Abstract
SUMMARY: Isolated dissection of the posterior cerebral artery (PCA) is a rare but important cause of stroke in younger patients, particularly women. We present 3 cases of dissection of the P2 segment of the PCA. In 2 patients, an association with minor axial head trauma was documented, suggesting shearing injury of the PCA as it crosses over the free edge of the tentorium. The clinical and imaging findings are discussed, and the therapeutic management is reviewed.
Extracranial and intracranial arterial dissection is an important cause of stroke in younger patients. Intracranial arterial dissections most often involve the vertebrobasilar system and, less commonly, the middle and anterior cerebral arteries.1-11 Isolated dissection of the posterior cerebral artery (PCA) is rare,12-18 but accurate diagnosis is important for appropriate clinical management. We present 3 cases of dissection of the P2 segment of the PCA, 2 of which occurred in association with minor head trauma.
Case Reports
Case 1
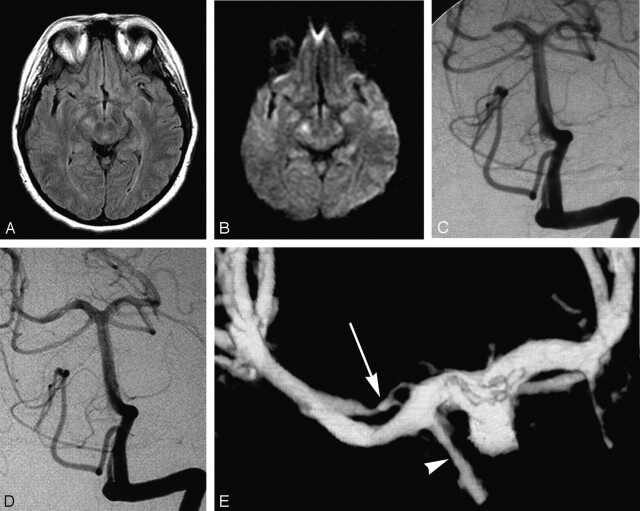
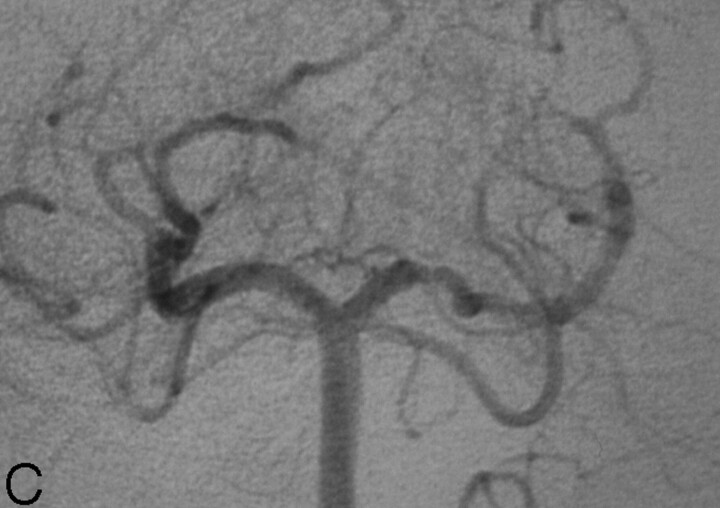
A 47-year-old woman with medically controlled arterial hypertension presented to the emergency department with occipital headache and acute left lower quadranopsia. She reported a 45-minute episode of left lower face and left-handed tingling that occurred 2 days before admission and resolved spontaneously. Additional history was significant for mild head trauma approximately 1 week before, when she had experienced a few minutes of dizziness after banging the top of her head on a wooden table. The general and neurologic examination at the time of presentation was unremarkable. In particular, the visual fields were full to confrontation. Findings of noncontrast brain CT were unremarkable. MR imaging demonstrated fluid-attenuated inversion recovery (FLAIR) hyperintensity (Fig 1A) with associated restricted diffusion in the right cerebral peduncle (Fig 1B) and to a lesser extent in the right occipital lobe, compatible with subacute infarcts. MR angiography demonstrated focal absence of flow-related enhancement in the P2 segment of the right PCA, which was attributed to slow or absent flow. Findings of transesophageal echocardiography and bilateral carotid duplex sonography were unremarkable, as were the results of the screening for hypercoagulability disorders. The patient was started on intravenous heparin, given the possibility of PCA thrombosis. Digital subtraction angiography (DSA) revealed an 80%–90%, smoothly tapered focal narrowing of the right PCA (P2 segment) approximately 5 mm distal to the termination of the posterior communicating artery (Fig 1C). The capillary and venous phases of the posterior fossa were unremarkable, as were the remainder of findings on the angiogram.
