Abstract
Summary: We report the case of an asymptomatic 2-month-old infant with 6-pyruvoyltetrahydropterin synthetase deficiency detected through a neonatal phenylketonuria screening program. MR imaging revealed symmetrical lesions in the central tegmental tract with reduced diffusion, which resolved after treatment. A possible explanation for these lesions is intramyelinic edema resulting from brain insults in utero.
Tetrahydrobiopterin (BH4) deficiency comprises a heterogenous group of disorders caused by mutations of one of the genes encoding enzymes involved in the synthesis or regeneration of BH4.1 It presents mostly with hyperphenylalaninemia (HPA) and a deficiency of neurotransmitter precursors l-dopa and 5-hydroxytryptophan and may be detected through neonatal phenylketonuria (PKU) screening programs. BH4 deficiency (1/1,000,000) is much rarer than PKU (1/10,000). 6-Pyruvoyltetrahydropterin synthetase (PTPS) deficiency is the most common enzyme defect of BH4 deficiency and causes neurologic symptoms, such as hypotonia, rigidity, seizures, and psychomotor retardation, because of a lack of catecholamine and serotonin. Treatment of PTPS deficiency should be initiated as early as possible by means of BH4 supplementation and replacement therapy with the neurotransmitter precursors (l-dopa and 5-hydroxytryptophan).1,2 We report the case of an infant with PTPS deficiency in whom MR imaging revealed reversible lesions in the central tegmental tract (CTT) with reduced diffusion.
Case Report
This girl, now 6 months old, was the first child born to healthy young nonconsanguineous parents. Her birth weight and head circumference were small for gestational age (ie, 2126 g and 29.5 cm for 37 weeks gestation). No prenatal, intrapartum, or perinatal difficulties were noted. Because of her low birth weight, she was cared for in a neonatal care unit until day 19. She was referred to our hospital on day 55 because of HPA detected through PKU screening, the blood phenylalanine (Phe) levels being 17.6 and 31.6 mg/dL (reference range, 1.01 ± 0.23 mg/dL) on days 18 and 44, respectively. On admission, she exhibited no physical or neurologic abnormal findings, and her weight was 3680 g. Laboratory findings on admission were as follows: blood and biochemical data, normal; blood amino acids: Phe, 43.0 mg/dL; tyrosine (Tyr), 1.20 mg/dL (reference range, 1.28 ± 0.41 mg/dL). A BH4 (10 mg/kg) load test revealed rapid normalization of the blood Phe value, 0.85 mg/dL, and a slightly increased blood Tyr level, 3.30 mg/dL, after 8 hours. Serum pteridine analysis before the BH4 load test showed an increased neopterin level, 830.0 nmol/L (reference range, 33.8 ± 4.9 nmol/L); a normal biopterin level, 14.2 nmol/L (reference range, 15.0 ± 1.6 nmol/L); and an increased neopterin/biopterin ratio, 58.3 (reference range, 2.5 ± 1.0). The erythrocytic PTPS activity in the patient was 4% of the control adult level, those in her father and mother being 38% and 34%, respectively, leading to the diagnosis of PTPS deficiency. BH4 supplementation and replacement therapy with l-dopa and 5-hydroxytryptophan was started on day 58.
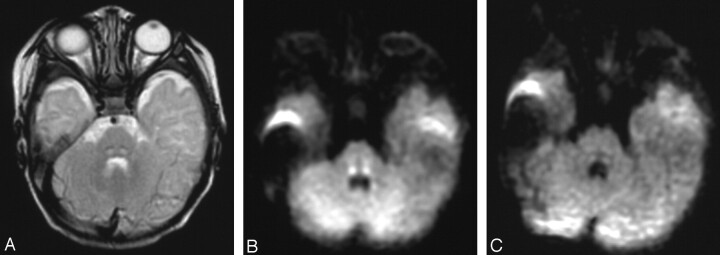
MR imaging was performed at 2 months of age, 2 days after the starting treatment to detect possible cerebral lesions. Fast spin-echo T2-weighted images (Fig 1 A, 4000/100) revealed faint T2 prolongation dorsal to lemniscus medialis. Diffusion-weighted images (DWIs; spin-echo echo-planar imaging; retention time, 5000; b = 1000; Fig 1B) revealed high intensity, and an apparent diffusion coefficient (ADC) map demonstrated a decreased ADC value of the symmetrical lesions in the dorsal brain stem from the red nucleus to the olivary nucleus, which suggested involvement of the CTT. No cerebral atrophy or white matter lesions were recognized. Follow-up MR imaging at 5 months showed no lesion, including in the CTT, on any sequence (Fig 1 C).
Fig 1.
At 2 months, fast spin-echo T2-weighted images (A) revealed faint T2 prolongation just posterior to the lemniscus medialis, compatible with the CTT. DWIs (B) revealed high intensity for the bilateral CTT. Follow-up MR imaging at 6 months (C) showed no lesion, including in the CTT.
