Abstract
SUMMARY: The neuropathologic diagnosis of brain tumors entails the microscopic examination of conventional formalin-fixed paraffin-embedded tissue samples surgically removed from a radiographically defined lesion. A preliminary diagnosis is often rendered with frozen sections, though the final or definitive diagnosis usually requires more elaborate studies. Typically, the tissue is first fixed for a minimum of several hours in 10% neutral buffered formalin, processed through a series of dehydrating and clearing reagents, and embedded in a hardening wax, such as paraffin. The latter enables the tissue to be thinly sliced with a microtome, transferred to a glass slide, and then stained with dyes such as hematoxylin-eosin (H&E) that contrast the different cellular elements. Pathologists rely on visual clues such as pattern recognition when examining the stained tissue with a microscope, much as radiologists rely on gray-scale patterns of densities and intensities on images. Some histologic patterns of cellular architecture are distinctive if not pathognomonic, whereas others are less specific, but nevertheless considerably narrow the differential diagnosis. The precise biologic bases for some of the observed microscopic patterns are poorly understood, though their recognition remains useful nonetheless. Although more advanced methods of tissue examination—such as histochemical and immunohistochemical profiling, genetic analysis, and electron microscopy—have been developed, the microscopic review of H&E–stained material remains a critical component of tumor diagnosis.
One commonly encountered neuropathologic histologic architectural pattern seen within certain tumors is the rosette. The purpose of this report is to review the patterns of rosettes and pseudorosettes in the context of such tumors as medulloblastoma/primitive neuroectodermal tumor (PNET), retinoblastoma, ependymoma, central neurocytoma, and pineocytoma.
What Are Rosettes?
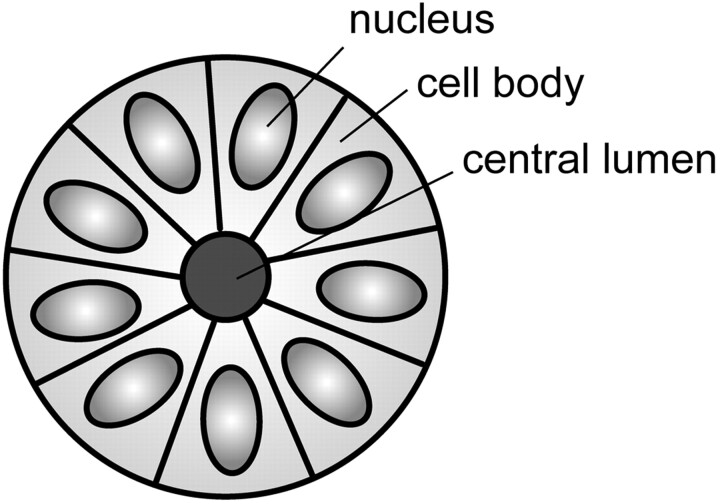
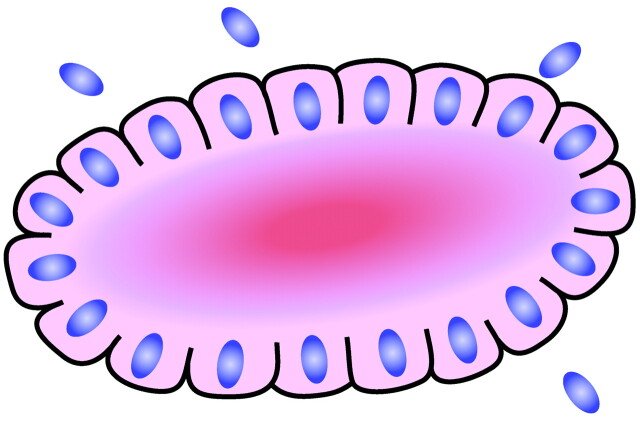
Rosettes consist of a halo or spoke-wheel arrangement of cells surrounding a central core or hub (Fig 1). The central hub may consist of an empty-appearing lumen or a space filled with cytoplasmic processes. The cytoplasm of each of the cells in the rosette is often wedge-shaped with the apex directed toward the central core; the nuclei of the cells participating in the rosette are peripherally positioned and form a ring or halo around the hub.1,2 Named for the flower-like architectural ornament, this pattern resembles the rose windows found in many gothic cathedrals (Fig 2). Rosettes may be considered primary or secondary manifestations of tumor architecture. Primary rosettes form as a characteristic growth pattern of a given tumor type, whereas secondary rosettes result from the influence of external factors on tumor growth. For example, in the latter instance, regressive cell swelling may centripetally displace the cytoplasm as the nucleus is squeezed to the periphery.2 Although the presence of primary rosettes may suggest a given diagnosis, usually this finding alone is not considered absolutely pathognomic for one specific tumor type. Ambiguous terminology and descriptions in the past have added to confusion of the usage of the term; however, several types of primary rosettes are generally recognized in the pathology literature.
