Abstract
BACKGROUND AND PURPOSE: The purpose of this study is to investigate the diagnostic utility of fluid-attenuated inversion recovery (FLAIR) in differentiating between glioma and metastasis by assessing for nonenhancing adjacent cortical signal intensity abnormality in patients who present with a solitary enhancing cerebral lesion.
METHODS: After approval from the institutional ethics committee was obtained, the MR imaging studies of 70 patients with a solitary enhancing lesion, without previous surgery or treatment, were reviewed. The axial FLAIR studies were initially reviewed for cortical involvement. If cortex involvement was detected, comparison with the axial T1, with and without gadolinium enhancement, was made to determine whether the cortex involvement was in an area without enhancement. If this was the case, the study was considered positive for glioma. Statistical analysis consisted of binary logistic regression and a 2 × 2 contingency table.
RESULTS: Involvement of the adjacent cortex with FLAIR signal intensity abnormality but without enhancement was seen in 19 of 70 patients; 16 were gliomas and 3 were solitary metastasis. The sensitivity and specificity of this finding were 44% and 91%, respectively. The positive predictive value for glioma was 84%.
CONCLUSION: FLAIR, when interpreted in concert with pre- and postgadolinium T1-weighted images, may be useful in differentiating glioma from metastasis when a solitary enhancing cerebral lesion is present. The presence of nonenhancing adjacent cortical involvement in a solitary enhancing lesion is a frequent and relatively specific sign.
The 2 most common malignant brain neoplasms are gliomas and metastases. In many cases, differentiation of the 2 neoplasms can be suggested from the clinical history or the presence of lesions elsewhere. Differentiation, however, may be difficult when patients present with a solitary enhancing lesion. In many, a biopsy is performed for histologic confirmation even when there is a history of a known primary malignancy.
Conventional MR imaging is said to be of limited value in making this distinction in most cases.1,2 Many exciting new developments in MR imaging techniques have been used to try and make this distinction, including the use of spectroscopy, diffusion and perfusion imaging, and absolute apparent diffusion coefficient (ADC) measurements.1–4 These new techniques may not be readily available in some centers, require additional imaging time and expense, and perhaps a further appointment as well as experience to perform and interpret. Overlap in tumoral and peritumoral values from these studies implies that even these techniques have limitations.
The key to making the distinction between these 2 entities appears to lie in detecting the changes within the peritumoral area, the area beyond the enhancing margin on imaging. In metastases, this consists essentially of vasogenic edema,5 while in glioma, this may also contain neoplastic cells.6,7 Many of the new MR imaging techniques currently used to differentiate glioma from a solitary metastasis are based on detecting these differences in the peritumoral area. The infiltrative nature of malignant cells in a glioma may demonstrate different spectroscopic, diffusion, and perfusion characteristics compared with the peritumoral vasogenic edema in a solitary metastasis.1–4,8 Furthermore, it has been suggested that there is a relatively greater extracellular water content in peritumoral vasogenic edema related to a metastasis compared with glioma, because of the presence of neoplastic cells.9 Thus, in glioma, a relative decrease in peritumoral T2 or fluid-attenuated inversion recovery (FLAIR) hyperintense signal intensity may be expected compared with metastasis.
Metastases characteristically involve the subcortical white matter and gray-white matter junction, have extensive white matter vasogenic edema, and the viable tumor component enhances when intravenous contrast is administered. Our hypothesis is that any nonenhancing signal intensity abnormality involving the cortex, an area relatively spared by vasogenic edema, should represent infiltration of glial cells.
In this study, we investigate the diagnostic utility of FLAIR in assessing for nonenhancing cortical signal intensity abnormality, to distinguish glioma from a solitary metastasis. To the best of our knowledge, no previous similar studies have considered the implications of these findings.
