Abstract
Summary: We report two patients with suspected primary angiitis of the CNS who underwent serial contrast-enhanced MR imaging of the spinal cord. MR abnormalities were multiple and enhancing, and located within the cervical and thoracic cord. Brain MR findings and brain biopsy specimens were positive for primary angiitis of the CNS. On follow-up MR studies, obtained after steroid and immunosuppressive therapy, a significant decrease in the number and size of the enhancing and nonenhancing abnormalities was observed, along with clinical improvement. Numerous small and enhancing abnormalities with a primarily posterior location, seen at the onset of the disease and resolved on follow-up studies, may be considered suggestive of a diagnosis of primary angiitis of the CNS.
Primary angiitis of the CNS is a vasculitis exclusively affecting the CNS, without association with systemic processes. Clinical features of primary angiitis of the CNS can be focal or nonfocal. Focal symptoms include transient or persistent hemiparesis, sensory loss, ataxia, seizures, cranial neuropathies, and paraparesis. Nonfocal symptoms include headache, cognitive decline, confusion, fluctuating levels of consciousness, lethargy, and malaise. Both acute (stroke and headache) and chronic (meningitis syndrome) presentations have been described (1). A vasculitis similar to primary angiitis of the CNS has been reported in association with a variety of conditions and diseases, including herpes zoster, HIV infection, non-Hodgkin's lymphoma and Hodgkin's disease (2–4). These same diseases are, however, included in major differential diagnoses. Many diseases, such as systemic vasculitides; bacterial, fungal, and viral infections; and antiphospholipid antibodies syndrome, mimic the clinical and radiologic CNS features of primary angiitis of the CNS (5, 6). Laboratory indicators of systemic vasculitic disease activity in primary angiitis of the CNS should be normal. CSF analysis frequently shows elevation of cell count and protein level. Positive cerebral angiographic findings may be useful in making a presumptive diagnosis of cerebral vasculitis; however, angiograms have produced normal findings in up to 40% of pathologically documented cases (5).
Symptomatic spinal cord involvement has been documented much less frequently than intracranial involvement. Acute transverse myelitis (4) is rarely a mode of presentation of primary angiitis of the CNS; however, progressive paraparesis and myelopathy are the most common manifestations when spinal cord involvement becomes clinically symptomatic. We report two patients with primary angiitis of the CNS and involvement of the spinal cord whose diagnosis was established by brain biopsy.
Case Reports
Case 1
A previously healthy 29-year-old woman presented with subacute severe headache, diplopia, and ataxia. Neurologic examination revealed bilateral papilledema, paresis of the sixth left cranial nerve, and loss of position and vibratory sense. Routine chemistries, serum profile for collagen vascular disease, angiotensin-converting enzyme (ACE), antiphospholipid antibodies, antibodies to different viruses (HIV and HSV-1 and−2, varicella-zoster, influenza, parainfluenza, Coxackievirus) and to several pathogens (Borrelia, Treponema pallidum) were normal. A CSF study showed protein at 70 mg/dL, glucose at 50 mg/dL, 12 cells/mm3 (80% lymphocytes), absent oligoclonal bands, and normal cytologic findings. Cultures of blood, urine, and CSF were sterile. Digital subtraction angiography of the brain was normal.
Brain MR studies revealed multiple small lesions of the supratentorial white matter, hyperintense on T2-weighted images, associated with enlarged perivascular spaces. These abnormalities were all simultaneously and homogeneously enhancing on contrast-enhanced T1-weighted images. One month later, the patient had gradual onset of progressive paraparesis. A spinal MR examination showed abnormal signal within a normal-sized spinal cord (Fig 1A–C). On postcontrast T1-weighted images, small enhancing abnormalities were evident (Fig 1D and F). A brain biopsy was then performed. Steroids (prednisone 60 mg/day) combined with immunosuppressive therapy (cyclophosphamide 100 mg/day) for 6 weeks, and afterward only prednisone, progressively tapered, resulted in clinical improvement after 2 months. The patient was then treated with azathioprine (100 mg/day). A neurologic examination 12 months later showed only hyperreflexia of the lower limbs and Babinski sign. A follow-up MR study showed marked improvement of the radiologic pattern (Fig 1G–I). On the long-term clinical follow-up, 4 years later, the patient was clinically stable and still receiving treatment with azathioprine.
fig 1.