Fig 1.
Diagram of a typical rosette demonstrating a halo of cells surrounding a central lumen. Adapted from Ellison et al (2004)34 with permission.
Fig 2.

Example of a cathedral rose window. Photo courtesy of the Cathedral Basilica of St. Louis, used with permission.
Homer Wright Rosette
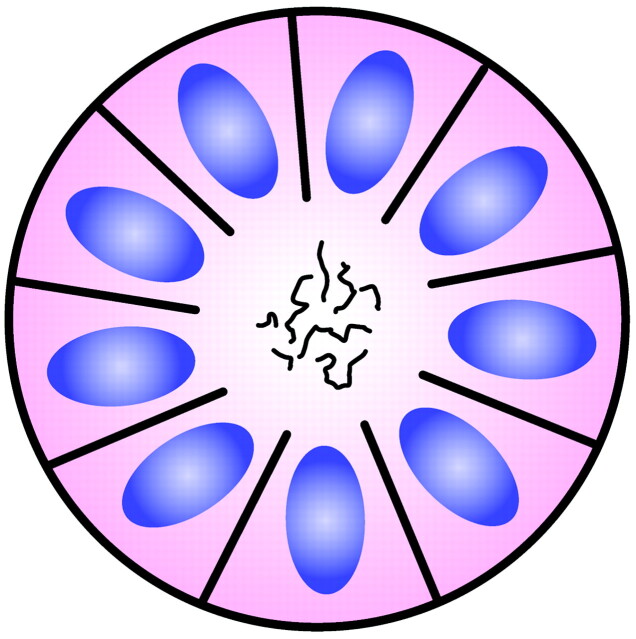
The Homer Wright rosette is named for James Homer Wright (1869–1928), the first director of the Massachusetts General Hospital Pathology Laboratory and developer of the Wright stain.3 He recognized a group of adrenal and sympathetic nervous system tumors, which became known as neuroblastomas, and described within these lesions characteristic ball-like arrangements of cells that enclosed meshworks of fibers (Fig 3). These fibers did not stain like those associated with neuroglia, and he postulated that they represented primitive neuronal processes resembling those in the developing sympathetic nervous system.4
Fig 3.
Diagram of Homer Wright rosette. A halo of cells surrounds a central hub that contains a meshwork of fibers. Adapted from Ellison et al (2004)34 with permission.
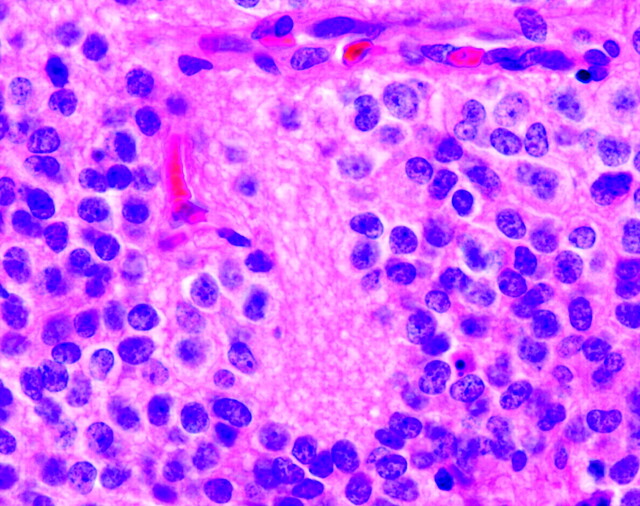
The typical Homer Wright rosette with its central lumen or hub filled with fiber-like processes can also be found in medulloblastomas and histologically similar tumors occurring outside of the cerebellum, designated PNETs (Fig 4). Although the cellular mechanisms responsible for the formation of rosettes within medulloblastomas and the significance of these rosettes are not fully understood, most investigators believe that their presence indicates neuronal differentiation.5,6 The delicate fibrillary material found within the central lumen of the Homer Wright rosette is composed of neuropil, which contains primitive neuronal processes or neurites.
