Abstract
BACKGROUND AND PURPOSE: Vertebral venography has been advocated before bone cement injection when performing percutaneous vertebroplasty (PV) for benign or malignant lesions of the spine. Although venography can document sites of potential leakage during subsequent cement application, stagnant contrast agent renders the cement injection more difficult to monitor, and an allergic reaction to contrast agent remains a potential risk. We evaluated our experience with PV without prior venographic evaluation.
METHODS: Two hundred five consecutive PV procedures performed in 137 patients without pretreatment venography were evaluated for complications linked to bone cement injection. Treated lesions were 172 benign compression fractures, 27 metastases, two hemangiomas, and four multiple myelomas. PV was performed with a single-pedicle technique in 146 cases and a two-pedicle technique in 59 cases.
RESULTS: No major complication occurred in our series. Three minor complications (1.5%) were documented: One patient had a transient episode of arterial hypotension during cement injection, without cement leak; one patient had a spontaneously resolving patch of cutaneous hypoesthesia at the puncture site; and one patient had a radiculopathy four levels above the treated level, not caused by cement deposition, and successfully treated with a nerve block. None of these three minor complications were related to cement leakage.
CONCLUSION: PV can, in our experience, be performed safely without prior angiographic evaluation of the vertebral venous system.
Percutaneous vertebroplasty (PV) is a minimally invasive procedure consisting of the injection of polymethyl methacrylate (PMMA) into a vertebral body lesion under radiologic guidance (1). Initially described in 1987 (2), PV has shown a constantly growing popularity among the radiology community and has now gained recognition as a safe interventional procedure when performed in an appropriate technical environment (3). The principal indications for PV are management of pain associated with benign compression fractures, vertebral metastatic lesions, multiple myelomas, lymphomas, and vertebral hemangiomas (4). Although combining CT and fluoroscopy has been promoted by some authors (5), most PV procedures are performed under fluoroscopic guidance alone.
Angiographic evaluation of the vertebral venous system (venography) before injection of bone cement has been advocated as a means of identifying sites of potential venous leakage during the procedure. However, the need for venography is debated (6), and some authors use it only in selected situations such as during treatment of vertebral hemangioma (1). Vertebral venography is not without drawbacks. Pooling of contrast agent in the area to be treated renders subsequent fluoroscopic monitoring of the cement injection more difficult, whereas administration of an iodine-based agent carries the risk of potentially severe allergic reactions.
The purpose of this study was to evaluate our experience with 205 consecutive PV procedures performed without prior venographic evaluation.
Methods
Patient Population
Two hundred five consecutive PV procedures were performed at our institution in 137 patients between January 1999 and October 2001. Indications for PV were benign compression fractures (n = 171), metastases (n = 27), multiple myelomas (n = 4), hemangiomas (n = 2), and osteogenesis imperfecta (n = 1). One-, two-, and three-level treatments were performed in 143, 28, and two instances, respectively, for a total of 173 PV sessions. The patient population was composed of 96 women and 41 men (age range, 18–99 years; mean, 73 years; median, 76 years). All patients were referred for pain resistant to conventional medical management, including bed rest, opioid analgesics, and/or braces. In cases of compression fractures, the delay between fracture documentation and PV was 6 weeks to 10 years. The distribution of vertebral levels (n = 205) was as follows: T4, 3; T5, 3; T6, 5; T7, 10; T8, 13; T9, 13; T10, 18; T11, 16; T12, 36; L1, 43; L2, 12; L3, 13; L4, 14; and L5, 6. PV was approved by our institutional review board, and informed consent was obtained from each patient after a detailed review of the potential risks and benefits of the procedure.
Preprocedural Imaging and Selection Criteria
Most patients referred for PV had been previously examined with MR imaging or, less often, with a combination of conventional radiography, CT, and/or nuclear imaging. MR imaging or CT was performed in patients, consulting with conventional radiographs only. Compression fractures with severe loss of vertebral body height (ie, vertebra plana) and/or posterior wall retropulsion were not considered contraindications in our series. In fact, owing to our referral pattern as a tertiary center, a large portion of our patients was referred for lesions previously deemed untreatable according to these criteria. Patients with multiple compression fractures, with chronic or ill-defined pain, or with discordant history and imaging findings had their back examined under fluoroscopy. In these cases, PV was performed if the pain elicited by palpation grossly corresponded to a fractured vertebral body. During the study period, 24 patients initially considered as potential candidates for PV were not treated because of lack of correspondence between the fractures and the pain produced by palpation.
