Abstract
BACKGROUND AND PURPOSE: Therapeutic intervention during the early stages of an intracerebral hemorrhage (ICH) might have value in improving clinical outcomes. During the 73-site International Recombinant Activated Factor VII Intracerebral Hemorrhage Trial, CT techniques were used to monitor the change in hematoma volume in response to treatment. The use of CT imaging technology served 3 functions: to provide accurate measurements of the change in hematoma volume, intraventricular volume (IVH), and edema volume; to evaluate the use of CT scans as a predictor of patient outcomes; and to demonstrate that hematoma volume can serve as a surrogate marker for ICH clinical progression.
METHODS: The multicenter clinical trial received institutional review board approval and obtained informed consent from the patient or a legally acceptable representative (waived in a few cases of incapacity, according to local and national regulations). CT scans were used to quantify volumes of hemorrhage and to monitor evolution over a 72-hour period in patients with ICH treated with placebo or 40, 80, or 160 μg/kg of recombinant activated factor VII (rFVIIa). CT image data were transmitted digitally to an imaging laboratory and analyzed by 2 readers masked to patient and treatment data, by using Analyze software, a fully integrated toolkit for interactive display, processing, and measurement of biomedical image data. The use of this software enabled the evaluation of intraclass variability of CT scan interpretations.
RESULTS: Interpretations of ICH and IVH volumes of CT scans in patients treated in this study showed minimal intraclass variability. Variability was greatest for interpretations of edema volume.
CONCLUSION: These CT assessments of lesions could have value in future early hemostatic interventions in ICH patients.
Imaging of the central nervous system has continued to advance with the establishment of new technology and techniques (eg, CT perfusion, diffusion-weighted imaging/perfusion-weighted imaging) and provides increasingly detailed information regarding brain injury. Recent studies have demonstrated the utility of MR imaging to detect intracerebral hemorrhage (ICH),1 but CT is currently the imaging technique of choice to detect early hematoma of this condition. Our report describes the use of CT to quantify hematoma volume in patients with intracerebral hemorrhage and to evaluate the expansion/extension of hematomas by using established software packages.
Intracerebral hemorrhage affects an estimated 37,000 individuals in the United States annually,2 and only 38% of these individuals survive the first year.3 A prospective study of 103 patients by Brott et al2 used serial CT scans to evaluate hematoma growth during the first 20 hours after the onset of symptoms of ICH. This study demonstrated that 38% of patients had a >33% increase in hemorrhage volume in the first 20 hours from baseline CT. This window of maximal hematoma expansion was further refined in retrospective analyses conducted by Fujii et al4 and Kazui et al5 on ICH patients. Their results indicated that hematoma volume increased substantially within the first 2 or 3 hours after ICH onset, with the rate of volume change decreasing by 6 hours after onset. These findings were in contrast to previous observations that bleeding was completed within minutes of ICH onset.6 Additional studies established that the early expansion in hematoma volume was associated with increased neurologic deterioration and was a critical determinant in 30-day mortality rates in patients with ICH.2,7 Therefore, in patients with ICH, for whom there are no well-accepted diagnostic or biochemical indicators to evaluate the therapeutic effects of a hemostatic agent or to predict clinical outcomes, measurements of hematoma volume may have potential as a surrogate marker. As such, a reliable and accurate methodology for determination of lesion volume is of value in therapeutic intervention clinical trials.
CT scan technology, currently used to confirm a diagnosis of ICH, is fast and patient-friendly. However, the utility of CT imaging to function both as a prognostic tool and an accurate marker of therapeutic efficacy is limited by the inherent variation present in the determination of the hematoma volumes by the CT image reader. The degree of discrepancy in the interpretations of CT images between emergency department staff, staff radiologists, radiology interns, and neuroradiologists has recently been the focus of investigation. Reports indicate that the rate of deviation in the interpretation of emergency CT scans was between 1.7% and 4.2%.8–10 In a recent clinical trial using recombinant activated coagulation factor VII (rFVIIa, NovoSeven) to test the possibility of reducing hematoma growth, the inter- and intra-reader variability of CT scan interpretations was also evaluated. The CT lesion assessment methods used in this trial are presented here and may have value in future ICH clinical trials that investigate the effects of early hemostatic interventions.