Fig 4.
Photomicrograph from a PNET demonstrating multiple Homer Wright rosettes. The halo-like cluster of cells in each rosette surrounds a central area of fiber-rich neuropil (H&E; original magnification 400×).
Fig 6.
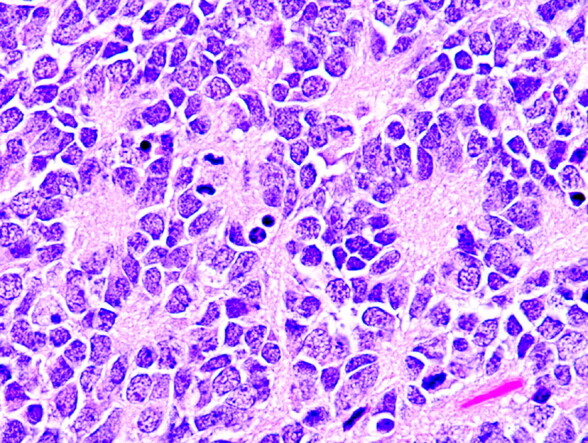
Photomicrograph from a retinoblastoma showing multiple Flexner-Wintersteiner rosettes. The halo-like cluster of cells in each rosette surrounds a nearly empty appearing central lumen containing fine cytoplasmic processes (H&E; original magnification 400×). Photomicrograph generously donated by Dr. Morton Smith, Ophthalmic Pathology, Washington University, St. Louis.
Although most medulloblastomas are considered primitive, variable degrees of differentiation are nearly always encountered at the microscopic, immunohistochemical, and ultrastructural level.5–7 Moreover, some medulloblastoma cells in specimens and cultures have demonstrated microtubules, attenuated-core neurosecretory and clear-centered synaptic vesicles, and synaptic junctions, all of which are characteristic of neuroblasts, as well as mature neurons.8,9 Regardless of the medulloblastoma subtype, immunohistochemical evidence of neuronal differentiation is found in nearly all cases with neuronal markers such as synaptophysin, neuron-specific enolase, and neurofilament protein.5,10 Some medulloblastomas may also display other forms of differentiation as demonstrated by the presence of the astrocytic marker glial fibrillary acidic protein.8,11 Skeletal muscle and melanocytic differentiation are considerably less common and define the medullomyoblastoma and melanotic medulloblastoma variants, respectively.
Although the significance of the rosette in medulloblastomas would seem to reflect neuronal differentiation in portions of an otherwise primitive tumor, the mechanism for the formation of the characteristic rosette pattern itself is not completely understood. The cell populations exhibiting neuronal differentiation are believed to secrete surface glycoproteins and glycolipids, which mediate cell-to-cell recognition and adhesion.7,9,12–17 One hypothesis is that these sticky cell surface markers cause the developing cell bodies to cluster or aggregate and their primitive neurites to tangle. As the cells grow, the neurite tangle remains centrally located and the cell bodies are squeezed to the periphery, thus explaining the rosette pattern.5
Although the identification of Homer Wright rosettes in a posterior fossa tumor is nearly pathognomonic of the diagnosis of medulloblastoma, the rosettes are encountered in only a third of these tumors. Moreover, Homer Wright rosettes may be found in other tumors such as supratentorial PNETs and pineoblastomas.18 For tumors composed of nothing more than primitive-appearing cells, the definitive diagnosis relies on additional techniques such as special stains and immunohistochemical analysis.
