Abstract
BACKGROUND AND PURPOSE: The imaging features of metastatic melanomas are distinctive due to the presence of melanin and the propensity for hemorrhage. Both hemorrhage and melanin can produce T1-weighted hyperintensity and T2*-weighted signal intensity loss. We hypothesized that T2*-weighted images would improve detection of metastatic melanoma.
METHODS: The T2* and T1 characteristics of 120 newly detected metastatic brain lesions from 31 patients with malignant melanoma were compared with those of 120 brain metastases from 23 patients with lung cancer.
RESULTS: Melanoma metastases were 5 times more likely to demonstrate prominent T2*-related signal intensity loss (susceptibility effect) than were lung metastases (42% vs 8%; P < .01), and 4.5 times more likely to demonstrate T1 hyperintensity (55% vs 12%; P < .01). Patients with melanoma had lesions that were either hypointense on T2*-weighted images, hyperintense on T1 images, or both, in 71% (85/120), compared with 19% (23/120) of lung carcinoma metastases (P < .01). Melanoma lesions were 16 times more likely than lung cancer lesions to show combined T2* related signal intensity loss and T1 hyperintensity (P < .01). Remarkably, 8 melanoma lesions (7%) in 3 patients were detectable principally on the T2*-weighted sequences, whereas no lung cancer lesion was detected solely on susceptibility images. We found a direct correlation between melanin content and T1 hyperintensity but no correlation between T2* intensity and melanin.
CONCLUSION: T2*-weighted images improve lesion detection in patients with melanoma metastases, and in conjunction with T1-weighted sequences, can suggest melanoma as the etiology of an intracranial mass. This sequence should be employed for evaluation of possible brain metastasis in patients without a known primary malignancy and in studies for melanoma staging.
Brain metastasis is a common and devastating consequence of metastatic malignant melanoma.1 MR imaging is the most sensitive diagnostic technique in the assessment of patients with cerebral metastases but in general lacks specificity for type of primary. Melanoma can produce distinctive MR findings,2 however, because approximately half of melanoma metastases are hyperintense on T1-weighted images before the administration of gadolinium whereas other cerebral metastases rarely demonstrate T1 hyperintensity.2–4 Potential explanations for this intrinsic T1 hyperintensity include the presence of blood products and melanin.5 Blood products and melanin can also produce T2*-weighted signal intensity loss,6 but the characteristics of metastatic melanoma on susceptibility images have not been well described in the literature. We hypothesized that T2*-weighted images would improve detection of metastatic melanoma and that together with T1-weighted images might be used to increase suspicion of melanoma as the source of a potential metastatic intracranial lesion. To test this hypothesis, we compared the findings of metastases from melanoma to those from lung cancer.
Materials and Methods
Patients.
The medical records and neuroimaging studies of patients with known malignant melanoma who had newly detected brain metastases were reviewed. Inclusion criteria included the availability of preirradiation T1-weighted MR images with and without gadolinium enhancement and T2*-weighted images. We examined 120 lesions from 31 patients with melanoma. For a comparison group, the imaging findings of 120 lesions from 23 patients with brain metastases from lung carcinoma were reviewed. Very large lesions with extensive necrosis and markedly heterogeneous signal intensity changes were excluded. Approval for this study was obtained from the institutional review board.
Imaging.
MR imaging was performed with a 1.5T unit. The MR imaging scans included T2*-weighted gradient-echo (susceptibility; repetition time, 750 milliseconds; effective echo time, 25 milliseconds; flip angle, 20°; number of excitations, 2); T1-weighted (repetition time, 400–625 millisecond; effective echo time, 14–17 milliseconds; number of excitations, 1), T1-weighted with gadolinium (repetition time, 400–625 milliseconds; effective echo time, 14–17 milliseconds; number of excitations 1), and fluid-attenuated inversion recovery (FLAIR; repetition time, 9000 milliseconds; effective echo time, 120 milliseconds; inversion time, 2200 milliseconds; number of excitations, 1) sequences. All sequences were performed with a section thickness of 5 mm with a 1-mm gap.
Data Analysis.
Axial T1-weighted images before and following the administration of gadolinium, FLAIR images, and T2*-weighted images were retrospectively reviewed in a nonblinded fashion by 2 neuroradiologists. Lesions were scored as (1) not detectable, mildly conspicuous, or very conspicuous; (2) hypointense, isointense, or hyperintense; (3) contrast enhancing or not contrast enhancing; and (4) with or without vasogenic edema. Lesions that demonstrated heterogeneous signal intensity were judged to be predominately hyperintense or not (T1-weighted images) and predominately hypointense or not (T2*-weighted sequences).
Histopathologic Analysis.