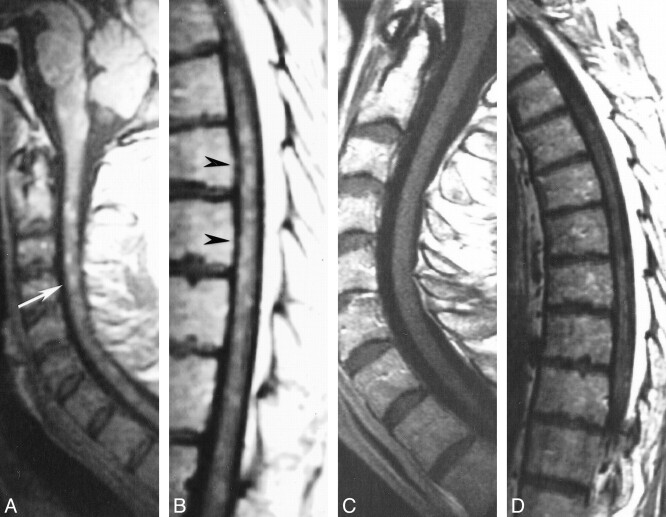
Patient 1: 29-year-old woman with primary angiitis of the CNS whose progressive paraparesis started 1 month after the onset of cerebral symptoms.
A–F, First MR study. Sagittal FSE T2-weighted (4700/112) images (A–C) show diffuse increased signal intensity within the cervical cord (arrows, A). Skip areas of slightly increased signal intensity are detectable within the upper and lower thoracic cord (arrows, B and C). Sagittal contrast-enhanced SE T1-weighted (500/12) images (D and E) show multiple small homogeneously enhancing areas of the cervical and thoracic spinal cord (arrows), primarily posterior in location, and pial enhancement of the conus. Axial contrast-enhanced SE T1-weighted (500/15) image (F) shows two posteriorly located punctate areas of homogeneous enhancement (arrows).
Case 2
A 50-year-old man presented with moderate progressive weakness of both legs, which progressed to severe paraparesis over 6 months. His medical history was remarkable only for headaches, which had started 2 years earlier. His family history was not significant. On neurologic examination the patient had hyperreflexia and paraparesis. Routine chemistries, collagen screening tests, ACE, antiphospholipid antibodies, antibodies to different viruses (HIV and HSV-1 and -2, varicella-zoster, influenza, parainfluenza, Coxackievirus, Echovirus) and to several pathogens (Borrelia, Treponema pallidum) were normal. CSF study showed protein of 140 mg/dL, glucose of 45 mg/dL, 30 cells/mm3 (80% lymphocytes), and absent oligoclonal bands. Cytologic studies, bacterial and viral cultures, and cryptococcal antigen tests were negative.
A brain MR study showed small multiple lesions of the deep and subcortical white matter located supra- and infratentorially. The lesions enhanced, and perivascular spaces were enlarged. An MR angiographic examination was normal. A spinal MR study showed punctate multiple enhancing abnormalities of the cervical and thoracic cord (Fig 2A and B). A brain biopsy was then performed. Steroids (prednisone 60 mg/day) combined with immunosuppressive therapy (cyclophosphamide 100 mg/day) for 6 weeks and then only prednisone, progressively tapered over 6 months, were instituted. During the following months, after therapy, paraparesis improved. Afterward, further neurologic symptoms developed, which were related to the brain involvement and to the intermittently received steroids. Three years later, while the patient was still receiving low-dose steroids, he had a mild relapse of the paraparesis and underwent follow-up spinal MR imaging (Fig 2C and D).
fig 2.
Patient 2: 50-year-old man with primary angiitis of the CNS who presented with progressive paraparesis.