Flexner-Wintersteiner Rosette
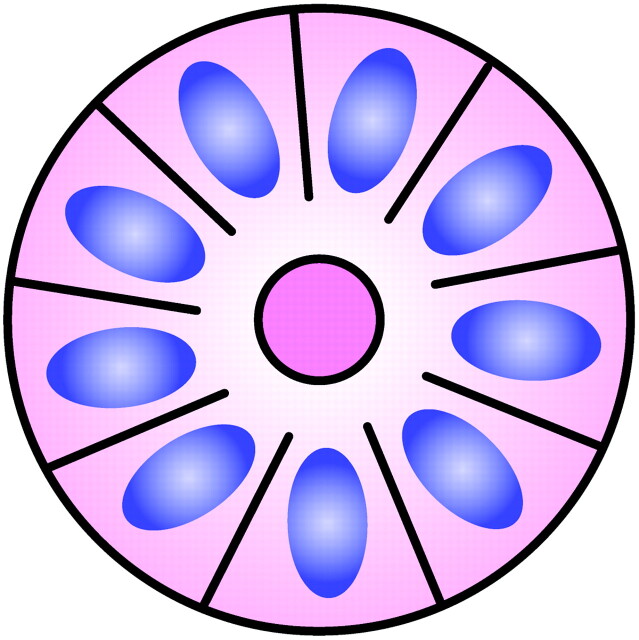
Simon Flexner (1863–1946), whose brother Abraham Flexner wrote the famous report on medical education reform that bears his name, was an innovative pathologist in his own right. He was named the head of the pathology department and later director of the then newly founded Rockefeller Institute. In addition to describing the bacillus responsible for dysentery, Flexner noted distinctive clusters of cells in an infantile ocular tumor that he termed retinoepithelioma.19–21 Several years later, in 1897, Austrian ophthalmologist Hugo Wintersteiner (1865–1946) agreed with Flexner’s observations and noted that the cell clusters resembled rods and cones.22 These cellular aggregates were termed rosettes and were subsequently recognized as important features of retinoblastomas. The tumor cells that form the Flexner-Wintersteiner rosette circumscribe a central lumen that contains small cytoplasmic extensions of the encircling cells; however, unlike the center of the Homer Wright rosette, the central lumen does not contain the fiber-rich neuropil (Fig 5). Like the Homer Wright rosette, the Flexner-Wintersteiner rosette signifies a specific form of tumor differentiation.23–26 This contention is supported by electron microscopy where the tumor cells forming the Flexner-Wintersteiner rosette have ultrastructural features of primitive photoreceptor cells.27 In addition, special staining properties of the rosette lumen resemble those seen in rods and cones.28 Although this type of rosette is particularly characteristic of retinoblastomas (Fig 6), it may also be seen in pineoblastomas and medulloepitheliomas, where it is similarly thought to represent retinal differentiation.18,23
Fig 5.
Diagram of Flexner-Wintersteiner rosette. A halo of cells surrounds a largely empty central hub. Small cytoplasmic extensions from the cells project into the lumen. Adapted from Ellison et al (2004)34 with permission.
True Ependymal Rosette
Another rosette pattern may be demonstrated in a subset of ependymomas and has been termed the “true ependymal rosette”6,29,30 (Fig 7). In contrast to the Homer Wright and the Flexner-Wintersteiner rosettes, the empty-appearing lumen of the true ependymal rosette resembles a tubule lumen and contains no fiber-rich neuropil or central cytoplasmic projections. These tubule-like structures, as well as more elongated versions known as ependymal canals, may represent an attempt by the tumor cells to recapitulate the formation of ventricles with ependymal linings.6,18 This rosette provides strong evidence of ependymal differentiation at the light microscopic level.29 Unfortunately, true ependymal rosettes and canals are found in only a minority of the most well-differentiated ependymomas and most commonly in infratentorial examples29 (Fig 8). Ironically, a similar structure known as the ependymoblastic rosette is encountered in a rare form of PNET known as ependymoblastoma, where they are thought to denote ependymal differentiation in an otherwise highly primitive malignancy.
Fig 7.
Diagram of true ependymal rosette. A halo of cells surrounds an empty lumen. Adapted from Ellison et al (2004)34 with permission.
Fig 8.
