging findings evaluated were those that could be consistently assessed by all three imaging techniques. These included ventricular size, extraaxial CSF, subdural collections, and focal space-occupying lesions. Table 2 summarizes the imaging findings of the initial neuroimaging examinations.
TABLE 2:
Summary of findings on initial imaging study
Univariate and Multivariate Clinical and Imaging Predictors of Disorders Requiring Surgery
Univariate analysis of the clinical and imaging variables was performed to identify predictors that could help distinguish patients with disorders requiring surgical treatment from those with disorders requiring nonsurgical treatment. Thirteen statistically significant clinical and imaging univariate predictors were identified (Table 3).
TABLE 3:
Univariate predictors of surgical disorders in children with macrocrania
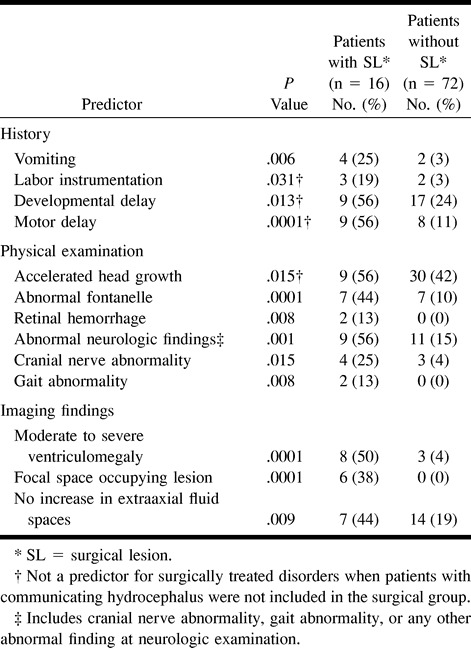
In addition, other variables analyzed included seizures, headache, torticollis, gestational age, multiple pregnancy, history of trauma, history of maternal drug abuse, family history of macrocrania, congenital heart disease, social developmental delay, craniofacial anomaly, positional molding, split sutures, abnormal eye movement, auditory abnormality, motor abnormality, abnormal tone (ie, hypotonia or hypertonia), and abnormal reflexes. None of these variables helped distinguish surgical patients from medically treated patients (data not shown).
A similar univariate analysis was performed, excluding from the surgical group those patients who underwent shunting for communicating hydrocephalus to assess potential surgical treatment bias from those patients who may have had benign idiopathic external hydrocephalus but who underwent shunting. Nine of the same univariate predictors were identified (Table 3).
Because the diagnosis of a disorder requiring surgery resulted in a substantial change in management, these patients were analyzed further to identify independent multivariate predictors that could help distinguish them from patients with disorders requiring medical treatment. Multiple logistic regression analysis identifies independent multivariate predictors by controlling for potential confounders.
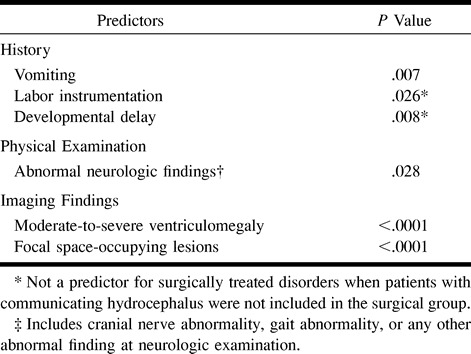
To establish the independent variables in the multiple logistic regression model, the inclusive predictor of abnormal neurologic findings was selected because this included any abnormal neurologic sign (ie, cranial nerve abnormality, gait abnormality, or any other abnormal finding at neurologic examination). Logistic regression analysis identified four clinical and two imaging independent multivariate predictors that helped differentiate patients with lesions requiring surgical treatment from those with lesions requiring medical treatment (Table 4). The Pearson χ2 test showed that an increase in the number of predictors was highly correlated with an increased risk of a lesion requiring surgery (P < .0001). The Hosmer-Lemeshow goodness-of-fit test did not reveal departure from fit (P = .44).