Materials and Methods
Study Overview
The evaluation of intra- and inter-reader variability in the measurement of hematoma volume by using CT scans was conducted as part of a multicenter (73 sites in 20 countries), randomized, double-blind, parallel group, placebo-controlled trial that evaluated the effects of treatment of acute ICH with rFVIIa versus placebo (399 patients).11 The trial was approved by local institutional review boards and by local and national ethics boards as applicable. A baseline CT scan was obtained within 3 hours after symptom onset to confirm the diagnosis of ICH. rFVIIa or placebo was administered within 1 hour after the baseline scan. CT scans were repeated at 24 hours and 72 hours posttreatment.
Patient Population
Three hundred ninety-nine patients, 18 years of age or older, with spontaneous intracerebral hemorrhage were randomized and received placebo or rFVIIa (40, 80, 160 μg/kg). Exclusion criteria included ICH secondary to aneurysm, arteriovenous malformation, or trauma. Patient demographics were previously reported for this study in the literature.11 Hemorrhages were most commonly located in the basal ganglia (53%), followed by the thalamus (34%) and frontal lobes (12%). Mean ICH volume at baseline (24 ± 25 mL) was similar in all 4 groups. The mean interval from ICH onset to baseline CT scan was 114 ± 35 minutes. A baseline CT scan was obtained in most patients (52%) within 1 to 2 hours after symptom onset. The mean interval from baseline CT scan to treatment was 54 ± 21 minutes, and the mean time from ICH onset to treatment was 167 ± 32 minutes. Seven percent of patients were treated within 2 hours of onset, and 63%, within 3 hours. Only 1 patient was treated more than 4 hours after onset of symptoms.
CT Technique
All study sites were required to provide documentation as to the standard parameters used for each CT scanner. The specific scan protocol/parameters of the initial CT evaluation were limited by the emergent nature at the time of admission. However, after a patient was enrolled, all follow-up scans were required to match the baseline CT scan with regard to section thickness, section spacing (overlap or no gap), matrix, field of view, and scan angulations. Consistency in these parameters across all CT evaluations for a patient allowed comparable measurements because of identical spatial resolution. Consistent section spacing was required to provide a contiguous dataset for volumetric calculations.
Image-Data Management
CT data were transmitted digitally to an imaging laboratory (Bio-Imaging Technologies Inc, Newtown, Pa) and analyzed by using Analyze software, a fully integrated toolkit for interactive display, processing, and measurement of biomedical image data developed by the Mayo Clinic (Rochester, Minn). The resulting images were evaluated for completeness, technical quality, and adherence to the protocol requirements. Analyze computed the volume results and listed them in a log, which was directly imported into the blind read data base. All digital images were stored in the format received, in Analyze format, and in Digital Imaging and Communications in Medicine (DICOM) format.
All acquisition values that related to spatial resolution as well as the display parameters were read directly from the header data elements that were recorded with each image. These parameters were used to calculate the pixel size of the image (field of view/matrix = pixel size), which allowed accurate volume determinations for section thickness and overlap variation in CT protocols directly from the image.
Masked Reading Method
The data base architecture allowed a logical progression design, forcing the readers to answer the appropriate questions and autochecking for missing or improper data. (Fig 1). Inter-reader variability was determined by having the CT image analyzed by 2 independent neuroradiologists who were masked to treatment. All CT images were randomly presented. Intra-reader variability was evaluated by randomly redisplaying 10% of previously read CT images. If there was a 10 mL or greater discrepancy between the 2 readers in the measurement of ICH, IVH, or edema volumes, then these CT images were returned to be reread together with 2 other randomly selected images to minimize bias. When a 24-hour scan was not available within the specified time range, the last available follow-up scan obtained within the 24 hours was analyzed, when available. Inadequate or missing data were identified and assigned a value of NA for calculation purposes. masked read interpretation data were collected via the electronic case report form.