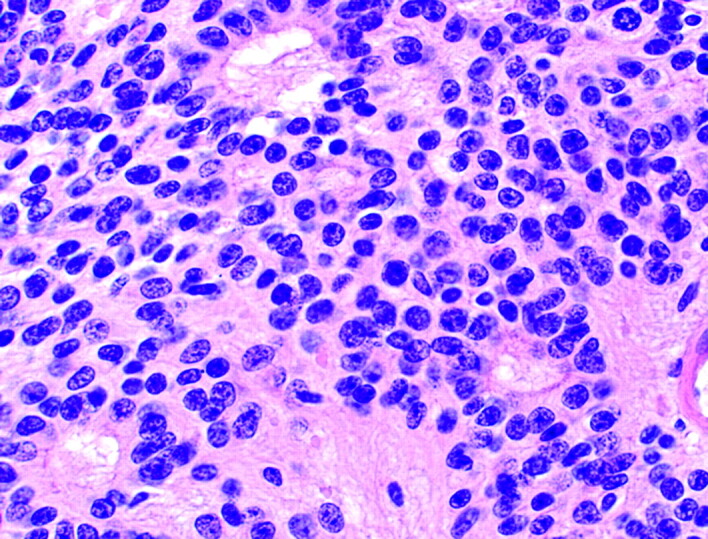
Photomicrograph from an ependymoma showing several true ependymal rosettes. The halo-like cluster of cells in each rosette surrounds an empty central lumen (H&E; original magnification 400×).
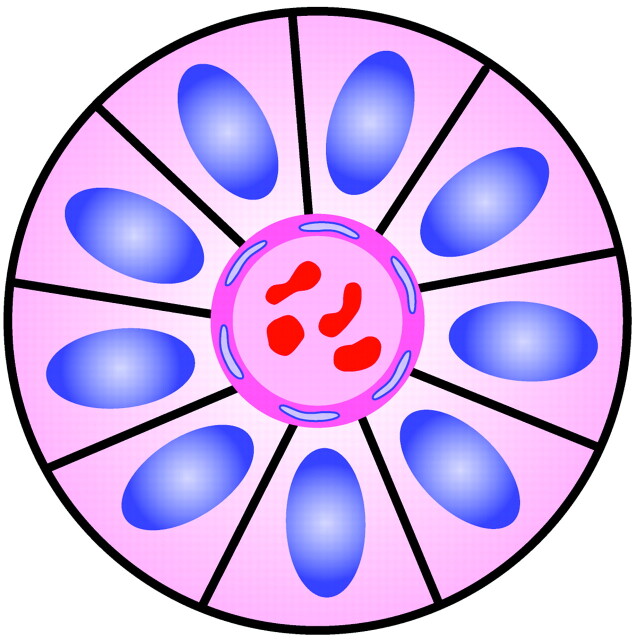
Perivascular Pseudorosette
A fourth type of rosette is the perivascular pseudorosette. In this pattern, a spoke-wheel arrangement of cells with tapered cellular processes radiates around a wall of a centrally placed vessel2,7,18 (Fig 9). The modifier “pseudo” differentiates this pattern from the Homer Wright and Flexner-Wintersteiner rosettes, perhaps because the central structure is not actually formed by the tumor itself, but instead represents a native, non-neoplastic element. Also, some early investigators argued about the definition of a central lumen, choosing “pseudo” to indicate that the hub was not a true lumen but contained structures.31 Nevertheless, this pattern remains extremely diagnostically useful and the modifier unnecessarily leads to confusion. Perivascular pseudorosettes are encountered in most ependymomas regardless of grade or variant. As such, they are significantly more sensitive for the diagnosis of ependymomas than true ependymal rosettes18,30,32 (Fig 10). Unfortunately, perivascular pseudorosettes are also less specific in that they are also encountered in medulloblastomas, PNETs, central neurocytomas, and less often in glioblastomas, and a rare pediatric tumor, monomorphous pilomyxoid astrocytomas.
Fig 9.
Diagram of perivascular pseudorosette. A halo of cells surrounds a blood vessel. Adapted from Ellison et al (2004)34 with permission.
Fig 10.
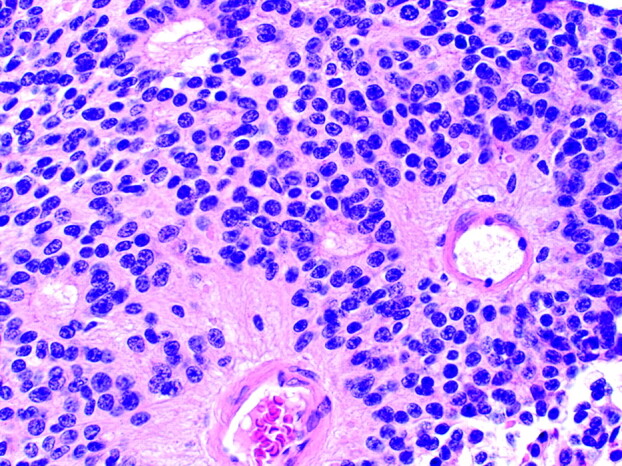
Photomicrograph from an ependymoma showing 2 prominent perivascular pseudorosettes. The halo-like cluster of cells in each rosette surrounds a blood vessel. Note the several smaller true ependymal rosettes (H&E; original magnification 200×).
