Abstract
Summary: This report describes 2-week and 20-month histopathologic findings in small aneurysms embolized with platinum coils. Electron microscopy showed the presence of endothelial cells encroaching on the platinum coils at the orifice of the aneurysm in both cases. We confirm that endothelial growth can be induced as early as 2 weeks after embolization of small human aneurysms with platinum coils, similar to previous observations in animal models and human cases.
The recent advances of endovascular techniques using catheter-delivered platinum coils in the treatment of intracranial aneurysms have dramatically increased their clinical use. Little is known, however, regarding the exact mechanism of aneurysmal occlusion and biologic responses at the neck of the aneurysm that seal it off from parent circulation. Several histologic studies have been reported in experimental animal models (1–4), yet similar human studies are very limited (5–12). Recently, we treated two patients by platinum coil packing of their aneurysms. One died as a result of massive cerebellar hemorrhage 20 months after coil treatment of an unruptured aneurysm. The second patient died as a result of acute renal failure 2 weeks after endovascular treatment of a ruptured aneurysm. This report details histopathologic findings in human small aneurysms obliterated with platinum coils.
Case Reports
Case 1
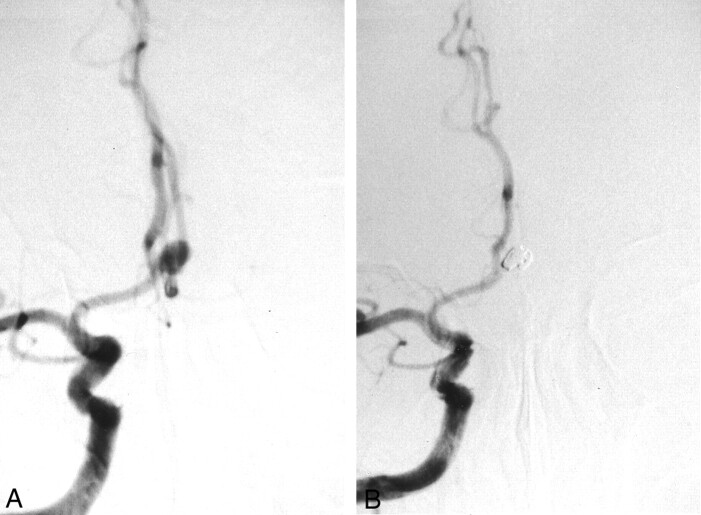
A 65-year-old man was admitted to our hospital for evaluation of a cerebral infarction that was diagnosed at another institution. He presented with mild dementia, dysarthria, and dysesthesia in his right hand. MR imaging revealed multiple lacunar infarctions in both basal ganglia and the brain stem, associated with mild cerebral cortical atrophy. Cerebral angiography showed an incidental anterior communicating artery aneurysm with a 5-mm dome diameter and a 3-mm neck (Fig 1A). No obvious stenosis was observed in any of the major cerebral vessels. Successful obliteration of the aneurysm with a 4 mm × 8 cm and a 3 mm × 6 cm interlocking detachable coil (Target Therapeutics) was achieved without incident (Fig 1B). The patient was treated with antiplatelet agents after endovascular treatment. A 12-month follow-up angiogram revealed complete obliteration of the aneurysm. Unfortunately, the patient died as a result of a massive cerebellar hemorrhage 20 months after the endovascular procedure. An autopsy limited to the brain was performed. The circle of Willis with the embolized aneurysm was carefully harvested for ultrastructural study.
Fig 1.
Images from the case of a 65-year-old man with an unruptured aneurysm (case 1).
A, Pretreatment cerebral angiogram shows the 5-mm anterior communicating artery aneurysm.
B, Post-treatment cerebral angiogram shows embolization by interlocking detachable coils (4 mm × 8 cm and 3 mm × 6 cm).