Percutaneous Vertebroplasty Technique
All procedures were performed with local anesthesia and conscious sedation monitored by the neurointerventional team. Sedation was obtained by intravenous administration of fentanyl (25–100 μg) and midazolam (0.25–2.00 mg). Prophylactic intravenous antibiotherapy (cefazolin 1.0 g) given at the beginning of the procedure was used for all but two patients with multiple antibiotic allergies. Patients were observed for 2–3 hours before discharge, except for two inpatients who were admitted before PV for nonrelated medical problems.
In one patient with severe kyphoscoliosis, two successive PV procedures performed with the patient in the lateral decubitus position. Another patient could not lie on the table at all and was therefore treated in a sitting position. All other patients were placed on the angiography table in a prone position. The fluoroscopy tube was positioned to obtain the best possible definition of the pedicle projecting over the targeted vertebral body. Local anesthesia using lidocaine 4% buffered with sodium bicarbonate 8.4% was obtained from the skin surface deep to the periostium of the targeted pedicle. An 11-gauge needle (Manan, MDTech, Gainesville, FL; or M1/M2, Cook, Bloomington, IN) was advanced into the vertebral body under anteroposterior and lateral fluoroscopic control. Thirteen-gauge needles were used for small pedicles, usually at the upper thoracic levels. Ideally, the tip of the trocar was placed in the lower anterior half of the vertebral body. Needle positions more posterior occasionally had to be acceptable when treating severely compressed vertebral bodies with a steep pedicle angulation. A lateral-to-medial angulation bringing the tip of the trocar close to the midline of the vertebral body (single-pedicle technique) was used in most cases. On a few occasions, a surgical hammer was used to help the needle penetrate denser bone.
Bone cement was prepared by mixing 10 mg of sterile barium powder (hospital pharmacy preparation) with 20 mg of powder copolymer (Osteobond; Zimmer, Warsaw, IN). Ten milliliters of the mixture (equivalent to 10 mg) was removed before addition of the 10 mL of liquid copolymer. The resultant mixture respects the 2:1 powder-to-liquid ratio intended by the manufacturer, but with a barium concentration increased to 40% that allows for adequate visualization of cement injection at fluoroscopy.
The needle stylet was removed from the trocar, and the needle hub was inspected for blood reflux possibly indicating needle tip position in a major venous channel of the vertebral body. Although theoretic concern about air embolism occurring during the stylet removal exists, we believe that the increase in central venous pressure resulting from the prone position of the patient makes it very unlikely. As a rule, slight blood reflux was observed at the needle hub. In cases showing substantial reflux, the needle was advanced slightly further.
The cement was then introduced into a 20-mL syringe, used in turn to fill 1-mL Luer-lok syringes. The cement was injected into the vertebral body under continuous fluoroscopic control in the lateral plane. The injection was discontinued when the degree of vertebral body filling was thought to be adequate. It was interrupted earlier if the cement reached the posterior fourth of the vertebral body, or if leakage into perivertebral venous structures was observed. In the latter situation, the injection of cement was usually resumed after a 20–30-second delay, allowing for completion of vertebral body filling without increasing the venous leak. When the degree of cross-filling from the initial injection was insufficient, a contralateral transpedicular approach was performed. As a rule, the amount of barium powder added to the bone cement was doubled for the contralateral injection. In patients with multiple compression fractures, the decision to address several levels during the same procedure was based on the degree of patient comfort.
A biopsy was performed in patients with suspicion of underlying malignant process, as well as in male patients with primary osteoporosis (ie, without an identifiable cause such as long-term steroid treatment). The biopsy was performed coaxially through the vertebroplasty needle. Core bone samples were obtained with a biopsy gun (Temno; Allegiance, McGaw Park, IL) and/or by means of 18- or 20-gauge needles (Franseen; Allegiance).
All patients were contacted 1 day and 1 week after PV for postprocedural assessment.
Results
Two hundred five vertebral lesions were treated in 137 patients during 173 sessions, for a total of 264 uneventful transpedicular needle placements (59 bilateral and 146 unilateral approaches). All PV procedures could be performed with local anesthesia and conscious sedation. The amount of PMMA injected per vertebral body (calculated from 70 procedures with reported volumes of injection) varied between 2.7 and 11.0 mL (mean, 5.4 mL; 5.0 mL at the thoracic level, 5.9 mL at the lumbar level).
Extravasation of a small amount of cement into the disk space was seen in 18 (8.8%) of the 205 procedures. It occurred only with severely compressed vertebral bodies in benign lesions (16 [9.3%] of 172 cases) (Figs 1 and 2) or with metastatic disease (two [7%] of 27 cases) (Fig 3). Disk space leaks went unnoticed by the patients and did not prevent successful completion of the procedure.