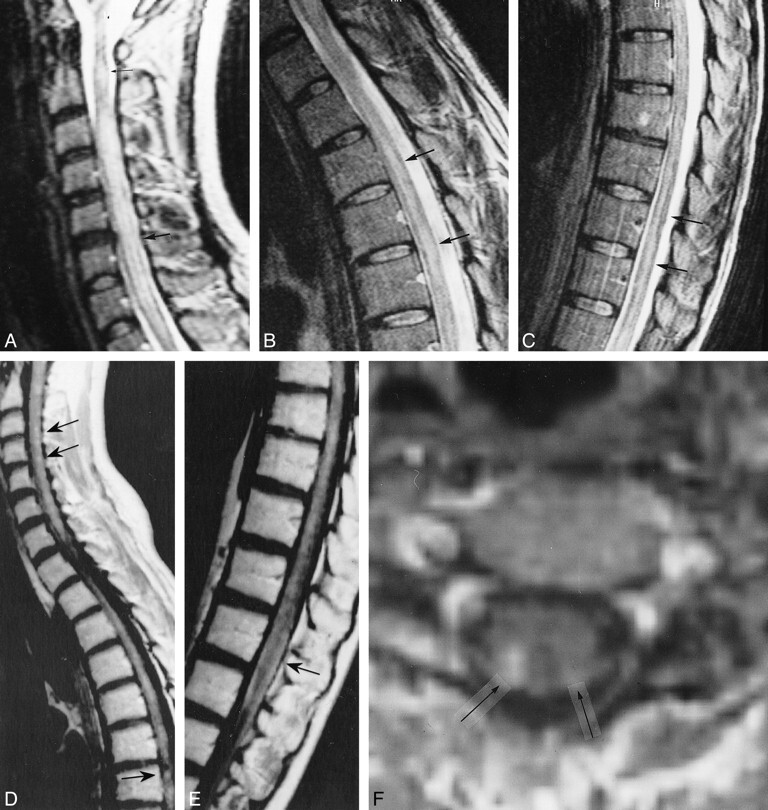
A and B, First MR study, obtained at 0.5 T. Sagittal contrast-enhanced SE T1-weighted images show numerous small homogeneously enhancing lesions located in the cervical and thoracic cord (arrow, A; arrowheads, B). Enhancing lesions can also be seen in the medulla, both dorsally and ventrally.
C and D, Follow-up MR study 36 months after the onset of disease. Sagittal contrast-enhanced SE T1-weighted (500/12) images show no evidence of contrast enhancement of the cord.
Pathologic Findings
Considering histories, laboratory and radiologic findings, and the lack of systemic diseases, primary angiitis of the CNS was suspected for both patients and a brain biopsy was performed after the first MR examinations, during the first month of disease. Brain biopsy specimens of parenchymal and leptomeningeal tissue of frontal brain regions showed vasculitis of parenchymal small vessels with fibrinoid necrosis and copious inflammatory infiltrate in patient 1 (Fig 1I and J), and inflammatory infiltrate with lymphocytes and granulocytes of small vessels in patient 2, confirming the diagnosis of primary angiitis of the CNS.
Discussion
Primary angiitis of the CNS is an uncommon disorder, which is also known as granulomatous angiitis of the CNS. A definite diagnosis can be made on the basis of previously published criteria (7). These include clinical history and findings that remain unexplained after a complete initial evaluation, angiographic or histopathologic features of angiitis within the CNS, and lack of evidence of systemic vasculitis or any other condition to which the angiographic or pathologic features could be secondary.
The major differential diagnoses include the systemic arthritides, such as periarteritis nodosa, Sjögren disease, sarcoidosis, infectious angiitis, especially herpes zoster, miliary tuberculosis, and granulomatous angiitis associated with Hodgkin's disease (1–7). Spinal cord involvement has been reported in 14% of patients (8). Previously reported cases of Hodgkin's disease associated with primary angiitis of the CNS included documentation of spinal cord involvement at autopsy (9) or at biopsy (3). In one of these cases the spinal cord involvement was not clinically evident, and in the remaining cases clinical features of spinal cord involvement were overshadowed by manifestations of cerebral involvement. On the other hand, another patient with primary angiitis of the CNS initially presented with spinal cord involvement then developed multifocal cerebral and cerebellar involvement (10). Primary angiitis of the CNS can also manifest as an isolated spinal cord arteritis (9) or as an isolated myelopathy (11). Several reported cases showed focal involvement of the spinal cord as the predominant manifestation of the vasculitis (7). Among our patients, the differential diagnoses included primarily sarcoidosis and acute disseminated encephalomyelitis (ADEM).