Gross Pathology.—
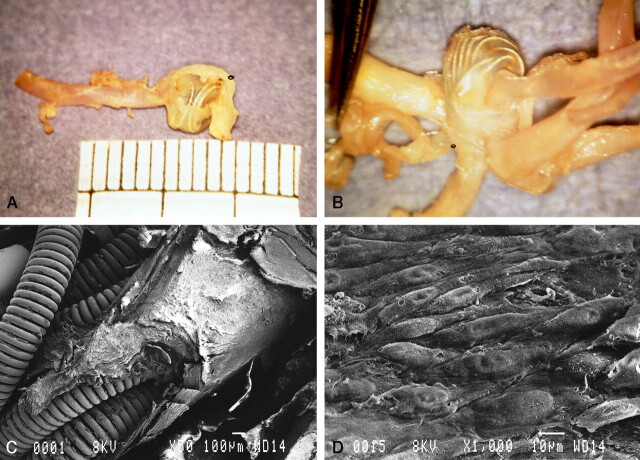
The macroscopic outlook examination of the aneurysm showed the coils to be embedded within its thin wall (Fig 2A). The anterior communicating artery was dissected to examine the orifice of aneurysm, and a window in the dome of the aneurysm was created to examine the coils. A thin transparent membrane covered the entire neck of the aneurysm and bridged over the underlying coils (Fig 2B). A segment of the membrane was removed for transmission electron microscopy studies. The window created in the fundus of the aneurysm enabled us to see that it was filled with white cotton-like fibrous tissue in which the coils were embedded. This fibrous tissue was fixed in formalin for light microscopy examination with hematoxylin and eosin stain. It revealed organized fibrous tissue with little inflammatory cellular reaction. No blood clot was seen in and around the coils within the aneurysm (Fig 3B).
Fig 2.
Gross pathologic findings in case 1.
A, Gross outlook appearance of the thin wall of the aneurysm through which embedded coils are seen.
B, Intraluminal view shows a thin transparent layer of membrane covering the whole orifice of the aneurysm and bridged over the underlying coils.
C, Scanning electron microscopy shows that the coils are covered by thick neointima at the orifice of the aneurysm. Part of the neointima was removed for transmission electron microscopic study (original magnification, ×50; bar = 100 μm).
D, Superficial layer of neointima with a cobblestone appearance (original magnification, ×1000; bar = 10 μm).
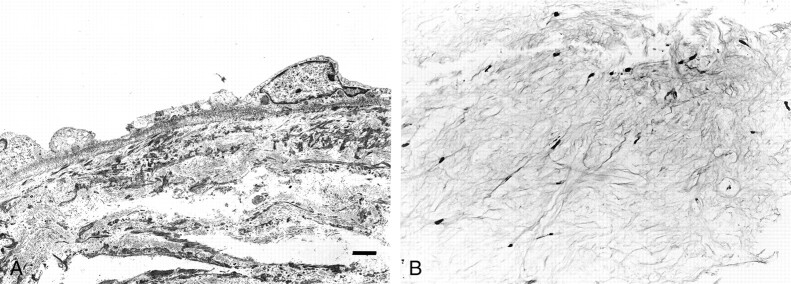
Fig 3.
Transmission electron microscopy and light microscopy (case 1).
A, Basal lamina runs contiguously along the basal endothelial cytoplasmic membrane (original magnification, ×2000; bar = 2 μm).
B, Light microscopy shows cotton-like white fibrous tissue in the dome of the aneurysm (hematoxylin and eosin stain; original magnification, ×74).
Scanning Electron Microscopy.—
The specimen was postfixed in 2.5% gluteraldehyde and was processed in the conventional manner. Although the manipulation of the specimen resulted in the artificial tearing and stripping of the neointima from the underlying coils, the neointima covered by endothelial cells was found at the neck of the aneurysm (Fig 2C). At high magnification, this neointima consisted of thickened layers of fibrous tissue covered by a layer of endothelial cells forming a typical cobblestone pattern (Fig 2D).