TABLE 4:
Independent multivariate predictors of surgical disor~ders in children with macrocrania
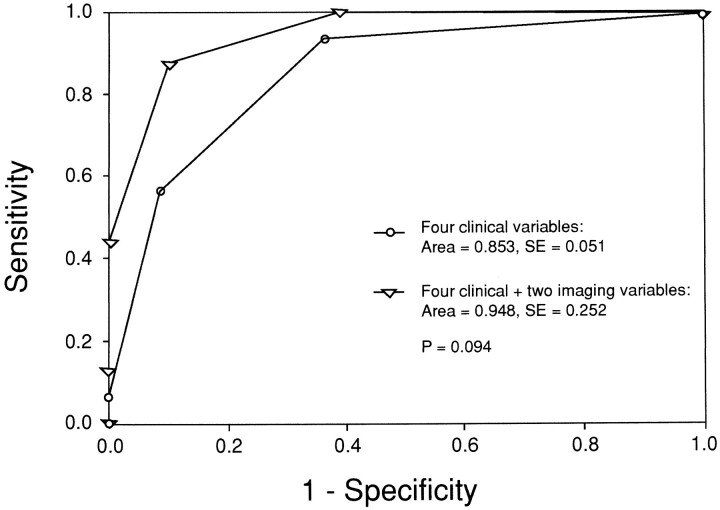
The diagnostic performance of the independent clinical predictors alone and the combination of the independent clinical and imaging predictors were evaluated using sensitivity, specificity, and receiver operating characteristic curves. Using the clinical predictors alone, of 16 patients with lesions requiring surgery, 15 had one or more predictors (sensitivity, 93.8% [95% confidence interval = 88.7%, 98.8%]) whereas 46 of 72 patients with disorders that required nonsurgical treatment had less than one clinical predictor (specificity, 63.9% [95% confidence interval = 53.8%, 73.9%]). Using the combination of independent clinical and imaging predictors of 16 patients with lesions requiring surgery, all 16 had one or more predictors (sensitivity, 100% [95% confidence interval = 96.9%, 100%]) whereas 44 of 72 patients with disorders that required nonsurgical treatment had less than one predictor (specificity, 61.1% [95% confidence interval = 50.9%, 71.3%]). The areas under the receiver operating characteristic curves for independent clinical predictors alone and the combination of independent clinical and imaging predictors were 0.85 and 0.95, respectively (P = .09) (Fig 1).
fig 1.
Comparison of receiver operating characteristic curves of four independent clinical predictors versus a combination of these clinical predictors plus two independent imaging predictors. The diagnostic performance, as illustrated by the area under the curve, was greater for the combination of clinical and imaging predictors than for clinical predictors alone, although the difference was not statistically significant (P = .09)
Multivariate logistic regression analysis was also conducted on the clinical predictors alone to determine the effect of the imaging predictors on the logistic regression model. Three clinical predictors remained the same: vomiting (P = .009), labor instrumentation (P = .018), and abnormal neurologic findings (P = .005). Motor delay (P < .001) was a predictor, whereas developmental delay was not. Multivariate analysis conducted with exclusion from the surgical group of those patients who underwent shunting for communicating hydrocephalus to assess potential surgical treated bias revealed the same clinical predictors: vomiting and abnormal neurologic findings.
Discussion
In our study, lesions requiring surgical treatment were detected in 18% of children with macrocrania. Univariate clinical predictors included vomiting, labor instrumentation, developmental delay, motor delay, accelerated head growth, abnormal fontanel, retinal hemorrhage, abnormal neurologic findings, and cranial nerve and gait abnormalities (Table 3). Univariate imaging predictors included moderate-to-severe ventriculomegaly, focal space-occupying lesion, and no increase in extraaxial fluid spaces (Table 3).
Independent multivariate predictors of surgical lesions included the clinical variables of vomiting, labor instrumentation, developmental delay, and abnormal results of the neurologic examination. Independent multivariate imaging predictors included focal space-occupying lesion and moderate-to-severe ventriculomegaly (Table 4). An increase in the number of predictors was highly correlated with an increased risk of surgical lesion (P < .0001).
Our study showed that the diagnostic performance of the combination of independent clinical and imaging predictors was better than that of the independent clinical predictors alone. This was supported by a better sensitivity of 100% (95% confidence interval = 96.9%, 100%) versus 93.8% (95% confidence interval = 88.7%, 98.8%) and area under the receiver operating characteristic curve of 0.95 versus 0.85, respectively. Although there was a trend for the area under the curve to be higher for the combination of independent clinical and imaging predictors, it was not statistically significant (P = .09). The added value of imaging of children with suspected macrocrania may be important in the current cost-conscious health care environment.