Fig 1.
47-year-old woman presenting with quadranopsia.
A, Axial FLAIR image demonstrating hyperintensity in the right cerebral peduncle.
B, Axial diffusion-weighted image (DWI) image showing corresponding abnormal increased signal intensity. There was respective low signal intensity on the apparent diffusion coefficient map (not shown).
C, DSA, left vertebral artery injection, transfacial view, demonstrates a smoothly tapered focal, near-occlusive narrowing of the proximal right PCA.
D, DSA, left vertebral artery injection, transfacial view. One-year follow-up demonstrating a “double lumen” sign of the right P2 segment.
E, 3D reconstruction of left vertebral angiogram rotational DSA which shows 2 patent lumens (double lumen sign) distal to the posterior communicating artery (arrowhead). Note the irregularly recanalized false lumen (arrow) with 2 separate inflow channels.
The patient was placed on enoxaparin (Lovenox) and warfarin (Coumadin) with a target international normalized ratio between 2.0 and 3.0. Lovenox was discontinued when the patient was at therapeutic levels on Coumadin. At the time of discharge, the visual field deficit had resolved. Follow-up CT angiography performed 6 months later demonstrated an improved, but still present, irregularity and narrowing of the P2 segment of the right PCA. The patient was asymptomatic, and findings of the neurologic examination were normal. The decision was made to continue anticoagulation and perform further follow-up imaging 6 months later. DSA, performed at 1 year, demonstrated a mild persistent irregular contour of the PCA as well as a flow in a second lumen parallel to the PCA, consistent with recanalization of a focal arterial dissection (“double lumen” sign) (Fig 1D). 3D DSA clarified the complex morphology of the true and false lumen of the right PCA, immediately distal to the posterior communicating artery (Fig 1E).
Case 2
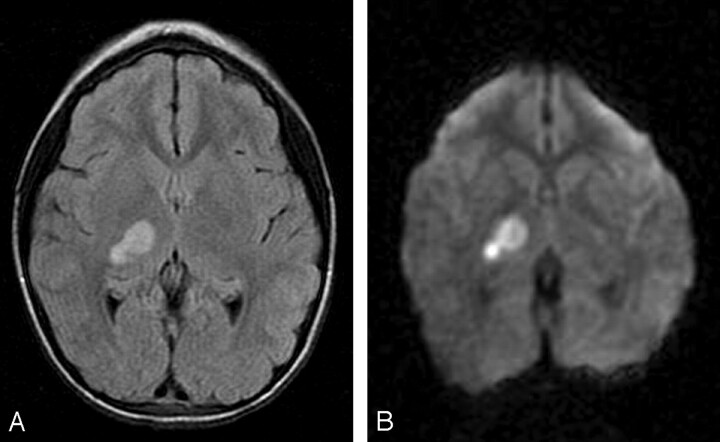
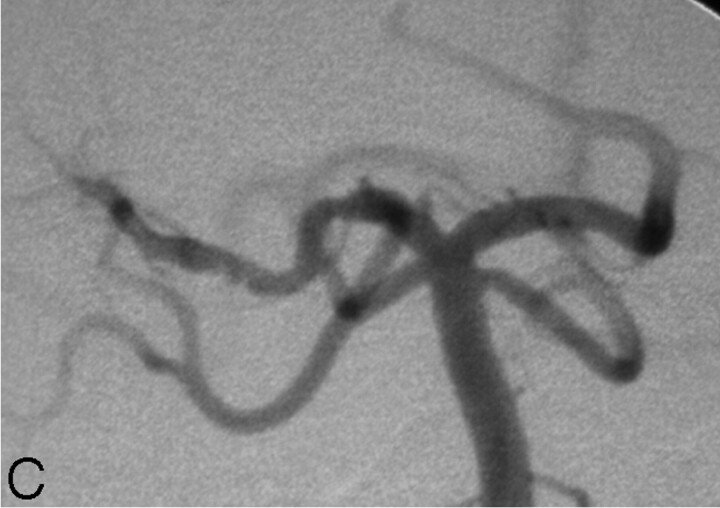
A 7-year-old girl with a history of attention deficit disorder and microcephaly presented to the emergency department with a 3-day history of left hemiparesis that appeared shortly after her older sister had accidentally dropped the edge of a mattress on the top of her head. There was no loss of consciousness. Head CT obtained 4 days after the onset of symptoms demonstrated an area of hypoattenuation in the right thalamus and basal ganglia. MR imaging demonstrated FLAIR hyperintensity in the right thalamus and posterior limb of the internal capsule (Fig 2A), associated with restricted diffusion (Fig 2B) and mild enhancement. MR angiography showed narrowing of the proximal right PCA. Findings of echocardiography, hypercoagulability screening, and autoimmune disease evaluation were negative. Cerebral DSA demonstrated a focal narrowing of the proximal P2 segment of the right PCA, distal to the posterior communicating artery termination (Fig 2C), with a slightly beaded appearance. During the capillary phase, a nodular blush compatible with luxury perfusion of a subacute infarct was visible in the posterior right thalamic region.