Fig 1.
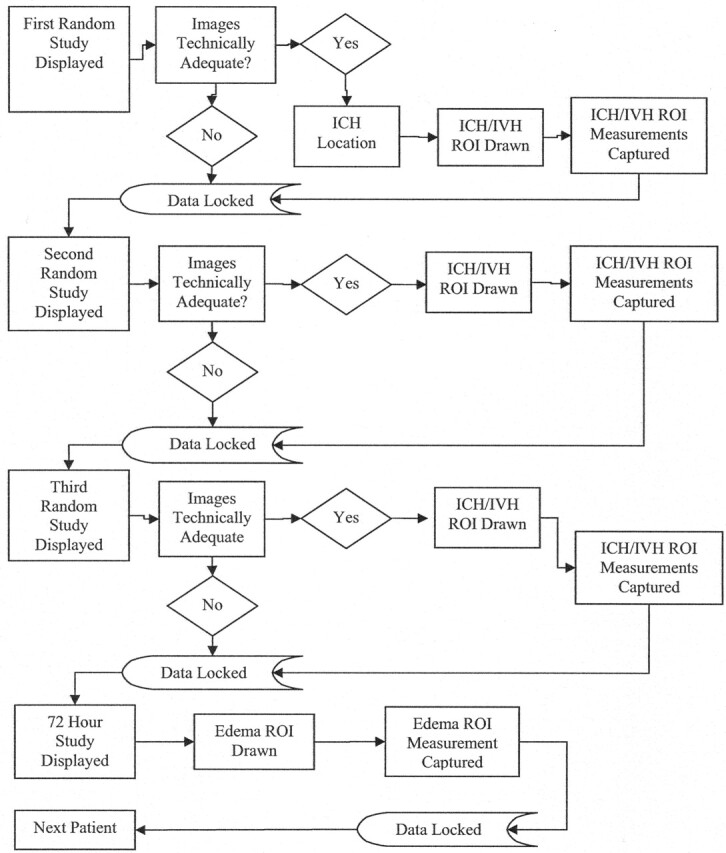
Masked read-flow diagram for each patient.
Hematoma Volume Measurements
The ICH, IVH, and edema volumes were defined by using the semiautomated segmentation and/or freehand tracing tools in the region of interest module from the Analyze software. This procedure was reproduced for each section as well as each separate target area as determined by the reader (Fig 2). After all areas were defined by the reader, the Analyze software provided area (square millimeters) and volume (cubic millimeters) measurements of each region of interest. The volume of the region of interest was calculated by multiplying the section thickness of the acquisition by the area. Because an object could include more than 1 section, the region of interest volume for each section was captured in a Stat Log Region of Interest file. These values were automatically imported into the masked read data base and used to calculate the volume (cubic millimeters) and percentage of change in ICH volume from the screening to the 24-hour CT scan, the ratio of edema to ICH volume for each CT scan time point, and the total hemorrhage for each CT scan. Volumes in cubic millimeters were converted to milliliters for purposes of reporting final results. The reader also identified the location of the ICH as midbrain, thalamus, basal ganglia, cerebellum, lobar, or other. Only areas of intraparenchymal hemorrhage were included in the ICH volume measurement. Areas of subarachnoid and subdural hematomas were excluded.
Fig 2.

CT scan showing region of interest. Region of interest, area 1, defines zones of ICH. Region of interest, area 2, defines zones of edema. From these reader-defined regions, the Analyze software calculated statistics of each region of interest (area in square millimeters and volume in cubic millimeters).
Masked Reading Calculations
The change in lesion (ICH, IVH, or edema) volume from the screening to the 24-hour CT scan was expressed in cubic millimeters and percentage:
Percentage change in lesion volume = [(lesion volume at 24 hours –lesion volume at screening) / lesion volume at screening] × 100.