We reviewed available biopsy (19) or autopsy (one) material from 20 of the patients with melanoma. The biopsies were derived from lymph nodes (8), skin (8), lung (2; both were cytology specimens), brain (3), bone (one), and maxillary sinus (one). Samples were scored as amelanotic (no melanin detected), slightly melanotic (1%–25% of tumor cells contained pigment), or heavily melanotic (>25% of tumor cells contained pigment).
Statistical Analysis.
To avoid the cluster effects associated with the intercorrelation of multiple lesions in the same patient, we reduced the information for each patient to a single number by calculating the fraction of lesions with a particular finding for each patient. Then, to assure that the assumption of a common standard deviation was met even if there was patient to patient variability in the rate, we applied the arcsine square root transformation to the values. In all comparisons of the frequencies of the imaging findings in melanoma and lung cancer metastases, we performed the Student t test on the transformed values (significance level P ≤.05).
Results
The T2*-weighted and T1-weighted imaging characteristics of 120 brain lesions in patients with metastatic melanoma were compared with 120 metastatic lesions in patients with carcinoma of the lung. The clinical characteristics of the 2 patient groups are presented in Table 1, and the general characteristics of the brain metastases are presented in Table 2. Patients with melanoma were more than twice as likely to have systemic metastases at the time of brain tumor detection than were patients with lung cancer (P < .01).
Table 1:
Clinical characteristics of study patients
| Melanoma | Lung | |
|---|---|---|
| Patients | 31 | 23 |
| Average age (y) | 59.6 (SD 14.0) | 62.5 (SD 10.2) (P = .40) |
| Sex (M:F) | 23:8 | 9:14 (P < .01) |
| Systemic metastasis* | 94% (29/31) | 35% (8/23) (P < .01) |
| Primary | ||
| Skin | 27 | |
| Ocular | 1 | |
| Unknown | 3 | |
| NSCLC | 20 | |
| SCLC | 3 |
Notes:—NSCLC indicated non-small cell lung carcioma; SCLC, small cell lung carcioma.
Present at time of brain metastasis diagosis.
Table 2:
Characteristics of metastatic brain lesions
| Melanoma | Lung | |
|---|---|---|
| Total lesions examined | 120 | 120 |
| Average lesions per patient | 3.9 | 5.2 (P = .36) |
| Single lesion | 12 patients | 7 patients |
| Average diameter (mm) | 9 | 8 (P = .18) |
| Supratentorial | 106 | 98 (P = .25) |
Lesion Characteristics on Imaging
On T2*-weighted sequences, 42% (50/120) of lesions in melanoma patients showed prominent hypointensity, compared with only 8% of lesions from lung cancer (P < .01). Thus, lesions in patients with melanoma were 5 times more likely to demonstrate T2*-weighted signal intensity loss than were lesions in patients with lung cancer.
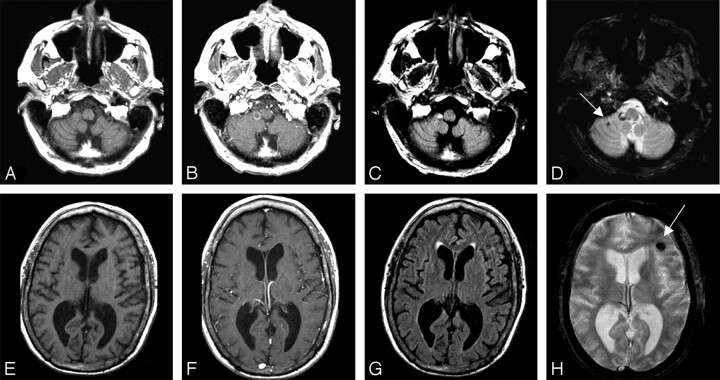
Eight lesions (7%) in 3 melanoma patients were detectable principally on the T2*-weighted sequence, whereas no lung cancer lesion was detected solely by susceptibility effect (Fig 1). These 8 lesions were small in size (3–8 mm in diameter), demonstrated no edema on FLAIR images, and did not enhance. In one patient, the presence of presumed melanoma brain metastasis would not have been detected without the T2*-weighted sequence. In the other 2 patients, additional lesions were identified on other sequences.
Fig 1.
Melanoma brain metastases were detected principally on T2*-weighted sequences in 7% of 120 lesions. T1 isointense, nonenhancing lesions are seen in the right cerebellar hemisphere lesion (top row) and left frontal lobe (bottom row). A subtle abnormality can be seen on the FLAIR image (G), but the lesion is markedly more conspicuous on T2*-weighted image (H). T1-weighted sequences before (A, E) and after (B, F) administration of gadolinium, FLAIR sequences (C, G), and T2*-weighted sequences (D, H) are shown.
Fifty-five percent (66/120) of melanoma metastases showed conspicuous T1 hyperintensity, compared with 12% (15/120) of lung cancer metastases (P < .01). Thus, T1 hyperintensity was 4.5 times more common in melanoma lesions than in lung carcinoma metastases.