Fig 2.
7-year-old girl presenting with left hemiparesis.
A, Axial FLAIR image demonstrating hyperintensity in the right thalamus and posterior limb of the internal capsule.
B, Axial DWI image showing corresponding restricted diffusion. There was respective low signal intensity on the apparent diffusion coefficient map (not shown).
C, DSA, left vertebral artery injection, transfacial image demonstrating a focal narrowing and irregularity of the proximal right PCA.
The patient was started on aspirin. At discharge, the patient had a mild residual left-sided weakness. At 1 month follow-up visit, the patient showed only slightly decreased fine fractionated movements in the left hand. Her gait was normal, with the exception of an occasional high-amplitude, low-frequency tremor that caused some difficulty when she was walking on her toes. This tremor was also noted when the patient had her arms outstretched and pronated. This tremor, characteristic after thalamic infarcts (Holmes’ tremor), began approximately 3 weeks after initial presentation.
Case 3
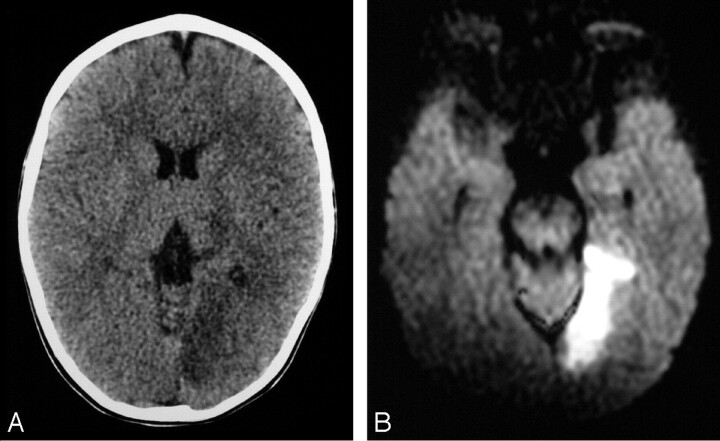
A 19-month-old boy with no significant medical history began complaining of left-sided headache after an uneventful day at daycare. He started to cry and vomited once before going to sleep for the night. He was difficult to arouse the next morning, was not using his right hand, and refused to bear weight on his legs. His primary care physician noted decreased use of his right hand and leg, decreased right visual field, and mild right-sided drooling. There was no definite history of trauma. At admission in the emergency department, he had a right homonymous hemianopsia and a mild right hemiparesis. Noncontrast brain CT showed hypoattenuation in the left occipital lobe as well as in the left thalamus, compatible with a left PCA territory infarct (Fig 3A). MR imaging demonstrated restricted diffusion compatible with an acute infarct in the medial aspect of the left occipital lobe and lateral aspect of the thalamus (Fig 3B). MR angiography showed absence of flow-related enhancement in the left PCA distal to the P1-P2 junction. DSA demonstrated a patent but irregular and narrowed left PCA, distal to the posterior communicating artery termination (Fig 3C), with delayed filling of its distal distribution and occlusion of the calcarine artery. Findings of transthoracic echocardiography were unremarkable, as was the result of screening for hypercoagulability disorders. The patient was maintained on 81 mg of aspirin per day throughout his hospital course and showed mild improvement of his hemiparesis and a decrease in his visual field deficit. The patient did not return for further follow-up at our institution.
Fig 3.
19-month old boy presenting with right hemiparesis.
A, Axial noncontrast head CT demonstrating hypoattenuation in the medial aspect of the left occipital lobe involving the cortex and subcortical white matter with loss of gray-white matter differentiation.