Absolute change in lesion volume = lesion volume at 24 hours –lesion volume at screening.
Ratio of edema / ICH volume = volume of edema / ICH volume.
Total hemorrhage volume = ICH + IVH.
Results
Inter-Reader Variation
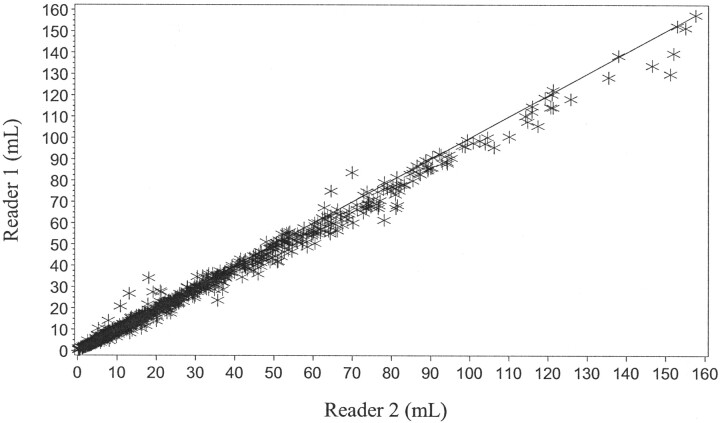
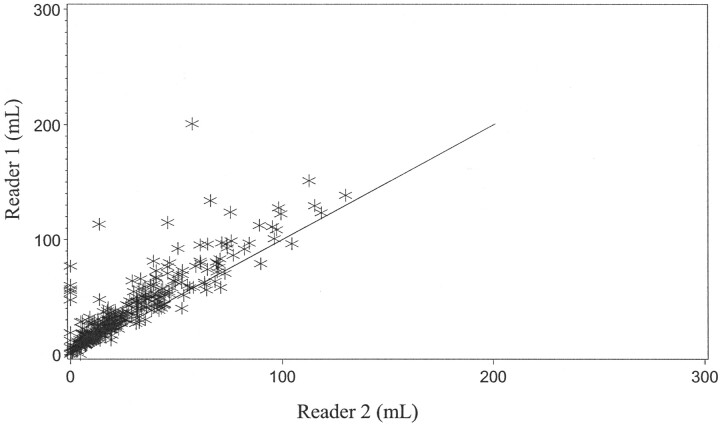
Of the 1144 CT scans evaluated for the determination of ICH volume, 82% demonstrated either only a 2-mL difference or no difference between the estimates for either reader (Fig 3). The intraclass correlation coefficient for the determination of ICH volume was 0.9596. In 84% of the CT scans evaluated for changes in IVH volume, there was either no difference or only a 2-mL difference between reader 1 and reader 2 (Fig 4). The intraclass correlation coefficient for the determination of IVH volume was 0.9468. Mean differences between the readers in estimates of the volume of the more ill-defined edematous area at 72 hours were greater, roughly 5–15 mL (Fig 5). The intraclass correlation coefficient for the determination of edema volume was 0.7363. With the exception of edema volume measurements for all visits and all treatment groups, ICH and IVH volume readings as estimated by reader 2 tended to be slightly greater than those of reader 1.
Fig 3.
Graph shows agreement between reader 1 and reader 2 (ICH volume measurement).
Fig 4.
Graph shows agreement between reader 1 and reader 2 (IVH volume measurement).
Fig 5.
Graph shows agreement between reader 1 and reader 2 (edema volume measurement).
Intra-Reader Variation
Ninety-one scans were reread, most of them by both readers, resulting in a total of 179 reread values. Mean variations in ICH and IVH volume estimates among reread CT scans are summarized for all visits combined in Table 1. Almost all the discrepancies between reread estimates of volumes of IVH, ICH, or edema were very small (<1 mL) within each treatment group and overall. An exception was the estimation of edema volume in the placebo group, in which intra-reader differences were approximately 10 mL greater and contributed to an overall variation that was slightly more than 3 mL. The intraclass correlation coefficient was 0.9844 for ICH volume, 0.9918 for IVH volume, and 0.7266 for edema volume.