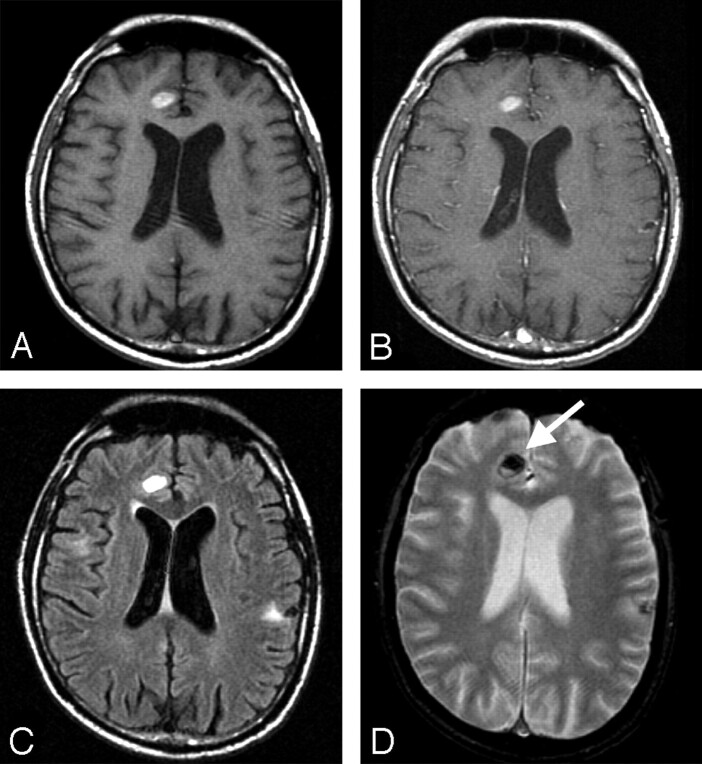
There was variation of T2*-weighted effect and T1 hyperintensity within the lesions of individual patients (that is, lesions in an individual patient exhibited a wide variation in imaging findings). Melanoma metastases were either hypointense on T2*-weighted images, hyperintense on T1-weighted images, or both in 71% (85/120) of melanoma lesions, compared with 19% of lung lesions (P < .01). Combined T1-weighted hyperintensity and T2*-weighted signal intensity loss were present in 26% (31/120) of melanoma lesions but in only 2% of lung lesions (P < .01; Fig 2). Therefore, this combination was 16 times more likely in lesions from melanoma than in lesions from lung cancer.
Fig 2.
Combined susceptibility effect and T1-weighted hyperintensity were seen in only one quarter of lesions, but this combination was 16 times more likely with melanoma than with lung cancer metastases. Intrinsic T1-weighted hyperintensity (A), minimal enhancement (B), minimal surrounding edema on a FLAIR image (C), and susceptibility effect (D) are shown in metastatic melanoma in the right frontal lobe.
Additional MR imaging characteristics of the metastatic lesions, including enhancement and presence of edema, are presented in Table 3. Contrast enhancement was less commonly detectable in patients with melanoma than with lung cancer (P =.02).
Table 3:
MR imaging features of metastatic brain lesions
| Melanoma | Lung | |
|---|---|---|
| Total lesion number | 120 | 120 |
| Susceptibility effect | 42% (50/120) | 8% (10/120) (P < .01) |
| Amelanotic/slightly | 49% (17/35)* | |
| Heavily melanotic | 33% (10/30)† (P = .4) | |
| Hyperintense T1WI | 55% (66/120) | 12% (15/120) (P < .01) |
| Amelanotic/slightly | 49% (17/35) | |
| Heavily melanotic | 83% (25/30) (P < .01) | |
| Enhancement | 78% (94/120) | 98% (118/120) (P = .02) |
| Edema | 68% (82/120) | 66% (80/120) (P = .56) |
Total of 35 lesions in 20 patients with biopsy material.
Total of 30 lesions in 20 patients with biopsy material.
Correlation of Melanin Content and Imaging Findings
Tumors were graded histopathologically as amelanotic (3), slightly melanotic (10), or heavily melanotic (7). The brain metastases of patients whose biopsies showed heavy melanin were more likely to demonstrate T1 hyperintensity than were the metastases in patients with amelanotic or lightly melanotic tumors (P < .01). A similar correlation was not observed for susceptibility effect.
Discussion
Our data demonstrate that T2*-weighted signal intensity loss and T1 shortening are both 5 times more common in melanoma metastases than in lung cancer metastases. Three quarters of melanoma metastases had either susceptibility effect or intrinsic T1 hyperintensity, whereas only 25% had both findings, demonstrating that individual metastatic lesions had considerable variation in the findings on these sequences. The combination of T2*-weighted signal intensity loss and T1 hyperintensity in a lesion, however, was 16 times more common with melanoma metastases than with lung cancer metastases. It is important to note that 7% of melanoma lesions were detected principally on T2*-weighted sequences. Finally, we found a direct correlation between melanin content in tumor cells from biopsy tissue and T1 hyperintensity, whereas this finding was not seen with susceptibility effect.