The reason for the tendency of some ependymoma cells to arrange themselves around blood vessels is poorly understood. Depending upon their location, ependymal cells may display 2 cell poles. A luminal pole projects to the ependymal lining of a ventricle and a “submesenchymal pole” projects toward the surface of the brain demonstrating glial processes and peripherally situated footplates.33 Frieda and Pollak conceptualize the architecture of ependymomas as a primitive neural tube turned inside out with the submesenchymal poles converging toward a central vessel, thus forming a pseudorosette rather than projecting centrifugally toward the pia.33 The blood vessels of the pseudorosette are often fibrotic and hyalinized.32
Pineocytomatous and Neurocytic Rosettes
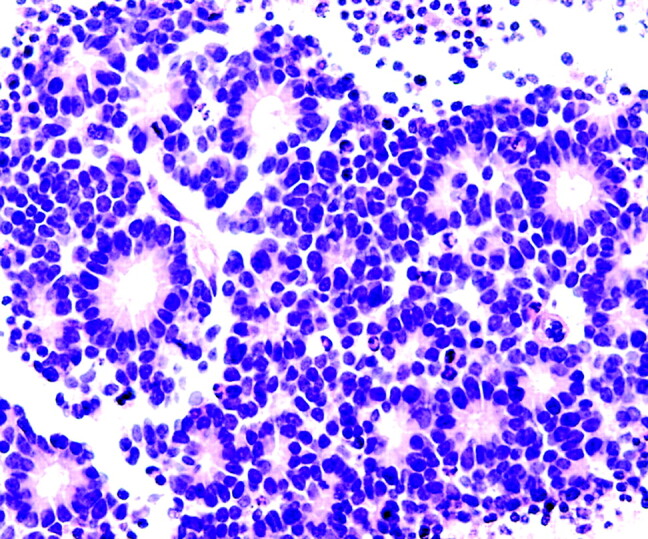
Pineocytomas and central neurocytomas represent well-differentiated neuronal neoplasms with small rounded nuclei, analogous to those normally encountered in the internal granular layer of the cerebellum or the dentate fascia of the hippocampus. Although they likely originate from slightly different precursors, the histologic features of these 2 tumors are virtually identical, including their tendency to form neuropil-rich rosettes, referred to as pineocytomatous rosettes in pineocytomas and neurocytic rosettes in central neurocytoma (Fig 11). Both are quite similar to the Homer Wright rosette, but they are generally larger and more irregular in contour34 (Fig 12). As in the other types of rosettes discussed, the presence of pineocytomatous or neurocytic rosettes is generally thought to reflect differentiation of the tumor, in this case neuronal.35,36 The cells of the pineocytomatous and neurocytic rosettes are also considered to be much more differentiated than the cells forming Homer Wright rosettes in that the nuclei are slightly larger, more rounded, much less mitotically active, and paler or less hyperchromatic.37 In rare cases, these rosettes may aggregate in a sheet of back-to-back clusters resembling field stone pavement.
Fig 11.
Diagram of neurocytic rosette. This rosette is similar to the Homer Wright rosette, but the central fiber-rich neuropil island is larger and more irregular. Adapted from Ellison et al (2004)34 with permission.
Fig 12.
Photomicrograph from a central neurocytoma containing an irregularly shaped neurocytic rosette with central neuropil (H&E; original magnification 400×).
Conclusion
In summary, rosettes and pseudorosettes represent a histologic architectural pattern seen within specific nervous system tumors (Table). The rosette pattern consists of a halo or spoke-wheel arrangement of cells surrounding a central core or hub which may be empty or contain fibers, cytoplasmic processes, or a blood vessel. Although the significance of the presence of rosettes is not always understood, most authorities agree that they represent various forms of tumor differentiation. The presence of rosettes is rarely if ever pathognomic of a specific tumor, though identification of rosettes is often helpful in the histologic diagnosis of medulloblastoma/PNET, retinoblastoma, ependymoma, central neurocytoma, and pineocytoma.