Sarcoidosis can cause patchy multifocal broad-based areas of enhancement adjacent to the surface of the cord in the first phase, with linear leptomeningeal enhancement detectable along the surface of the spinal cord. The inflammatory process may then involve parenchyma with or without enhancement of Virchow-Robin perivascular spaces on MR images (12). Enlargement of the cord and cord swelling, with faint or no enhancement, mimicking an intramedullary tumor, are common during the later stages of this disease (13). Lesions are frequently located in the cervical spine (14). Our patients, however, did not show swelling of the cord and had cervical and thoracic lesions. Development of spinal cord atrophy over time can be another finding suggestive of neurosarcoidosis; however, this was not observed during the follow-up period in our patients, although moderate atrophy was already present on the first MR study of patient 2. Typical findings of brain sarcoidosis, such as involvement of the hypothalamus and pituitary stalk, leptomeningeal enhancement, or dural masses (14), were absent in our patients. Serum ACE levels were within the normal range.
Primary angiitis of the CNS is distinguished from sarcoidosis by the absence of necrosis in sarcoidosis, a greater tendency for sarcoidosis to involve the base of the brain, and involvement of systemic organs. The composition of the inflammatory infiltrate, partially similar to that found in patients with sarcoidosis and delayed hypersensitivity reaction, suggests that a disorder of cell-mediated immunity may play a role in the pathogenesis of primary angiitis of the CNS (6). The pathologic findings can be indistinguishable from those of varicella-zoster herpetic angiitis; however, the distinction lies in the antecedent herpetic eruption and intracranial involvement, usually of the ophthalmic division of the trigeminal nerve (6). Various viral infections most likely participate in the production of CNS vasculitis (7).
The pathogenesis of primary angiitis of the CNS is unknown, although it is known to be associated with lymphoma or leukemia with a frequency of 10%, and viruslike particles or mycoplasmalike structures have been seen at electron microscopy in mononuclear giant cells of these patients (7). Neurologic symptoms are the most common nonpulmonary manifestations of Mycoplasma pneumoniae infections, and transverse myelitis has recently been described in children infected by M. pneumoniae (15). These associated infectious agents may be considered as coincidental or consequent rather than causal, since patients with primary angiitis of the CNS may be immunocompromised. Our patients did not have infections or abnormalities of the immune system, nor did any develop during the follow-up period. Younger at al (16) suggested that primary angiitis of the CNS is a nonspecific inflammatory reaction and not a unique disease.
Cryptococcosis could be a further differential diagnosis. In fact, Cryptococcus produces small pseudocysts that extend along dilated perivascular spaces and show varying contrast enhancement on MR studies (17). Biological investigations on serum and CSF cultures for infectious agents in our patients were negative. ADEM was also considered as a differential diagnosis; however, no antecedent infectious diseases or vaccinations were reported by our patients before the onset of neurologic symptoms. Moreover, brain MR images did not show the large white matter lesions with asymmetrical distribution that are typical of ADEM.
Histologic findings in primary angiitis of the CNS may be variable, even in the same specimen. Leptomeningeal and parenchymal blood vessels may be involved together or separately (1). Vessel walls can be infiltrated by lymphocytes, plasma cells, large mononuclear cells, and giant cells, with fibrinoid necrosis and polymorphonuclear leukocytes during the acute phases. Small arteries, arterioles, and venules are more frequently affected. Leptomeningeal involvement can occur with infiltration of the perivascular spaces. Inflammatory cells pass from the blood into either the subarachnoid space or the subpial space through the venous wall, particularly of smaller veins in the subpial space (18). Inflammatory cells entering the subpial space may be distributed along perivascular spaces of arteries and veins in the subarachnoid space (18). Infiltration can compress the vessel wall, inducing ischemic abnormalities.