B, Axial DWI showing corresponding restricted diffusion compatible with infarct. There was respective low signal intensity on the apparent diffusion coefficient map (not shown).
C, DSA, right vertebral artery injection, transfacial image demonstrating an irregular and narrowed left proximal PCA.
Discussion
Although most arterial dissections or dissecting aneurysms remain without detectable cause, a possible causative relation has been suggested with conditions such as syphilis, migraine headaches, cystic medial necrosis, Marfan syndrome, mixed connective tissue disease, fibromuscular dysplasia, homocystinuria, polycystic kidney disease, and trauma.6,14,16,18
Although arterial hypertension has commonly been associated with extracranial carotid dissection, few patients with posterior circulation dissection are found to be hypertensive.6,19 In 2 of our patients, the onset of symptoms shortly followed a minor head trauma without loss of consciousness. Interestingly, the mechanism of trauma was similar, yet somewhat unusual in both cases, with an axial impact on the top of the head. The temporal relationship between the traumatic event and the onset of symptoms suggests a causal link in these 2 patients. The third patient, a toddler who developed initial symptoms after returning from daycare, had no definite trauma history. It is unclear if hypertension played a role in the first patient, who had a history of medically controlled blood pressure.
Vertebrobasilar dissections are more commonly seen in males, whereas isolated PCA dissections are predominant in females, with a female-male ratio of 3.1:1.12,13 In general, intracranial arterial dissections occur in young adults, typically in the late third to fifth decade.
The most common symptom of vertebrobasilar and PCA dissection is headache, predominantly in the occipital and posterior cervical regions. Dissecting intracranial aneurysms usually present with large cerebral infarcts but may also present with subarachnoid hemorrhage or both.2,6,15 They can be associated with severe neurologic deficits, often leading to death. Isolated PCA dissections tend to present with ischemic symptoms and have a more benign clinical course and prognosis.13-15,20
Intracranial dissections typically occur between the intima or internal elastic lamina and the media,6 whereas in extracranial arteries, dissection occurs in the outer layers of the media or between the media and the adventitia.2,6 Although the reason for this difference in location of the intramural hematoma remains unclear, it has been suggested that some characteristics of the intracranial arteries may be playing a role, including the fact that they lack an external elastic membrane, have a thinner adventitia, have fewer elastic fibers in the media, and generally have a thicker internal elastic lamina compared with that in the extracranial arteries.6 In the cervical carotid arteries, these changes occur at the skull base, whereas in the vertebral arteries, the change occurs approximately 1 cm proximal to the dural perforation of the skull base. These respective anatomic characteristics tend to correlate with the angiographic appearance of the lesion—that is, focal stenosis in case of intracranial dissection and pseudoaneurysm development in case of extracranial dissection.6 A focal stenosis, with or without a poststenotic dilation, occurs as the hematoma tracks between the internal elastic membrane and the media, causing mass effect on the lumen without disrupting the outer media or adventitia. Rarely, intracranial dissections may involve the subadventitia and rupture into the subarachnoid space.2 In the extracranial arteries, the hematoma tracks in the outer media and between the media and adventitia, thus narrowing the vessel lumen but also leading to pseudoaneurysm formation, with the hematoma contained by the adventitia.
Absence or dysplasia of the internal elastic lamina, especially if the media is incomplete, is believed to predispose to intracranial dissection.5,13 Isolated PCA dissections most commonly occur near the P1-P2 junction, which is close to the free border of the tentorium cerebelli.17 It has been suggested that traumatic aneurysms, including dissections, may occur in mild head trauma secondary to a stretch or shear injury of the vessels.3,7 In 2 of our patients, the location of the PCA dissection and the association with mild head trauma lend credence to a posttraumatic cause of intracranial dissections. In the third patient, no definite trauma was documented but the dissection occurred in the same location.
The diagnosis of intracranial dissection and dissecting aneurysms is predominantly made by DSA, which remains the gold standard technique. There is narrowing of the vascular lumen secondary to the subintimal hematoma (string sign), with or without segmental dilation proximal or distal to the stenosis (string and pearl sign).1,2,3,15 The lumen is often irregular or asymmetrical. If the intramural hematoma becomes large enough, occlusion can occur.6 A pathognomonic double lumen sign, as documented in our first patient, is less frequently seen; it represents antegrade flow in both the true and false lumens, which are separated by the intimal flap.6,11,15 It has been suggested that the predominantly stenotic lesions more often present with ischemic symptoms, whereas the predominantly dilated lesions more likely present with bleeding.15 A change in the appearance of the vessel lumen with time is also characteristic.6,11,15 Noncontrast CT should be performed at initial presentation primarily to evaluate for hemorrhage and also to assess for large infarction. Subarachnoid hemorrhage is present in approximately 25% of cases.19 MR imaging should also be performed for definitive evaluation of stroke.