Summary of rosette patterns and associated tumors
| Rosette Type | Associated Tumors |
|---|---|
| Homer Wright rosette | Neuroblastoma, medulloblastoma, primitive neuroectodermal tumor, pineoblastoma |
| Flexner-Wintersteiner rosette | Retinoblastoma, pineoblastoma, medulloepithelioma |
| True ependymal rosette | Ependymoma |
| Perivascular pseudorosette | Ependymoma, medulloblastoma, primitive neuroectodermal tumor, central neurocytoma, glioblastoma, monomorphous pilomyxoid astrocytomas |
| Pineocytomatous rosette | Pineocytoma |
| Neurocytic rosette | Central neurocytoma |
Note: In this issue of the journal a new feature entitled “Special Report” appears. This series of short pathologic descriptions will be written by Jay Wippold and associates at the Washington University School of Medicine. The first of the series deals with rosettes and pseudorosettes and will be followed quarterly by other topics of interest, namely Rosenthal fibers; palisading/pseudopalisading; Alzheimer plaques and tangles; and fluorescence in situ hybridization (FISH).
Robert M. Quencer
Footnotes
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the United States Department of Defense.
References
- 1.Ellison D, Love S, Chimelli L, et al. Pathologic reactions in the CNS. In: Neuropathology: a reference text of CNS pathology. Edinburgh: Mosby,2004;3–25
- 2.Zulch KJ. Brain tumors: their biology and pathology. 3rd ed. Berlin:Springer-Verlag;1986. :118–34
- 3.Lee RE, Young RH, Castleman B. James Homer Wright: a biography of the enigmatic creator of the Wright stain on the occasion of its centennial. Am J Surg Pathol 2002;26:88–96 [DOI] [PubMed] [Google Scholar]
- 4.Wright JH. Neurocytoma or neuroblastoma, a kind of tumor not generally recognized. J Exp Med 1910;12:556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsetos CD, Liu HM, Zacks SI. Immunohistochemical and ultrastructural observations on Homer Wright (neuroblastic) rosettes and the “pale islands” of human cerebellar medulloblastomas. Hum Pathol 1988;19:1219–27 [DOI] [PubMed] [Google Scholar]
- 6.Rubinstein LJ. The cerebellar medulloblastoma: its origin, differentiation, morphological variants, and biological behavior. In: Vinken PJ, Bruyn GW, eds. Handbook of clinical neurology.Vol 18. Amsterdam: Elsevier ;1975. :167–93 [Google Scholar]
- 7.Liu HM, Bailey OT. Neuropathology. In: Amador LV, ed. Brain tumors in the young. Springfield, Ill: Charles C. Thomas;1983. :361–456
- 8.Herman MM, Rubinstein LJ. Divergent glial and neuronal differentiation in a cerebellar medulloblastoma in an organ culture system: in vitro occurrence of synaptic ribbons. Acta Neuropathol 1984;65:10–24 [DOI] [PubMed] [Google Scholar]
- 9.Moss TH. Evidence for differentiation in medulloblastomas appearing primitive on light microscopy: an ultrastructural study. Histopathology 1983;7:919–30 [DOI] [PubMed] [Google Scholar]
- 10.Burger PC, Grahmann FC, Bliestle A, et al. Differentiation in the medulloblastoma. Acta Neuropathol (Berlin)1987;73:115–23 [DOI] [PubMed] [Google Scholar]
- 11.Barnard RO, Pambakian H. Astrocytic differentiation in medulloblastoma. J Neurol Neurosurg Psychiatry 1980;43:1041–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai J. Studies on the mechanism determining the course of nerve fibers in tissue culture. Zeitschr Zellforschung 1960;52:427–49 [DOI] [PubMed] [Google Scholar]
- 13.Pannese E. The histogenesis of the spinal ganglia. Adv Anat Embryol Cell Biol 1974;47:7–16 [DOI] [PubMed] [Google Scholar]
- 14.Willis RA. The nervous system and sense organs. In: The borderland of embryology and pathology. London: Butterworths;1962. :121–23