Methods
Subjects
We retrospectively reviewed the MR imaging studies and patient charts of 70 patients with a solitary enhancing cerebral lesion, without previous surgery or treatment. The patients ranged in age from 23 to 75 years (mean age ± SD, 56.2 ± 12.4 years). The patients included 44 men and 26 women. Hospital ethics committee approval was obtained. The data were retrieved by using SORTS (Simple On-line Report Text Search), a vector space document-retrieval system that displays ranked results in a familiar Web-based user interface, developed by our PACS support team. All MR imaging scans were performed in our adult tertiary referral teaching hospital between June 2001 and December 2004. The studies were reviewed by a radiologist and an MR imaging fellow, and a consensus was reached in regard to the presence of each sign. Reviewers were blinded to the histologic diagnosis and clinical history, including age and sex. A histopathologic diagnosis was available from a brain specimen in all cases.
MR Imaging
All MR imaging was performed on a 1.5T whole body unit (LX platform, SIGNA, General Electric, Milwaukee, Wisc) with a quadrature head coil. Axial fast FLAIR MR imaging was performed as follows: TR, 9000 milliseconds; TE, 140 milliseconds; TI, 2200 milliseconds; field of view (FOV), 240 mm; matrix = 256 × 192; number of excitations (NEX), 1; section thickness, 5 mm; intersection gap, 2 mm; scan time, 3 minutes 36 seconds; and 20 sections covering the whole brain. In addition, axial spin-echo T1-weighted imaging, pre- and postgadolinium injection (10 mL) with no delay, were also performed, as follows: TR, 500 milliseconds; TE, 14 milliseconds; FOV, 240 mm; matrix, 256 × 192; scan time, 1 minute 46 seconds; and NEX, 1.
The FLAIR images were independently reviewed for nonenhancing signal intensity abnormalities in the cortex adjacent to the enhancing lesion.
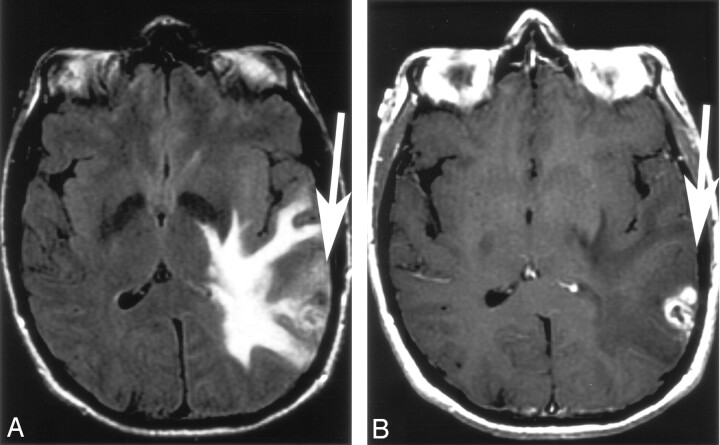
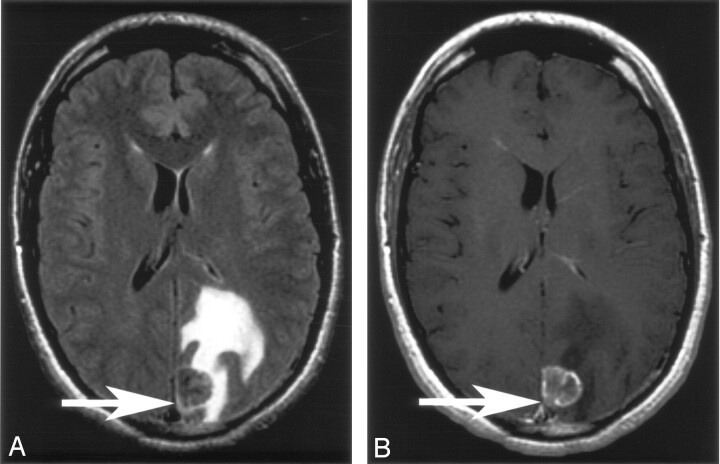
Where adjacent cortical involvement was identified, the T1 pre- and postcontrast images were reviewed to assess for the presence or absence of enhancement in these areas. The presence of cortical involvement without enhancement was determined; if present, it implied glioma (Figs 1 and 2).
Fig 1.
Solitary enhancing lesion in the left parietal lobe.