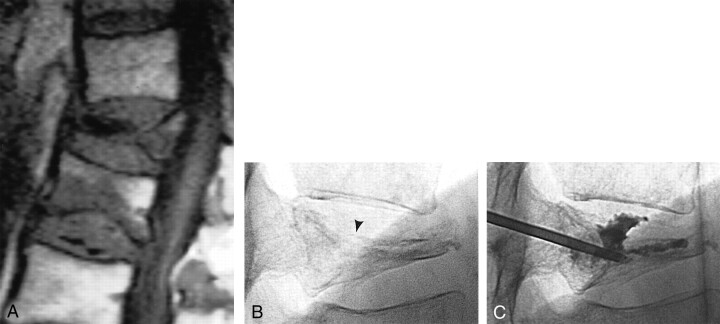
Fig 1.
84-year-old man with osteoporosis and compression fractures at T12 and L1.
A, Sagittal T1-weighted MR image shows a severe T12 compression fracture with minor posterior wall displacement and an inferior endplate fracture at L1 with bone marrow edema.
B, Lateral fluoroscopic view of the T12 vertebral body confirms severe loss of vertebral height and documents a superior endplate fracture (arrowhead).
C, Lateral fluoroscopic view of T12 after placement of an 11-gauge needle by means of a right transpedicular approach. Because of the pedicle angulation and the superior endplate fracture, a suboptimal posterior needle tip position had to be accepted. The cement distributed within the vertebral body both anteriorly and posteriorly and crossed the superior endplate fracture plane to reach the disk space.
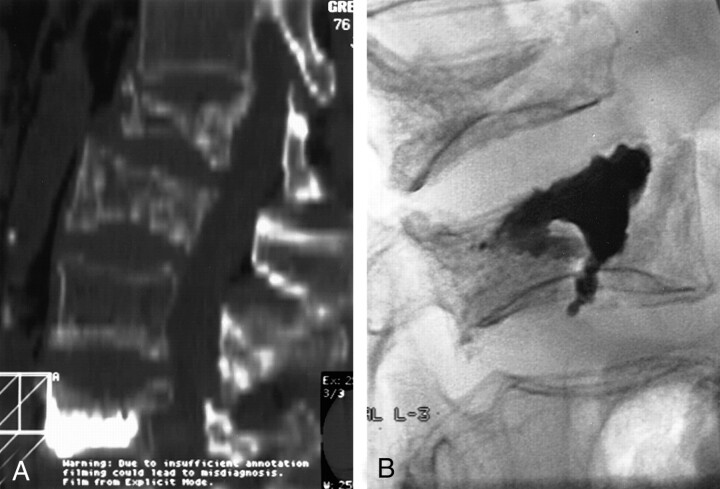
Fig 2.
76-year-old woman with osteoporosis and multiple compression fractures.
A, Two-dimensional CT reconstruction in the sagittal plane shows L2 and L3 compression fractures, as well as a previously treated L5 fracture. Note the inferior and superior endplate fractures at L3.
B, Lateral fluoroscopic view of L3 after PV. Note the extension of the cement into both the inferior and superior endplate fracture lines. The L2 fracture was treated during the same session.
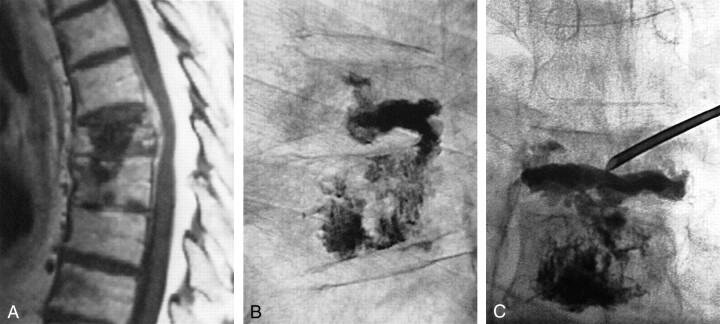
Fig 3.
81-year-old man with T7 and T8 biopsy-proved metastases from unknown primary carcinoma.
A, Sagittal T1-weighted MR image shows a T7 metastatic lesion extending into the epidural space and into the T8 vertebral body through the T7–T8 disk space.
B, Lateral fluoroscopic view after T8 PV. The cement injected at T8 followed the extension pathway of the lesion and reached the inferior aspect of T7 via the T7–T8 disk space.