On follow-up MR examinations, the enhancement regressed, even for several abnormalities detectable on T2-weighted images. These reversible findings on T2-weighted images may be explained by considering these fluctuating abnormalities as small areas of mainly venular focal congestion related to small venous engorgement induced by perivascular infiltration. Theoretically, even biochemically induced segmental vasoconstriction could be responsible for reversible abnormalities located around small arteriolar vessels (19). However, the posterior location of the lesions in the cord in our patients seems to be more likely related to a perivascular inflammatory infiltration, mainly around small veins. Predominant posterior involvement of the spinal cord, primarily at the thoracic and lumbar levels, has also been described in some reported cases (11). Kattah et al (10) found atrophy of the conus at myelography in a patient with paraparesis, urinary retention, global sensory level at T12, absent vibration, and position and pain sensation. These signs were suggestive of posterior and lateral involvement of the cord. Inwards et al (2) reported a patient with paraplegia and thoracic sensory levels in whom MR images revealed an enlarged thoracic cord with abnormal signal. In a patient described by Caccamo et al (11), angiitis was evident at autopsy throughout the entire cord, but was especially pronounced in the lower thoracic and lumbar levels, resulting in degeneration of ascending tracts, especially the posterior columns. A massive perivascular inflammatory infiltration and a severe breakdown of the blood-brain barrier may explain the intense and simultaneous enhancement of the cord abnormalities reported at the onset of neurologic symptoms in our patients.
Conclusion
During the initial phase of primary angiitis of the CNS, MR studies in our patients revealed multiple abnormalities involving the entire cord and enhancement of these small abnormalities, primarily posterior in location, in conjunction with similar lesions in the brain. During the follow-up period, most of the abnormalities, both enhancing and nonenhancing, resolved. These MR findings may be considered suggestive of primary angiitis of the CNS with spinal cord involvement.
fig 1.
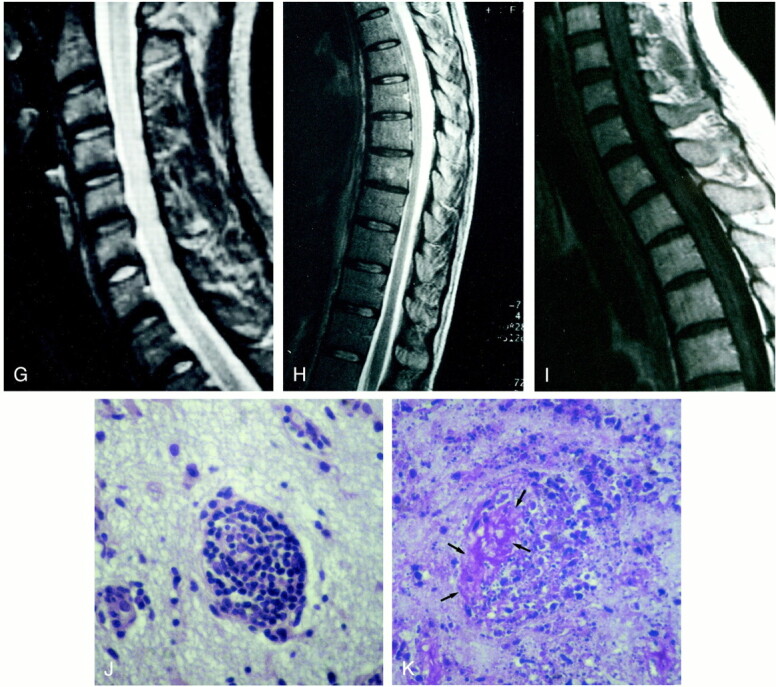
fig. 1. Continued.
G–I, Follow-up MR study 12 months later. Sagittal FSE T2-weighted (4700/112) images (G and H) show regression of the increased signal intensity within the entire cord. Sagittal contrast-enhanced SE T1-weighted (500/12) image (I) shows no evidence of contrast enhancement of the cervico-thoracic cord.
J and K, Microscopic sections show a small cortical vessel with its wall infiltrated by a proliferation of mononuclear cells in rarefied gliotic cerebral cortex (J) and fibrinoid necrosis with nuclear and cytoplasmic debris (arrows, K) and loss of normal structure of the white matter (K) (hematoxylin-eosin, original magnification ×275).
Footnotes
Address reprint requests to Adriana Campi, MD, Department of Neuroradiology, Scientific Institute and University San Raffaele, Via Olgettina 60, 20132 Milan, Italy.