Intra-reader variation of ICH, IVH, and edema volumes by treatment
| Placebo | 40 mcg | 80 mcg | 160 mcg | Total | r2 | |
|---|---|---|---|---|---|---|
| ICH volume (mL) | ||||||
| N | 44 | 37 | 50 | 48 | 179 | .9844 |
| Mean (SD) | −0.2 (1.6) | 0.1 (0.5) | −0.1 (2.0) | −0.2 (2.5) | −0.1 (1.9) | |
| IVH Volume (mL) | ||||||
| N | 44 | 37 | 50 | 48 | 179 | .9918 |
| Mean (SD) | −0.1 (0.2) | −0.1 (0.1) | 0.4 (2.2) | 0.0 (0.2) | 0.1 (1.2) | |
| Edema volume (mL) | ||||||
| N | 14 | 14 | 10 | 12 | 50 | .7266 |
| Mean (SD) | −12.6 (36.2) | 0.0 (0.2) | −0.2 (0.7) | −0.6 (2.3) | −3.7 (19.5) | |
Note:—ICH indicates intracerebral hemorrhage; IVH, intraventricular volume.
Discussion
Neurologic deterioration in patients with ICH was previously thought to be due to hemorrhage-induced edema and mass effect. However, recent studies have shown that early neurologic deterioration actually correlates well with early hematoma growth. Accurate lesion measurements may allow the use of hematoma sizes and changes as clinical correlates or surrogate end points for therapeutic outcomes in clinical intervention trials.
CT is currently the imaging technique of choice to detect early hematoma associated with intracerebral hemorrhage. This technique has the potential to provide quantitative hematoma data, with implications for use as a predictor of treatment benefit. Differences in scanning equipment and experience levels of attending radiologists, neurologists, and neurosurgeons require assurance that the detection and estimation of hematoma volume and location are accurate and that CT interpretations are reliable. Minimal inter-reader variability will ensure validity and reliability in the interpretations of CT scans.
In the current study, blinded readers used CT and established software packages to measure and quantify changes in early hematoma volume and to monitor treatment effects in patients with ICH. The results showed minimal variation in the inter- and intra-reader measurements and excellent agreement with respect to early hematoma volume, expansion/extension of hematomas, and extent of treatment benefit. The high degree of inter- and intraobserver agreement was particularly impressive given the complex appearance of many hematomas, especially those involving cortical and subcortical regions (Fig 1). Focal areas of hemorrhage of varying sizes were interspersed with normal or edematous nonhemorrhagic brain. The lower agreement rates for edema volume are likely a consequence of 2 factors. First, differentiation of normal and edematous brain was more difficult than that of hemorrhagic and nonhemorrhagic brain. Second, edema volumes had to be traced by hand, whereas semiautomated software was used to define regions of hemorrhage.
These results suggest that CT scans can accurately quantify hematoma volume and monitor hemorrhage evolution in ICH patients, supporting the validity of CT interpretations made by different readers. Hematoma assessments using CT methods may have value in future clinical trials of early hemostatic intervention in ICH.