A and B, Axial FLAIR and T1 postgadolinium images. There is cortical FLAIR hyperintensity with both the enhancing and nonenhancing components of the lesion. The presence of nonenhancing cortical FLAIR hyperintensity (arrow) adjacent to the enhancement implies glioma. The histopathology was glioblastoma multiforme.
Fig 2.
Solitary enhancing lesion in the left posterior parietal lobe.
A and B, Axial FLAIR and T1 postgadolinium images. There is cortical FLAIR signal intensity abnormality related to this lesion, which enhances postgadolinium (arrow). There is no nonenhancing cortical FLAIR signal intensity abnormality to imply glioma. Histology showed this to be a metastasis.
Exclusion Criteria
Solitary enhancing lesions were excluded if they involved the corpus callosum (5 patients) or the posterior fossa (9 patients), because of the high likelihood of these representing glioma and metastasis respectively. Patients without histologic confirmation or FLAIR, pre- and postgadolinium, T1 sequences were also excluded.
Statistical Analysis
A binary logistic regression analysis was performed by using MedCalc for Windows, version 8.1.0.0 (MedCalc Software, Mariakerke, Belgium). The histologic diagnosis was coded 1 for glioma and 0 for metastasis and entered as the dependent variable. The consensus reading for the presence of cortical signal intensity abnormality (1 = present; 0 = absent) was entered as the independent variable. Subject age and sex were also included in the model as covariates. A P value <.05 was considered to be statistically significant. The sensitivities, specificities, and positive and negative predictive values were calculated by using the 2 × 2 contingency table.
Results
Seventy subjects were included in this study. Of the 70 patients, 36 had a glioma and 34 had a metastasis. The gliomas consisted of glioblastoma multiforme (24), anaplastic astrocytoma (5), oligodendroglioma (6: 2 WHO grade II and 4 WHO grade III), and gliosarcoma (1).
Nineteen of 70 patients (incidence of 27%) had abnormal signal intensity in the adjacent cortex that was nonenhancing. Of these 19 patients, 16 had a histologic diagnosis of a glioma (anaplastic astrocytoma, 4; glioblastoma multiforme, 7; oligodendroglioma; grade II, 1; and grade III, 4), and 3 were metastases. The metastases included 2 melanomas and a poorly differentiated adenocarcinoma; all had a history of known metastatic disease.
The logistic regression analysis demonstrates a statistically significant relationship between the presence of nonenhancing cortical signal intensity abnormality and glioma (P =.0023; regression coefficient, 2.1; odds ratio, 8.2; confidence interval, 2.1–32.0). There was no statistically significant contribution from patient age (P =.70) or sex (P =.85).
The presence of nonenhancing adjacent cortical signal intensity abnormality had a sensitivity of 44% and specificity of 91%, which indicates that this sign is specific but not particularly sensitive. The positive predictive value for glioma was 84%, and the negative predictive value was 61%. A summary of these results are displayed in the Table.
Results for presence of nonenhancing adjacent cortical FLAIR signal abnormality
| Nonenhancing adjacent cortical FLAIR signal abnormality | Glioma | Metastasis | |
|---|---|---|---|
| Present | 16 | 3 | PPV = 84% |
| Not present | 20 | 31 | NPV = 61% |
| Sensitivity 44% | Specificity 91% |
Note:—FLAIR indicates fluid-attenuated inversion recovery MR sequences; PPV, positive predictive value; NPV, negative predictive value.
Discussion
Gliomas and metastases are the 2 most commonly encountered malignant neoplasms of the brain.10 When intracranial neoplasms are encountered, the clinical history or the presence of multifocal lesions may assist in differentiating gliomas from metastases in many cases. In general, high-grade gliomas tend to be heterogeneous, with central necrosis and an enhancing component whereas metastases are usually multiple and well circumscribed with nodular or ring enhancement on conventional MR imaging.2 There is, however, considerable overlap of appearances such that, for a solitary enhancing lesion, differentiation between a glioma from a solitary metastasis may be difficult, and conventional MR imaging is said to be of limited value.1,2 The quest to differentiate these 2 common brain neoplasms has involved many exciting new MR imaging techniques, including the use of spectroscopy, diffusion and perfusion imaging, and absolute ADC measurements.1–4
In this study, we attempted to assess the diagnostic utility of conventional MR imaging techniques in distinguishing glioma from a solitary metastasis. The key in differentiating the 2 neoplasm types appears to lie in the peritumoral areas, beyond the enhancing margins of the lesion. In a glioma, the peritumoral region may be infiltrated with malignant cells in addition to vasogenic edema,6,7 whereas in a metastatic deposit, the surrounding peritumoral areas comprise predominantly vasogenic edema.5 Detection of changes in the metabolites and perfusion in the peritumoral area are the basis of many of the previously mentioned advanced MR imaging techniques. Many of these techniques, however, are not readily available and require extra time and expertise.