Transmission Electron Microscopy.—
A segment of the neointima was removed for transmission electron microscopy studies, which revealed that the neointima consisted of three layers. The most superficial layer was formed by endothelial cells and displayed several characteristic morphologic features: continuous basal lamina, numerous pinocytotic vesicles, and tight junctions (Fig 3A). Smooth muscle cells and collagen fibers formed the other two layers of the neointima.
Case 2
A 62-year-old man was admitted to our hospital with subarachnoid hemorrhage documented on a CT scan (Fisher group 3). Clinically, the patient’s condition was grade III on the Hunt and Hess scale. An angiogram showed a 4-mm aneurysm arising from the left superior cerebellar artery and basilar artery bifurcation distal to the superior cerebellar artery (Fig 4A). Based on the patient’s poor general condition (especially respiratory failure due to neurogenic pulmonary edema), coil embolization was selected for treatment of the ruptured aneurysm.
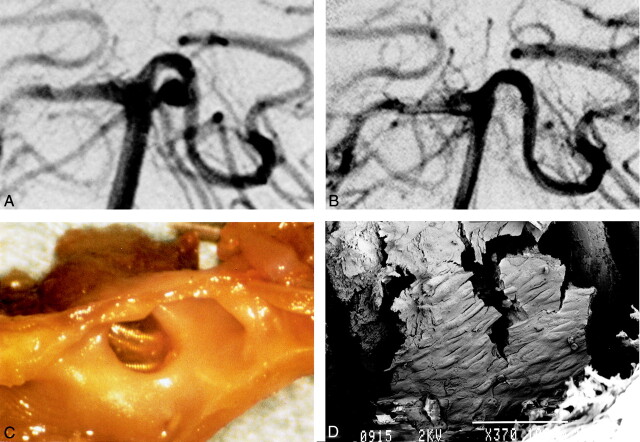
Fig 4.
Images from the case of a 62-year-old man with subarachnoid hemorrhage (case 2).
A, Right pre-embolization vertebral angiography shows 4-mm left basilar artery-superior cerebellar artery aneurysm.
B, After embolization by GDC (3 mm × 4 cm and 2 mm × 4 cm), almost total obliteration was achieved.
C, Gross examination shows thin membrane on coils at orifice of aneurysm. The thin fragile covering bridges the whole space across the coils at the orifice.
D, Scanning electron microscopy shows neointima partially covering the coils (GDC-10 soft). A cobblestone pattern can be seen (original magnification, ×370; bar = 100 μm).
A microcatheter was positioned in the aneurysm, and two GDC (3 mm × 4 cm and 2 mm × 4 cm) were placed in the aneurysmal lumen without systemic heparinization. Post-treatment angiography showed almost complete obliteration of the aneurysm (Fig 4B). Although the endovascular treatment was successful, the patient developed acute renal failure and died 2 weeks after the embolization procedure. An autopsy was performed, and the embolized aneurysm was evaluated with scanning electron microscopy.
Gross Pathology.—
Gross examination of the aneurysm showed its transparent wall and packed coils embedded in clot. The basilar artery was dissected to visualize the neck of the aneurysm. A very thin membrane was visible over the coils, and blood clot in the lumen was observed (Fig 4C). Because of the fragility of the specimen, we decided to limit its evaluation to scanning electron microscopy.
Scanning Electron Microscopy.—
The specimen was fixed and was processed according to standard protocols. Even with artificial tissue tearing, neointima, which was relatively thin, was detected at the neck of the aneurysm, covering the coils. At higher magnification, elongated cells arranged in a cobblestone pattern were visible on the surface of the neointima, contiguous with the adjacent parent vessel, indicating that the endothelial cell layer developed over the coils (Fig 4D).