C, Anteroposterior fluoroscopic view shows that the cement distributed only along the inferior aspect of T7. To complete the treatment, a second needle was placed in the T7 vertebral body by means of a right transpedicular approach. Since both pedicles were involved by the metastatic process and not identifiable fluoroscopically, the medial wall of the pedicles located above and below were used as osseous landmarks (“virtual pedicle” technique).
Minor passage of cement into perivertebral veins (not including the epidural venous plexus) was recorded in 34 cases (16.6%): 29 in benign lesions (16.9%) (Fig 4), and five in metastatic lesions (18%). No disk space or perivertebral leaks were noted in the cases of hemangioma (n = 2) and multiple myeloma (n = 4). When a leak occurred and the degree of cement filling was considered insufficient, the cement injection was resumed after a 15–20-second delay without changing the needle tip position. Cement extravasations remained asymptomatic in all patients. In two cases of severe osteoporotic compression fractures (1.0%), a small amount of cement reached the perivertebral soft tissues by a lateral wall bone dehiscence. One of these two patients reported complete relief from her back pain but complained of a newly occurring radicular pain on the side of the extravasation after the procedure. Although PV was performed at T12, the radicular pain followed a T8 distribution. Postprocedural CT confirmed the anterolateral location of the cement leak, at a distance from the neural structures. The radicular pain disappeared after a right T8 nerve block was performed. Cement leak toward the neural foramina or spinal canal never occurred in our series. In one case (0.5%), a minute amount of cement reached the inferior vena cava. Although this event remained totally asymptomatic and no cement could be detected fluoroscopically over the lung region, the deposition of a minute amount of PMMA in the pulmonary circulation was not excluded, and the procedure was stopped. The same pedicle was reaccessed 7 weeks later and the fracture successfully treated without further extravasation.
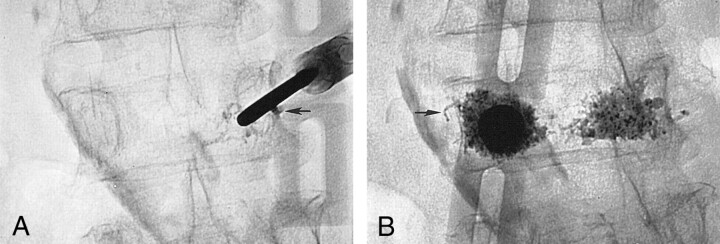
Fig 4.
63-year-old woman with osteoporosis and a compression fracture at L3 associated with a superior endplate Schmorl’s node.
A, Fluoroscopic view shows the 11-gauge needle that was placed by means of a right transpedicular approach. The presence of the Schmorl’s node precluded a median position of the needle tip. During PMMA injection, a leak (arrow) in the perivertebral venous system was immediately observed. The injection was resumed after a 20-second delay and completed uneventfully.
B, Fluoroscopic view shows a second needle that was placed by means of a left transpedicular approach. Again, note a small leak (arrow) in the perivertebral venous system. The Schmorl’s node is outlined by the cement deposition.
One patient reported a focal patch of dysesthesia appearing around the puncture site approximately 48 hours after the procedure; this progressively resolved over the next 4 weeks. No other signs or symptoms, in particular no evidence of infection, were noted. This was interpreted as resulting from a subcutaneous nerve branch lesion that had occurred during needle placement.
Another patient had a transient episode of arterial hypotension during the cement injection, without evidence of extravertebral PMMA leak. The blood pressure went back to baseline values after approximately 5 minutes without specific treatment other than intravenous fluid perfusion, and the procedure could be successfully completed.
Discussion
The principal complication of PV is leakage of PMMA into the vertebral venous system, potentially resulting in radiculopathy, spinal cord compression, and/or pulmonary embolism (1, 3, 6–8). Vertebral venography performed before PV has proved useful in the identification of vertebral venous outflow pathways, indicating potential leakage sites during cement injection (7). However, the usefulness of venography, either routinely or for selected cases only, is debated (6). Venography has been advocated before PV for any type of spine abnormalities (ie, lytic lesions, hemangiomas, and osteoporotic compression fractures), while some authors use it only before treating vertebral hemangiomas (1). Vertebral venography itself presents some drawbacks, such as pooling of the contrast agent in the area to be treated and potential allergic reactions to iodine-based contrast agents. Although the latter might be considered rare, one of our patients suffered an anaphylactic reaction during prevertebroplasty venography performed previously at another institution. We report our series of 205 consecutive vertebral lesions treated with PV without prior vertebral venogram.