When the lesion is in close proximity to the cortical gray matter, any nonenhancing or enhancing signal intensity change within this cortex is likely to be due to neoplastic infiltration as vasogenic edema preferentially involves the white matter and largely spares the gray matter. The enhancing component of the glioma and metastasis will, however, be indistinguishable. Metastases enhance at their advancing margin, and thus any nonenhancing signal intensity abnormality in the adjacent cortex is hypothesized to favor infiltration by glial tumor.
In this study, involvement of the adjacent cortex with FLAIR signal intensity abnormality without gadolinium enhancement was seen in 19 of 70 solitary enhancing cerebral lesions and in 16 of 36 gliomas (sensitivity, 44%; specificity, 91%; positive predictive value, 84%; and negative predictive value, 61%). Therefore, when present, this is a relatively specific sign favoring glioma over a solitary metastasis, though its absence does not imply metastasis or exclude glioma.
Conclusion
When interpreted in concert with pre- and postgadolinium-enhanced T1-weighted images, FLAIR may be useful in differentiating glioma from metastasis in patients with a solitary enhancing cerebral lesion. The detection of nonenhancing involvement of the adjacent cortex with signal intensity abnormality on FLAIR in a solitary enhancing cerebral lesion is a frequent and specific sign for glioma.
Acknowledgments
We thank Lawrence Sim and Michael Bowden for their valuable contribution to the statistical analysis of this project. We also thank our PACS support team, especially Ken Manthey, for the development and use of the SORTS program.
References
- 1.Chiang IC, Kuo YT, Lu CY, et al. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imaging. Neuroradiology 2004;46:619–27 [DOI] [PubMed] [Google Scholar]
- 2.Opstad K, Murphy M, Wilkins P, et al. Differentiation of metastases from high-grade gliomas using short echo time 1H spectroscopy. J Magn Reson Imaging 2004;20:187–92 [DOI] [PubMed] [Google Scholar]
- 3.Ishimaru H, Morikawa M, Iwanaga S, et al. Differentiation between high-grade glioma and metastatic brain tumor using single-voxel proton MR spectroscopy. Eur Radiol 2001;9:1784–91 [DOI] [PubMed] [Google Scholar]
- 4.Law M, Cha S, Knopp EA, et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002;222:715–21 [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Olsson Y. Hematogenous metastases of the human brain–characteristics of peritumoral brain changes: a review. J Neurooncol 1997;35:81–89 [DOI] [PubMed] [Google Scholar]
- 6.Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 1987;66:865–74 [DOI] [PubMed] [Google Scholar]
- 7.Burger P. Classification, grading, and patterns of spread of malignant gliomas. In: Apuzzo ML, ed. Neurosurgical topics: malignant cerebral glioma. Park Ridge, Ill: American Association of Neurological Surgeons;1990. :3–17
- 8.Tien RD, Felsberg GJ, Friedman H, et al. MR imaging of high-grade cerebral gliomas: value of diffusion-weighted echoplanar pulse sequences. AJR Am J Roentgenol 1994;162:671–77 [DOI] [PubMed] [Google Scholar]
- 9.Lu S, Ahn D, Johnson G, et al. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 2003;24:937–41 [PMC free article] [PubMed] [Google Scholar]
- 10.DeAngelis LM. Brain tumors. N Engl JMed 2001;344:114–23 [DOI] [PubMed] [Google Scholar]