Acknowledgments
The following primary investigators provided clinical sites of the Recombinant Activated Factor VII Intracerebral Hemorrhage Trial: Australia: S. Davis, Parkville, Victoria; G. Donnan, Heidelberg, Victoria; D. Freilich, Footscray, Victoria; R. Gerraty, Fitzroy, Victoria; T. Kimber, Adelaide, South Australia; D. Shutz, Bedford Park, South Australia; Austria: F. Fazekas, Graz; Belgium: S. Blecic, Brussels; P.P. De Deyn, Antwerp; V. Thijs, Leuven; Canada: P. Bailey, Saint John, NB; M. Hill, Calgary, AB; D. Selchen, Mississuaga, ON; C. Voll, Saskatoon, SK; A. Woolfenden, Vancouver, BC; Croatia: V. Demarin, Zagreb; Denmark: G. Andersen, Aarhus; G. Boysen, Copenhagen; Finland: M. Kaste, Haartmaninkatu; Germany: O. Busse, Minden; A. Ferbert, Kassel; M.A. Grond, Siegen; R. Haberl, Munich; M. Hennerici, Mannheim; D. Schneider, Leipzig; T. Steiner, Heidelberg; Italy: C. Argentino, Rome; V. Gallai, Perugia; D. Guidetti, Reggio Emilia; G. Miceli, Pavia; Malaysia: R.A. Adman Zurin, Kuala Lumpur; Netherlands: J.U.R. Niewold, Emmen; M. Vermeulen, Amsterdam; New Zealand: C. Anderson, Auckland; A. Barber, Auckland; Norway: U. Waje-Adnreassen, Bergen; Singapore: I. Ng, Singapore; Spain: A. Chamorro, Barcelona; A. Davalos, Girona; J. Egido, Madrid; J.V. Osorio, Madrid; J.A. Sabin, Barcelona; Sweden: M. Callander, Linkoping; T.B. Kall, Stockholm; N.G. Walgren, Stockholm; Switzerland: J. Bogousslavsky, Lausanne; Taiwan: Y.H. Chang, Taipei; T.K. Lin, Taoyuan; Y.K. Tu, Taipei; United Kingdom: J. Barrett, Merseyside; G. Ford, Newcastle Upon Tyne; M.J. Macleod, Glasgow; United States: B.F. Fitzsimmons, NY; C. Graffagnino, Durham, NC; D. Green, Honolulu, Hawaii; J. Grotta, Houston, Tex; S.E. Kastner, Philadelphia, Pa; R. Libman, New Hyde Park, NY; T. Lowenkopf, Portland, Ore; F. McGee, Richmond, Va; B. Meyer, San Diego, Calif; J. Rosand, Boston, Mass; C. Wijman, Palo Alto, Calif.
This study received the financial support of Novo Nordisk, Inc.
References
- 1.Fiebach JB, Schellinger PD, Gass A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke 2004;35:502–06 [DOI] [PubMed] [Google Scholar]
- 2.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5 [DOI] [PubMed] [Google Scholar]
- 3.Dennis MS, Burn JP, Sandercock PA, et al. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke 1993;24:796–800 [DOI] [PubMed] [Google Scholar]
- 4.Fujii Y, Tanaka R, Takeuchi S, et al. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg 1994;80:51–57 [DOI] [PubMed] [Google Scholar]
- 5.Kazui S, Naritomi H, Yamamoto H, et al. Enlargement of spontaneous intracerebral hemorrhage: incidence and time course. Stroke 1996;27:1783–87 [DOI] [PubMed] [Google Scholar]
- 6.Herbstein DJ, Schaumberg HH. Hypertensive intracerebral hematoma: an investigation of the initial hemorrhage and rebleeding using chromium Cr 51-labeled erythrocytes. Arch Neurol 1974;30:412–14 [DOI] [PubMed] [Google Scholar]
- 7.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93 [DOI] [PubMed] [Google Scholar]
- 8.Erly WK, Ashdown BC, Lucio RW, et al. Evaluation of emergency CT scans of the head: is there a community standard? AJR Am J Roentgenol 2003;180:1727–30 [DOI] [PubMed] [Google Scholar]
- 9.Mucci B, Brett C, Huntley LS, et al. Cranial computed tomography in trauma: the accuracy of interpretation by staff in the emergency department. Emerg Med J 2005;22:538–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wysoki MG, Nassar CJ, Koenigsberg RA, et al. Head trauma: CT scan interpretation by radiology residents versus staff radiologists. Radiology 1998;208:125–28 [DOI] [PubMed] [Google Scholar]
- 11.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85 [DOI] [PubMed] [Google Scholar]