Although MR angiography has been reported to be a reliable method for establishing the diagnosis of intracranial arterial dissection,11 the technique is limited by the small caliber of the intracranial vessels and often by patient motion artifact. In addition, the appearance of vessel wall irregularity or narrowing is often a nonspecific finding. T1-weighted images may show high signal intensity within the dissected vessel wall, but the signal intensity of the hematoma changes with chronologic age of the bleed and is seen in a minority of cases. Although Hirai et al11 reported a high degree of accuracy in the detection of intracranial dissection on the MR angiography source images in 7 patients, none of the dissections involved the PCA. At the current state of image resolution, the accuracy of MR angiography in the evaluation of PCA dissection is limited and should not be the primary diagnostic technique. In cases 1 and 3, MR angiography showed no flow in the proximal P2 segment, falsely suggesting lumen occlusion likely to be caused by an embolic event. In case 2, MR angiography showed a narrowed right P2 segment, initially interpreted as a variant hypoplastic vessel. The beaded appearance documented by DSA was not appreciated on MR angiography. DSA was required to make an accurate diagnosis in all cases. CT or MR angiography may play a role in follow-up imaging to avoid repeat DSA in selected cases.
Anticoagulation is the recommended treatment for an extracranial arterial dissection, but its use in intracranial arterial dissection is debated in the literature. Anticoagulation has not been promoted in the treatment of intracranial arterial dissecting aneurysms because of the potential risk of mural hemorrhage, rupture, or extension of the lesion.6,12,15 Lazinski et al17 recommend anticoagulation, specifically heparin alone, for patients with intracranial dissection presenting with stable ischemic symptoms. Our 47-year-old patient responded favorably to intravenous heparin. We opted for more conservative antiplatelet therapy with low-dose aspirin in our 7-year-old and 19-month-old patients. Pozzati et al8 reported 2 cases of nonhemorrhagic PCA dissection that resolved spontaneously, without anticoagulation. They suggested not treating patients who are asymptomatic or have resolving neurologic deficits and do not have an associated aneurysm. In hypertensive patients, it is important to maintain adequate blood pressure control to prevent progression of the dissection and possible rebleeding or worsened ischemia.
Patients with ruptured dissecting aneurysms and associated subarachnoid hemorrhage or progressive neurologic deficits typically require treatment by surgical or endovascular means.17 Surgical treatment of dissecting aneurysms includes excision, trapping, proximal ligation, and reinforcement with or without intracranial-extracranial bypass.6,9,18,21 Repeated intramural hemorrhage or enlargement of an associated aneurysm also requires surgical intervention.18 However, Pozzati et al8 noted spontaneous healing in 2 patients with subarachnoid hemorrhage, who had fusiform dissecting aneurysms of the PCA. They suggested that this shape of dissecting aneurysm lies at the more benign end of the clinical spectrum.
Maillo et al15 also reported a case of spontaneous resolution with complete recanalization of a dissecting PCA aneurysm. Endovascular options for the treatment of arterial dissections include stent placement or therapeutic occlusion by using detachable microcoils and/or balloons.
Endovascular treatment for dissecting aneurysms of the posterior circulation and specifically for PCA dissecting aneurysms has been described.17,22-24 Although endovascular treatment has been performed only in a small number of patients, it seems to offer a reasonable alternative to surgery.