Discussion
Recent developments in the endovascular treatment of aneurysms, including the introduction of detachable coils, has made this technique an available alternative to surgical clipping, even though the efficacy of this technique has not yet been sufficiently studied. Recently, Viñuela et al (13) reported a series of 403 patients with ruptured cerebral aneurysms that were treated with GDC. In their series, complete aneurysm occlusion was observed in 70.8% of small aneurysms with small necks, 35% of large aneurysms, and 50% of giant aneurysms. Similar clinical studies using Dacron-coated microcoils have been reported by Casasco et al (14). They achieved complete occlusion in 85% of their cases and subtotal (>90%) occlusion in 15%. However, the long-term outcome of endovascular treatment with coils is still not well known.
Several experimental animal studies documenting the histology of embolized aneurysms have been reported. Mawad et al (1) described 6-month histopathologic changes in canine aneurysms obliterated with GDC. Their studies showed that both completely obliterated and recanalized aneurysms were excluded from the parent artery by an endothelialized layer of connective tissue. The fundus of the aneurysm was completely obliterated by heavy reactive fibrous tissue surrounding the coils. The neointima is composed of three readily identifiable layers: the most superficial layer of endothelial cells, forming a cobblestone pattern; the smooth muscle cell layer; and a layer of collagen fibers. The histologic studies performed on our first patient (case 1) showed very similar histopathologic findings. More recently, Mawad et al (15) confirmed the same results in an 18-month experimental study. They emphasized, however, that not all their histologic data reflected the early changes of coiled aneurysms.
Tenjin et al (2) conducted carotid artery aneurysm experiments in Japanese monkeys. In their study, 19 aneurysms were occluded with GDC and histopathologic studies were obtained at various time intervals. They concluded that the GDC initiated a cellular response within several hours of aneurysm occlusion. Endothelialization was noted at 2 weeks and at 3 months after packing, and remodeling of the aneurysm had progressed to produce a media-like structure in the former aneurysm. Histologic data obtained 2 weeks after treatment (case 2) showed a cobblestone appearance on the coils at the neck of aneurysm. The shape of these fusiform cells and continuation with the adjacent parent artery suggested that neoendothelialization was occurring. The fundus did not contain any organized clot.
Our findings in human cases concur with those of Tenjin et al (2). Other experiments reported by Ruel et al (3) and Spetzger et al (4) showed less chance of endothelialization in 3- to 6-month rabbit models.
Moreover, especially in humans, very little is known regarding the histology of embolized aneurysms and the cellular reaction to coils (5–12). Among reported human cases, only five, including ours, showed endothelial covering at the orifice of the aneurysm. All five cases exclusively involved small neck aneurysms. A more recent informative report presented by Bavinzski et al (12) showed only one endothelial lining among 17 obtained at autopsy or surgery. They concluded that endothelialization of the aneurysm orifice after coil placement can occur, but it seems to be the exception rather than the rule. Considering the neck size of the aneurysms in our cases, we think that small aneurysms or aneurysms with small necks can be successfully obliterated by coils. Compared with larger aneurysms, these small aneurysms have a higher chance to develop neointima over the coils at the neck of the aneurysm. The number of coils present across the neck of the aneurysm may be the key factor to induce endothelial growth, which excludes the lumen of the aneurysm from parent circulation. Even relatively wide neck aneurysms can be successfully treated with coils if dense packing is obtained across the neck.
What are the physical factors that can affect growth of a new intimal layer over the neck of an aneurysm obliterated by coils? Reul et al (3) recently reported that perioperative anticoagulation did not have any influence on the long-term results of coiled aneurysms in their animal model. In general, an intravascular foreign body induces clot formation. The extent of the blood clot depends on the chemical composition, electrical charge, and surface properties of the foreign body as well as the extent of intimal injury (2). Early thrombus formation involves attachment of platelets and leukocytes to an injured vessel surface, and fibrin formation then occurs. Thrombosis of the aneurysm eventually occurs but may happen because a turbulent flow within the fundus activates the clotting cascade. For long-term permanent obliteration of an aneurysm, the blood clot needs to be replaced by fibrous tissue to become organized thrombus. To better understand the histology of embolized aneurysms, we should always take into consideration the role of adjuvant factors such as the coagulation profile of the patient, drugs used in the course of treatment, and duration of the observation period. In the experimental or clinical setting, the modification of coil thrombogenicity can enhance the healing mechanism. Several experimental studies are being conducted to that effect.