References
- 1.Calabrese LH, Duna GF, Lie JT. Vasculitis of the central nervous system. Arthritis Rheum 1997;40:1189-1201 [DOI] [PubMed] [Google Scholar]
- 2.Inwards DJ, Piepgras DG, Lie JT, O'Neill BP, Scheithauer BW, Habermann TM. Granulomatous angiitis of the spinal cord associated with Hodgkin's disease. Cancer 1991;68:1318-1322 [DOI] [PubMed] [Google Scholar]
- 3.Rosen CI, DePalma L, Morita A. Primary angiitis of the central nervous system as a first presentation in Hodgkin's disease: a case report and review of the literature. Neurosurgery 2000;46:1504-1510 [DOI] [PubMed] [Google Scholar]
- 4.Yuen RW, Johnson PC. Primary angiitis of the central nervous system associated with Hodgkin's disease. Arch Pathol Lab Med 1996;120:573-576 [PubMed] [Google Scholar]
- 5.Calabrese LH, Furlan AJ, Gragg LA, Ropos TJ. Primary angiitis of the central nervous system: diagnostic criteria and clinical approach. Cleve Clin J Med 1992;59:293-306 [DOI] [PubMed] [Google Scholar]
- 6.Koo EH, Massey EN. Granulomatous angiitis of the central nervous system: protean manifestations and response to treatment. J Neurol Neurosurg Psychiatry 1988;51:1126-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese LH, Mallek JA. Primary angiitis of the central nervous system: report of 8 new cases, review of the literature and proposal for diagnostic criteria. Medicine 1988;67:20-39 [DOI] [PubMed] [Google Scholar]
- 8.Duna GF, George T, Rybicki L, Calabrese LH. Primary angiitis of the central nervous system: an analysis of unusual presentations. Arthritis Rheum 1995;38:S340-S1122 [Google Scholar]
- 9.Lie JT. Angiitis of the central nervous system. Curr Opin Rheumatol 1991;3:36-45 [DOI] [PubMed] [Google Scholar]
- 10.Kattah JC, Cupps TR, Di Chiro G, Manz HJ. An unusual case of central nervous system vasculitis. J Neurol 1987;:344-347 [DOI] [PubMed] [Google Scholar]
- 11.Caccamo DV, Garcia JH, Ho KL. Isolated granulomatous angiitis of the spinal cord. Ann Neurol 1992;32:580-582 [DOI] [PubMed] [Google Scholar]
- 12.Waubant E, Manelfe C, Bonafé A, Berry I, Clanet M. MRI of intramedullary sarcoidosis: follow-up of a case. Neuroradiology 1997;39:357-360 [DOI] [PubMed] [Google Scholar]
- 13.Junger SS, Stern BJ, Levine SR, Sipos E, Marti-Masso JF. Intramedullary spinal sarcoidosis: clinical and magnetic resonance imaging characteristics. Neurology 1993;43:333-337 [DOI] [PubMed] [Google Scholar]
- 14.Christoforidis GA, Spickler EM, Recio MV, Mehta BM. MR of CNS sarcoidosis: correlation of imaging features to clinical symptoms and response to treatment. AJNR Am J Neuroradiol 1999;20:655-669 [PMC free article] [PubMed] [Google Scholar]
- 15.Smith R, Eviatar L. Neurologic manifestations of Mycoplasma pneumoniae infections: diverse spectrum of diseases: a report of 6 cases and review of the literature. Clin Pediatr 2000;39:195-201 [DOI] [PubMed] [Google Scholar]
- 16.Younger DS, Hays AP, Brust JCM, Rowland LP. Granulomatous angiitis of the brain: an inflammatory reaction of diverse etiology. Arch Neurol 1988;45:514-518 [DOI] [PubMed] [Google Scholar]
- 17.Matthews VP, Alo PL, Glass JD, Kumar AJ, McArthur JC. AIDS-related CNS cryptococcosis: radiologic-pathologic correlation. AJNR Am J Neuroradiol 1992;13:1477-1486 [PMC free article] [PubMed] [Google Scholar]
- 18.Weller RO. Anatomy and pathology of the subpial space. Riv Neuroradiol 1994;17:15-21 [Google Scholar]
- 19.Harris KG, Tran DD, Sickels WJ, Cornell SH, Yuh WTC. Diagnosing intracranial vasculitis: the roles of MR and angiography. AJNR Am J Neuroradiol 1994;15:317-330 [PMC free article] [PubMed] [Google Scholar]