Anatomic factors that might increase the risk of cement leakage, such as severe collapse of the vertebral body and posterior wall retropulsion, were not considered exclusion criteria in our series. We observed minor intradiskal leaks in 18 cases (8.8%), perivertebral soft tissue leaks in two cases (1.0%), and perivertebral venous leakage in 34 cases (16.6%), including one case in which a minute amount of cement reached the inferior vena cava (0.5%). These numbers probably underestimate the true frequency of minute PMMA leakage into the perivertebral venous system, since a minor leak may go undetected and/or may not be recorded as such in the procedural report. We consider minor leaks in the perivertebral venous system as clinically insignificant. All the leaks observed in our practice remained totally asymptomatic.
In our series, three minor complications (1.5%) and no major complications were experienced. All the minor complications were transient. One patient had a patch of dysesthesia at the puncture site that spontaneously resolved over a 4-week period. This was interpreted as a subcutaneous nerve lesion produced by the passage of the vertebroplasty needle into the soft tissues of the back. One patient had a transient episode of arterial hypotension during the PMMA injection, without evidence of cement leakage. Although there was no definite explanation for this episode, allergic or toxic reaction to PMMA was considered. One of the patients with a minor perivertebral soft tissue extravasation complained of a newly occurring radicular pain at T8 after a PV performed at T12. In that case, the postprocedural CT scan showed the cement leak to be at a distance from neural structures and confirmed the absence of cement at other vertebral levels, including T8. This radicular pain, which resolved completely after a right T8 nerve block, was in our opinion not related to the cement injection, but to the prone position of the patient during the procedure. None of the three minor complications were therefore caused by PMMA extravasation.
Cement leakage into the spinal canal or neural foramina never occurred in our series, and no neurologic deficits appeared during or after the procedures.
Our experience indicates that careful fluoroscopic monitoring of the PMMA injection allows performance of PV safely without prior venography. When the cement reaches the posterior fourth of the vertebral body, or if a leak is detected, the injection must be interrupted. In our practice, the injection usually was resumed after a short period of time (15–20 seconds), and the procedure was completed without further leakage. The risk of leakage is considered to be greater during the treatment of lytic metastatic lesions or vascular tumors (7, 9). Although we did not observe a major difference in the rate of extraosseous PMMA leakage between benign and malignant lesions in our series, it can be noted that venous leaks occurred slightly more frequently in cases of metastatic lesions and disk space leaks in cases of benign fractures. There was, on the other hand, a clear relation between disk space leaks and severely compressed vertebral bodies with fractured endplate(s). In this type of situation, we believe that the passage of a moderate amount of PMMA through the fracture plane has a beneficial effect on fracture consolidation and should not interrupt the procedure. It should be remembered, however, that a large amount of cement in the disk space, in particular when it assumes a round “ball-like” shape rather than a flat “pancake-like” distribution, may increase the risk of endplate fracture at the adjacent vertebral level (Avery Evans, personal communication, 2000). In summary, although the number of lytic or vascular lesions treated in our series (27 metastases, two hemangiomas, four multiple myelomas) is relatively small, both the benign and malignant lesions were treated safely without venographic evaluation.
Conclusion
Our experience indicates that PV can be performed safely without pretreatment vertebral venography. In our opinion, the most critical element in ensuring a safe bone cement injection lies in the visualization of the PMMA deposition and, therefore, is linked to adequate radiologic equipment and physician training.
References
- 1.Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications, and results. Radiol Clin North Am 1998;36:533–546 [DOI] [PubMed] [Google Scholar]
- 2.Galibert P, Deramond H, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty [in French]. Neurochirurgie 1987;33:166–168 [PubMed] [Google Scholar]
- 3.Martin JB, Sugiu K, San Millan D, Murphy KJ, Piotin M, Rufenacht DA. Vertebroplasty: clinical experience and follow-up results. Bone 1999;25(suppl):11–15 [DOI] [PubMed] [Google Scholar]
- 4.Murphy KJ, Deramond H. Percutaneous vertebroplasty in benign and malignant disease. Neuroimaging Clin N Am 2000;10:535–545 [PubMed] [Google Scholar]
- 5.Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol 1994;15:83–86 [PMC free article] [PubMed] [Google Scholar]
- 6.Mathis JM, Barr JD, Belkoff SM, Barr MS, Jensen ME, Deramond H. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol 2001;22:373–381 [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JB, Sugiu K, Delavelle J, et al. Vertebroplasty: the interest of pretreatment contrast material injection. Presented at the 37th annual meeting of the American Society of Neuroradiology, San Diego, 1999.
- 8.Padovani B, Kasriel O, Brunner P, Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:375–377 [PMC free article] [PubMed] [Google Scholar]
- 9.Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology 1996;200:525–530 [DOI] [PubMed] [Google Scholar]