- 15.Pfenninger KL, Rees RP. From the growth cone to the synapse. In: Barondes SH, ed. Neuronal recognition. New York: Plenum;1976. :131–78
- 16.Moscona AA. Cell recognition in embryonic morphogenesis and the problem of neuronal specificities. In: Barondes SH, ed. Neuronal recognition. New York: Plenum;1976. :205–26
- 17.Gilula NB. Gap junctions and cell contacts. In: Karkinen-Jaaskelainen M, Saxen L, Weiss L, eds. Cell interaction in differentiation. New York: Academic Press;1977. :325–38
- 18.Smith TW, Folkerth RD, Poirer J, et al. Tumors of the nervous system. In: Gray F, De Girolami U, Poirer J, eds. Escourolle and Poirer manual of basic neuropathology. Philadelphia: Butterworth Heinemann;2004;21–56
- 19.Flexner S. A peculiar glioma (neuroepithelioma?) of the retina. Johns Hopkins Hosp Bull 1891;2:115–19 [Google Scholar]
- 20.Schatski SC. Simon Flexner. AJR Am J Roentgenol 1997;169:1395–96 [DOI] [PubMed] [Google Scholar]
- 21.Bendiner E. Simon Flexner: his “rock” was for the ages. Hosp Pract 1988;23:213–66 [DOI] [PubMed] [Google Scholar]
- 22.Wintersteiner H. Die zellen der geschwulst. In: Das neuroepithelioma retinae: Eine anatomische und klinishe studie. Leipzig: Franz Deuticke;1897. :12–16
- 23.McLean IW, Burnier MN, Zimmerman LE, et al. Tumors of the retina. In: Atlas of tumor pathology: tumors of the eye and ocular adnexa. Washington, DC: Armed Forces Institute of Pathology;1994. :97–154
- 24.Donoso LA, Shields CL, Lee EY-H. Immunohistochemistry of retinoblastoma. Ophthal Paediatr Genet 1989;10:3–32 [DOI] [PubMed] [Google Scholar]
- 25.Vrabec T, Arbizo V, Adamus G, et al. Rod cell-specific antigens in retinoblastoma. Arch Ophthalmol 1989;107:1061–63 [DOI] [PubMed] [Google Scholar]
- 26.Kivela T. Glycoconjugates in retinoblastoma: a lectin histochemical study of ten formalin-fixed and paraffin embedded tumours. Virchows Arch A 1987;410:471–79 [PubMed] [Google Scholar]
- 27.Ts’o MOM, Fine BS, Zimmerman LE. The Flexner-Wintersteiner rosettes in retinoblastoma. Arch Pathol 1969;88:664–71 [PubMed] [Google Scholar]
- 28.Zimmerman LE. Retinoblastoma and retinocytoma. In: Spencer WH, ed. Ophthalmic pathology: an atlas and textbook. Philadelphia: WB Saunders;1985. :1292–351
- 29.McLendon RE, Enterline DS, Tien RD, et al. Tumors of central neuroepithelial origin. In: Bigner DD, McLendon RE, Bruner JM, eds. Russell and Rubinstein’s pathology of tumors of the nervous system. 6th ed. London: Arnold1998. :307–571 [Google Scholar]
- 30.Burger PC, Scheithauer BW. Tumors of neuroglia and choroid plexus epithelium. In: Atlas of tumor pathology: tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology;1994;25–159
- 31.Rubinstein LJ. Tumors of neuroglial cells. In: Tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology;1970. :19–126
- 32.Bailey P. A study of tumors arising from ependymal cells. Arch Neurol Psychiatry 1924;11:1–27 [Google Scholar]
- 33.Friede RL, Pollak A. The cytogenetic basis for classifying ependymomas. J Neuropathol Exp Neurol 1978;37:103–18 [DOI] [PubMed] [Google Scholar]
- 34.Ellison D, Love S, Chimelli L, et al. Neoplasms of the pineal gland. In: Neuropathology: a reference text of CNS pathology. Edinburgh: Mosby;2004. :677–84
- 35.Hirato J, Nakazato Y. Pathology of pineal tumors. J Neuro-oncol 2001;54:239–49 [DOI] [PubMed] [Google Scholar]
- 36.Jouvet A, Saint-Pierre G, Fauchon F, et al. Pineal parenchymal tumors: a correlation of histological features with prognosis in 66 cases. Brain Pathol 2000;10:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burger PC, Scheithauer BW. Tumors of the pineal gland. In: Atlas of tumor pathology: tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology;1994. :227–49