Discussion
MR imaging revealed no parenchymal lesions in 7 of 8 patients with PTPS deficiency younger than 4 years of age who were treated immediately after the diagnosis through neonatal PKU screening.3 There are, however, no imaging data for a neonatal or early infant patient with PTPS deficiency having HPA and a lack of catecholamine and serotonin, which may affect the central nervous system (CNS). The most striking MR finding in this infant with PTPS deficiency was symmetrical involvement of the CTT, which resolved after prompt treatment. The CTT is one of the earliest regions of myelination; its myelination begins at 9 postconceptional months.4 Reflecting myelination, the CTT is not visualized as high signal intensity on T2-weighted images or DWI at any time after birth. The CTT lesions have been noted in severely asphyxiated infants and in patients with leukoencephalopathy with vanishing white matter, nonketotic hyperglycinemia, methionine adenosyltransferase (MAT) I/III deficiency, and Leigh syndrome.5–8 The CTT lesions in these disorders, however, are inevitably associated with other parenchymal lesions in the white matter, basal ganglia, or corticospinal tract. Therefore, isolated CTT lesions might be a useful imaging clue for PTPS deficiency in early infancy.
There are higher incidences of lower birth weight and microcephaly at birth in PTPS deficiency,1,2 as seen in the present patient. The average intelligence quotient score is lower than normal, even in patients with PTPS deficiency who were treated immediately after diagnosis through neonatal screening.2 Therefore, possible prenatal brain insults can occur in patients with PTPS deficiency, which might be explained by reduced exposure to neurotransmitter amines in utero.2 The CTT lesions seen in the present patient suggested that the CTT might be vulnerable to the prenatal brain insults and that the lesions could resolve with treatment. The reversibility may explain why no CTT lesion was recognized in the patients reported elsewhere.3
The DWI and ADC map in this patient revealed that the CTT lesions exhibited homogenous reduced diffusion. One possible mechanism for the reduced diffusion is cytotoxic edema in cellular energy failure, such as in acute infarction or encephalopathy, which is nearly always irreversible and causes severe clinical manifestations. This seems unlikely in the present patient, because the CTT lesions were reversible and the patient presented no neurologic signs or symptoms suggesting involvement of the CTT, such as palatal myoclonus or an uncontrollable tremor presumably caused by loss of inhibitory control after focal lesions in the dentato-rubro-olivary pathway. We postulate another possible mechanism for the decreased ADC of the lesions, intramyelinic edema. High signals on DWI and decreased ADC values for white matter and CTT lesions were observed in patients with MAT I/III deficiency.7 A possible explanation proposed for this phenomenon is intramyelinic edema caused by separation of the myelin layers.7 It is interesting that ADC and T2 abnormalities of the white matter in MAT I/III deficiency have been shown to be reversible. Further study, involving untreated neonatal or infantile patients with PTPS deficiency or another type of BH4 deficiency, is needed to clarify the pathogenesis of the CTT lesions.
Acknowledgments
We thank Dr. Haruo Shintaku, Department of Pediatrics, Osaka City University Graduate School of Medicine, for analyzing serum pteridine and erythrocytic PTPS activity and his valuable comments. We appreciate clinical support by Drs. Hiroko Tada and Atsushi Ogawa at Department of Pediatrics, Graduate School of Medicine, Chiba University.
References
- 1.Blau N, Thony B, Cotton RGH, et al. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill;2001. :1725–76
- 2.Chien YH, Chiang SC, Huang A, et al. Treatment and outcome of Taiwanese patients with 6-pyruvoyltetrahydropterin synthetase gene mutation. J Inherit Metab Dis 2001;24:815–23 [DOI] [PubMed] [Google Scholar]
- 3.Chien YH, Peng SF, Wang TR, et al. Cranial MR spectroscopy of tetrahydrobiopterin deficiency. AJNR Am J Neuroradiol 2002;23:1055–58 [PMC free article] [PubMed] [Google Scholar]
- 4.Brody BA, Kinney HC, Kloman AS, et al. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 1987;46:283–301 [DOI] [PubMed] [Google Scholar]
- 5.Van der Knaap MS, Barth PG, Gabreëls FJM, et al. A new leukoencephalopathy with vanishing white matter. Neurology 1997;48:845–55 [DOI] [PubMed] [Google Scholar]
- 6.Khong PL, Lam BCC, Chung BHY, et al. Diffusion-weighted MR imaging in neonatal nonketotic hyperglycinemia. AJNR Am J Neuroradiol 2003;24:1181–83 [PMC free article] [PubMed] [Google Scholar]
- 7.Tada H, Takanashi J, Barkovich AJ, et al. Reversible white matter lesion in methionine adenosyltransferase I/III deficiency. AJNR Am J Neuroradiol 2004;25:1843–45 [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi A, Biancheri R, Bruno C, et al. Leigh syndrome with COX deficiency and. SURF1 gene mutations: MR imaging findings. AJNR Am J Neuroradiol 2003;24:1188–91 [PMC free article] [PubMed] [Google Scholar]