In our study, four (25%) of our 16 patients with surgical lesions experienced vomiting, seven (44%) had bulging fontanels, and nine (56%) experienced accelerated head circumference growth. Bodensteiner and Chung (7) reported emesis, accelerated head circumference growth beyond the normal growth curve, and a tense, bulging, or full anterior fontanel as predictors of raised intracranial pressure. Several studies have recommended neuroimaging if the clinical history and physical examination suggest increased intracranial pressure (7, 15, 16). Gait abnormality was also identified as a clinical predictor of a lesion requiring surgery. One patient with gait abnormality had a posterior fossa medulloblastoma. Abnormal gait has been reported as a common sign in children with infratentorial tumors (17).
In our study, the presence of accelerated head circumference growth in two patients with associated retinal hemorrhages was proved to be caused by subdural hemorrhages secondary to child abuse. Retinal hemorrhages are present in nearly all cases of infant abuse in which shaking or shaking impact is documented (18). Retinal hemorrhages seldom occur with accidental trauma, and such events usually are severe (19). Retinal hemorrhages are commonly identified at birth, and hence, the high specificity of this sign for child abuse is valid only beyond the neonatal period (18). Retinal hemorrhages occurring with normal deliveries do not seem to be associated with subdural hemorrhagic collections (16).
Labor instrumentation was one of the univariate predictors for a surgical disorder. Furthermore, once all potential confounders were controlled on the multivariate analysis, instrumentation was an independent predictor. Instrumentation with forceps and vacuum extraction may cause intracranial hemorrhage, which may predispose to noncommunicating or communicating hydrocephalus.
The imaging findings provided important predictors of lesions requiring surgery. In our study, moderate-to-severe ventriculomegaly was an imaging predictor of a lesion requiring surgery, whereas mild ventricular dilation was not. Several authors have reported surgical management for children with macrocrania if there is significant ventriculomegaly suggestive of obstructive hydrocephalus (20).
Increased extraaxial CSF was relatively more frequent in the nonsurgical (78%) than the surgical group (44%). Hence, the lack of increased extraaxial subarachnoid fluid was an univariate predictor of a lesion requiring surgery. The explanation for this finding is that the presence of an intraaxial focal space-occupying lesion or moderate-to-severe ventriculomegaly decreases the extraaxial subarachnoid space. Furthermore, several studies have noted that enlarged extraaxial CSF spaces in children younger than 2 years may be a normal variant (21, 22). Other studies have also shown that children with macrocrania and enlarged extraaxial CSF spaces have a good prognostic outcome (4, 6, 23, 24).
Regarding our study design, our results may reflect a higher risk population, considering that all patients were referred to a pediatric neurosurgeon or neurologist at a tertiary care center where neuroimaging was deemed necessary. This selection bias allowed us the opportunity to study a population likely enriched in neurologic signs and symptoms to determine which clinical and imaging variables best help predict the presence of lesions requiring surgery. Hence, the results herein cannot be generalized to all children with macrocrania. However, the clinical and imaging variables of lesions requiring surgery described may be useful to neurosurgeons, neurologists, and neuroradiologists who may ultimately decide whether surgical treatment is warranted.
Our series did not include patients with neurodegenerative disorders, such as Canavan and Alexander disease, with macrocrania (25). These neurodegenerative disorders are uncommon in comparison with hydrocephalus and focal intracranial space-occupying lesions. In addition, these neurodegenerative disorders rarely present because of macrocrania but rather because of neurodevelopmental abnormalities. Very large series of children with macrocrania may be required to study the predictors of these important but rare disorders.
The clinical variables obtained from the charts may be limited, because this study was based on retrospective data. In evaluation of certain clinical variables, we assumed that if a feature was not mentioned to be present, it was absent. We think this is a reasonable assumption, because all of the patients were evaluated by a staff neurosurgeon, a neurologist, or a neurologist-in-training (along with a staff neurosurgeon or neurologist), all of whom were trained to assess specifically the predictors in question.
The patients in this study did not all undergo the same imaging technique. Comparison of different imaging procedures is difficult. However, we limited the imaging findings evaluated by sonography, CT, and MR imaging to those consistently assessed by all three imaging techniques.
Inter- and intraobserver agreement for the different imaging procedures was not determined. The prospectively generated written report was used as the final imaging interpretation. Future prospective studies should include inter- and intraobserver agreement so that variability among readers can be determined.
In summary, baseline neuroimaging is indicated for children with macrocrania because the combination of clinical and imaging predictors has the best diagnostic performance in determining the need for surgical versus nonsurgical management.
Footnotes
Address reprint requests to L. Santiago Medina, MD, MPH, International Health Outcomes and Policy Center, Department of Radiology, Children's Hospital, 3100 SW 62 Avenue, Miami, FL 33155.