Despite its relative rarity as a systemic neoplasm, melanoma is the third-most-common primary to produce brain metastasis and thus must be considered as the cause of newly detected brain masses in patients with no known primary.7 Our findings demonstrate that T2*-weighted and T1 sequences can be used to increase the level of suspicion for melanoma as the source of brain metastases. Contrast-enhanced MR imaging, however, remains the gold standard for the diagnosis of brain metastasis, and biopsy of a brain or extracranial lesion is required to establish a specific histopathologic diagnosis.
The observation that occasional melanoma lesions are detected primarily or solely on T2*-weighted sequence was unexpected. Although other lesions, such as cavernous angioma, hypertensive hemorrhage, or hemorrhagic infarction, would be a possible cause of these isolated T2*-weighted findings,8 it is more likely that the changes were produced by small melanoma metastases. This finding underlines the value of the T2*-weighted sequence in patients with known melanoma who are undergoing CNS staging.
Two major explanations have been advanced to explain T1 relaxation in melanoma. Melanin itself may lead to T1 shortening.3, 6 Melanoma metastases have a well-known propensity for hemorrhage, and methemoglobin can also produce T1 shortening.5 In addition to T1 shortening, melanin and blood products may also produce susceptibility effect on T2* images because of the presence of metal ions including iron, copper, manganese, and zinc.6
To test the relationship between melanin content and findings on T2*-weighted and T1 images, we estimated the melanin content of the tumor cells in biopsy material.3 We found an association between tumors with heavy melanin content and the presence of marked T1 hyperintensity in the brain metastases. This analysis used the biopsy tissue from another site as a surrogate for the melanin content of the brain lesions, and it is known that the melanin content of primary and metastatic lesions within an individual patient’s body can vary.9 We found, however, that all biopsies from separate sites in 3 patients had the same melanin content, which suggests that marked variation between lesions is not common.
No correlation was found between melanin content and susceptibility effect, which suggests that T1 shortening correlates more closely with melanin content than does the T2*-weighted signal intensity loss. This finding might suggest that other characteristics of the metastasis, such as the metal content of melanin or the presence of hemorrhage, are important in determining susceptibility effect. In vitro analysis will be required to clarify this issue.
In conclusion, T2*-weighted images may improve detection of metastatic melanoma in patients undergoing tumor staging, because some lesions are detectable principally or solely on these images. In addition, T2*-weighted images can be used to suggest melanoma as the etiology of lesions with the appearance of brain metastases.
Footnotes
This work was supported by the Stephen E. and Catherine Pappas Brain Tumor Imaging Research Program.
References
- 1.McWilliams RR, Brown PD, Buckner JC, et al. Treatment of brain metastases from melanoma. Mayo Clin Proc 2003;78:1529–36 [DOI] [PubMed] [Google Scholar]
- 2.Escott EJ. A variety of appearances of malignant melanoma in the head: a review. Radiographics 2001;21:625–39 [DOI] [PubMed] [Google Scholar]
- 3.Atlas SW, Braffman BH, LoBrutto R. Human malignant melanoma with varying melanin content in nude mice: MR imaging, histopathology, and electron paramagnetic resonance. J Comput Assist Tomogr 1990;14:547–54 [DOI] [PubMed] [Google Scholar]
- 4.Atlas SW, Grossman RI, Gomori JM, et al. MR imaging of intracranial metastatic melanoma. J Comput Assist Tomogr 1987;11:577–82 [DOI] [PubMed] [Google Scholar]
- 5.Isiklar I, Leeds NE, Fuller GN, et al. Intracranial metastatic melanoma: correlation between MR imaging characteristics and melanin content. AJR Am J Roentgenol 1995;165:1503–12 [DOI] [PubMed] [Google Scholar]
- 6.Enochs WS, Petherick P, Bogdanova A, et al. Paramagnetic metal scavenging by melanin: MR imaging. Radiology 1997;204:417–23 [DOI] [PubMed] [Google Scholar]
- 7.Kalkman E, Baxter G. Melanoma. Clin Radiol 2004;59:313–26 [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, Kucharczyk W, Henkelman RM. Cavernous hemangiomas: dipolar susceptibility artifacts at MR imaging. Radiology 1993;187:735–41 [DOI] [PubMed] [Google Scholar]
- 9.Gupta RK, Lallu S. Cytodiagnosis of amelanotic metastatic malignant melanoma: an immunocytochemical study. Diagn Cytopathol 1997;16:238–41 [DOI] [PubMed] [Google Scholar]