Conclusion
The present report shows endothelialization of platinum coils at the orifice of human cerebral aneurysms at 2 weeks and at 20 months after endovascular treatment. This confirms that endothelial growth can be induced as early as 2 weeks after coil embolization in a small human aneurysm. Our findings are in agreement with those of previous similar reports that suggested only small aneurysms could be endothelialized after coil embolization. Among many possible factors that may influence the histologic reaction around coiled aneurysms, dense packing with coils across the neck may be most crucial for successful outcome. Further investigations are required to understand the healing process of coil-embolized aneurysms.
References
- 1.Mawad ME, Mawad JK, Cartwright J Jr, Gokaslan Z. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1995;16:7–13 [PMC free article] [PubMed] [Google Scholar]
- 2.Tenjin H, Fushiki S, Nakahara Y, et al. Effect of Guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke 1995;26:2057–2080 [DOI] [PubMed] [Google Scholar]
- 3.Reul J, Weis J, Spetzger U, Konert T, Fricke C, Thron A. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. AJNR Am J Neuroradiol 1997;18:35–42 [PMC free article] [PubMed] [Google Scholar]
- 4.Spetzger U, Reul J, Weis J, Bertalanffy H, Thron A, Gilsbach J. Microsurgically produced bifurcation aneurysms in a rabbit model for endovascular coil embolization. J Neurosurg 1996;85:488–495 [DOI] [PubMed] [Google Scholar]
- 5.Mizoi K, Yoshimoto T, Takahashi A, Nagamine Y. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery 1996;39:65–69 [DOI] [PubMed] [Google Scholar]
- 6.Otawara Y, Sugawara T, Seki H, Fujimura M, Tomichi N. An autopsy case of a ruptured cerebral aneurysm treated with interlocking detachable coils [in Japanese]. No Shinkei Geka 1997;25:829–833 [PubMed] [Google Scholar]
- 7.Manabe H, Fujita S, Hatayama T, Ohkuma H, Suzuki S, Yagihashi S. Embolization of ruptured cerebral aneurysms with interlocking detachable coils in acute stage. Intervent Neuroradiol 1997;3:49–63 [DOI] [PubMed] [Google Scholar]
- 8.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils: report of two cases with autopsy correlation. J Neurosurg 1996;83:129–132 [DOI] [PubMed] [Google Scholar]
- 9.Stiver SI, Porter PJ, Willinsky RA, Wallace MC. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils: case report and review of the literature. Neurosurgery 1998;43:1203–1208 [DOI] [PubMed] [Google Scholar]
- 10.Koizumi T, Kawano T, Kazekawa K, et al. Histological findings in aneurysm treated with IDC: scanning electron microscopical study [in Japanese]. No Shinkei Geka 1997. :25:1027–1103 [PubMed] [Google Scholar]
- 11.Horowitz MB, Purdy PD, Burns D, Bellotto D. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:688–690 [PMC free article] [PubMed] [Google Scholar]
- 12.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–293 [DOI] [PubMed] [Google Scholar]
- 13.Viñuela F, Duckwiler G, Mawad M, et al. Guglielmi detachable coil embolization in acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–482 [DOI] [PubMed] [Google Scholar]
- 14.Casasco AE, Aymard A, Gobin YP, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg 1993;79:3–10 [DOI] [PubMed] [Google Scholar]
- 15.Mawad ME, Mawad JK, Klucznik RP, Rose JE, Vermani R. Long-term (1-1 1/2 year) histopathological findings in experimental canine aneurysms treated with Guglielmi detachable coils: the presence of new endothelial layer. Intervent Neuroradiol 1997;3(Suppl 1):80 [Google Scholar]