References
- 1.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr 1979;32:607-629 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez LA, Maytal J, Shinnar S. Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocrania. Pediatrics 1986;77:901-907 [PubMed] [Google Scholar]
- 3.Gooskens RH, Gielen CC, Hanlo PW, Faber JA, Willemse J. Intracranial spaces in childhood macrocrania: comparison of length measurements and volume calculations. Dev Med Child Neurol 1988;30:509-519 [DOI] [PubMed] [Google Scholar]
- 4.Nickel RE, Gallenstein JS. Developmental prognosis for infants with benign enlargement of the subarachnoid spaces. Dev Med Child Neurol 1987;29:181-186 [DOI] [PubMed] [Google Scholar]
- 5.Babcock DS, Han BK, Dine MS. Sonographic findings in infants with macrocrania. AJR Am J Roentgenol 1988;150:1359-1365 [DOI] [PubMed] [Google Scholar]
- 6.Maytal J, Alvarez LA, Elkin CM, Shinnar S. External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. AJR Am J Roentgenol 1987;148:1223-1230 [DOI] [PubMed] [Google Scholar]
- 7.Bodensteiner JB, Chung EO. Macrocrania and megalencephaly in the neonate. Semin Neurol 1993;13:84-91 [DOI] [PubMed] [Google Scholar]
- 8.Vaughan VC III. Developmental pediatrics. In: Behrman RE, Vaughan VC III, eds. Nelson's Textbook of Pediatrics. Philadelphia: W.B. Saunders Company 1983;10-134
- 9.Breslow NE, Day NE. Statistical Methods in Cancer Research: The Analysis of Case-Control Studies.. vol 1. Lyon: IARC Scientific Publications 1980;192-246 [PubMed]
- 10.Hosmer DW, Lemeshow S. Applied Logistic Regression.. New York: Wiley 1989;106-108
- 11.Lemeshow S, Hosmer DW. A review of goodness of fit statistics in the development of logistic regression models. Am J Epidemiol 1982;115:92-106 [DOI] [PubMed] [Google Scholar]
- 12.Agresti A. Categorical Data Analysis.. New York: Wiley 1990;95-101
- 13.Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;7:283-298 [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36 [DOI] [PubMed] [Google Scholar]
- 15.Gooskens RH, Willemse J, Faber JA, Verdonck AF. Macrocephalies: a differentiated approach. Neuropediatrics 1989;20:164-169 [DOI] [PubMed] [Google Scholar]
- 16.Smith R, Leonidas JC, Maytal J. The value of head ultrasound in infants with macrocrania. Pediatr Radiol 1998;28:143-146 [DOI] [PubMed] [Google Scholar]
- 17.Zaki A, Natarajan N, Mettlin CJ. Patterns of presentation in brain tumors in the United States. J Surg Oncol 1993;53:110-112 [DOI] [PubMed] [Google Scholar]
- 18.Kleinman PK, Barnes PD. Head trauma. In: Kleinman PK, ed. Diagnostic Imaging of Child Abuse. St. Louis: Mosby; 1998;285-342
- 19.Duhaime AC, Alairo AJ, Lewander WJ, et al. Head injury in very young children: mechanisms, injury types and etiologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics 1992;90:179-185 [PubMed] [Google Scholar]
- 20.Kingsley D, Kendall BE. The value of computed tomography in the evaluation of the enlarged head. Neuroradiology 1978;15:59-71 [DOI] [PubMed] [Google Scholar]
- 21.Kleinman PK, Zito JL, Davidson RI, Raptopoulos V. The subarachnoid spaces in children: normal variations in size. Radiology 1983;147:455-457 [DOI] [PubMed] [Google Scholar]
- 22.Odita JC. The widened frontal subarachnoid space: a CT comparative study between macrocephalic, microcephalic, and normocephalic infants and children. Childs Nerv Syst 1992;8:36-39 [DOI] [PubMed] [Google Scholar]
- 23.Andersson H, Elfverson J, Svendsen P. External hydrocephalus in infants. Childs Brain 1984;11:398-402 [DOI] [PubMed] [Google Scholar]
- 24.Neveling EA, Truex RC Jr. External obstructive hydrocephalus: a study of clinical and developmental aspects in ten children. J Neurosurg Nurs 1983;15:255-260 [DOI] [PubMed] [Google Scholar]
- 25.Ruggieri PM. Metabolic and neurodegenerative disorders and disorders with abnormal myelination. In: Ball WS Jr, ed. Pediatric Neuroradiology. Philadelphia: Lippincott-Raven 1997;175-237