References
- 1.Scott GE, Neubuerger KT, Kenst J. Dissecting aneurysms of intracranial arteries. Neurology 1960;10:22–27 [DOI] [PubMed] [Google Scholar]
- 2.Yonas H, Agamanolis D, Takaoka Y, et al. Dissecting aneurysms of the intracranial arteries. Surg Neurol 1977;8:407–15 [PubMed] [Google Scholar]
- 3.Johnson AC, Graves VB, Pfaff JP Jr. Dissecting aneurysms of intracranial arteries. Surg Neurol 1977;7:49–52 [PubMed] [Google Scholar]
- 4.Fisher CM, Ojemann RG, Roberson AG. Spontaneous dissection of the cervico-cerebral arteries. Can J Neurol Sci 1978;5:9–19 [PubMed] [Google Scholar]
- 5.Kulla L, Deymeer F, Smith TW, et al. Intracranial dissecting and saccular aneurysms in polycystic kidney disease: report of a case. Arch Neurol 1982;39:776–78 [DOI] [PubMed] [Google Scholar]
- 6.Berger MS, Wilson CB. Intracranial dissecting aneurysms of the posterior cerebral circulation: report of six cases and review of the literature. J Neurosurg 1984;61:882–94 [DOI] [PubMed] [Google Scholar]
- 7.Kassel NF, Boarini DJ, Adams HP Jr. Intracranial and cervical vascular injuries. In: Cooper PR, eds. Head Injury. 2nd ed. Baltimore, Md: Williams & Wilkins;1987. :327–543508877
- 8.Pozzati E, Padovani R, Fabrizi A, et al. Benign arterial dissections of the posterior circulation. J Neurosurg 1991;75:69–72 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki O, Koike T, Takeuchi S, et al. Serial angiography in a spontaneous dissecting anterior cerebral artery aneurysm. Surg Neurol 1991;36:49–53 [DOI] [PubMed] [Google Scholar]
- 10.Pozzati E, Andreoli A, Limoni P, et al. Dissecting aneurysms of the vertebrobasilar system: a study of 16 cases. Surg Neurol 1994;41:119–24 [DOI] [PubMed] [Google Scholar]
- 11.Hirai T, Korogi Y, Murata Y, et al. Intracranial artery dissections: serial evaluation with MR imaging, MR angiography, and source images of MR angiography. Radiat Med 2003;21:86–93 [PubMed] [Google Scholar]
- 12.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology 2001;57:1155–60 [DOI] [PubMed] [Google Scholar]
- 13.Kawahara I, Hiu T, Onizuka M, et al. Isolated posterior cerebral artery dissection: case report and review of the literature [in Japanese]. No Shinkei Geka 2003;31:671–75 [PubMed] [Google Scholar]
- 14.Le Tu PT, Zuber M, Meder JF, et al. Dissection isolee de l’artere cerebrale posterieure. Rev Neurol (Paris)1996. :152, 542–47 [PubMed] [Google Scholar]
- 15.Maillo A, Diaz P, Morales F. Dissecting aneurysm of the posterior cerebral artery: spontaneous resolution. Neurosurgery 1991;29:291–94 [DOI] [PubMed] [Google Scholar]
- 16.Berthier E, Bourrat C. Dissecting aneurysm of the posterior cerebral artery: case report and review of the literature. Cerebrovasc Dis 1993;3:56–59 [Google Scholar]
- 17.Lazinski D, Willinsky RA, TerBrugge K, et al. Dissecting aneurysms of the posterior cerebral artery: angioarchitecture and a review of the literature. Neuroradiology 2000;42:128–33 [DOI] [PubMed] [Google Scholar]
- 18.Sasaki O, Koizumi T, Ito Y, et al. Dissecting aneurysm of the posterior cerebral artery treated with proximal ligation. Surg Neurol 1992;37:394–401 [DOI] [PubMed] [Google Scholar]
- 19.Nelson JW, Stryi O. Dissecting subintimal hematomas of the intracranial arteries: report of a case. J Am Osteopath Assoc 1968;67:512–17 [PubMed] [Google Scholar]
- 20.Ohki M, Nakajima M, Sato K, et al. A case of dissecting aneurysm associated with mixed connective tissue disease [in Japanese]. No To Shinkei 1994;46:855–58 [PubMed] [Google Scholar]
- 21.Mizutani T, Goldberg HI, Parr J, et al. Cerebral dissecting aneurysm and intimal fibroelastic thickening of cerebral arteries: case report. J Neurosurg 1982;56:571–76 [DOI] [PubMed] [Google Scholar]
- 22.Leibowitz R, Do HM, Marcellus ML, et al. Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. AJNR Am J Neuroradiol 2003;24:902–07 [PMC free article] [PubMed] [Google Scholar]
- 23.Xavier J, Vasconcelos C, Cruz R, et al. Endovascular treatment of dissecting aneurysms of the posterior cerebral artery [in Portuguese]. Acta Med Port 2001;14:65–70 [PubMed] [Google Scholar]
- 24.Yamaura I, Tani E, Yokota M, et al. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Gugliemi detachable coils. J Neurosurg 1999;90:853–56 [DOI] [PubMed] [Google Scholar]